Abstract
Nitric oxide- (NO) and hydrogen sulfide- (H2S) releasing naproxen (NOSH-naproxen) and NO and H2S-releasing sulindac (NOSH-sulindac) were synthesized and their cell growth inhibitory properties were evaluated in four different human cancer cell lines. These cell lines are of adenomatous (colon, pancreas), epithelial (breast), and lymphocytic (leukemia) origin. Using HT-29 human colon cancer cells, NOSH-naproxen and NOSH-sulindac increased apoptosis, and inhibited proliferation. NOSH-naproxen caused a G0/G1 whereas NOSH-sulindac caused a G2/M block in the cell cycle. Both compounds exhibited significant anti-inflammatory properties, using the carrageenan rat paw edema model. Reconstitution and structure-activity studies representing a fairly close approximation to the intact molecule showed that NOSH-naproxen was approximately 8000-fold more potent than the sum of its parts in inhibiting cell growth. Our data suggest that these compounds merit further investigation as potential anti-cancer agents.
1. Introduction
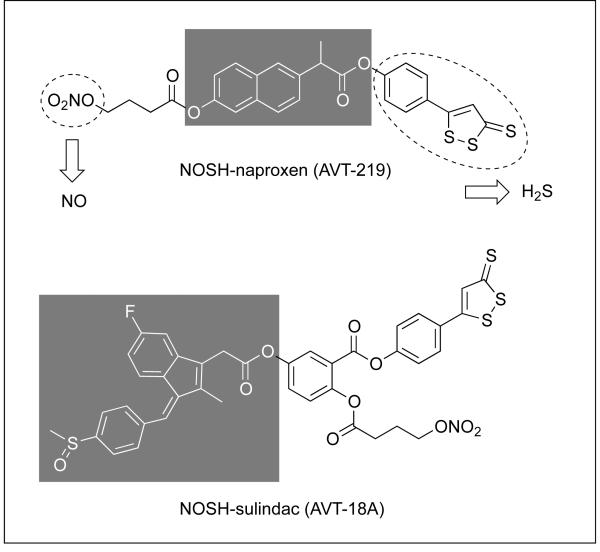
Non-steroidal anti-inflammatory drugs (NSAIDs) in general and aspirin in particular are recognized as the prototypical chemopreventive agents against cancer. However, long-term use of NSAIDs may lead to significant side effects, mainly gastrointestinal, ranging from dyspepsia to gastrointestinal bleeding, obstruction, and perforation; renal, and cardiovascular (reviewed in 1). Amongst patients using NSAIDs, it is estimated that about 16,500 deaths occur every year in the United States. This figure is considerably greater than the number of deaths from multiple myeloma, asthma, cervical cancer, or Hodgkin’s disease.2 Unfortunately, many physicians and most patients are unaware of the magnitude of the problem. The gastric damage is as a result of direct epithelial damage due to their acidic properties and also through the breakdown of mucosal defence mechanisms (leukocyte adherence, decreases in blood flow, bicarbonate and mucus secretions) due to a reduction of mucosal prostaglandin (PG) synthesis.3 In our search for a “better NSAID” we developed NOSH-aspirin,4 a hybrid entity capable of releasing both, nitric oxide (NO) and hydrogen sulfide (H2S), two gasotransmitters of physiological significance. Our rational was based on the observations that NO and H2S could enhance the local gastric mucosal defence mechanisms, thereby decreasing NSAID-induced gastric toxicity.5-8 Here we report on the synthesis and some of the biological properties of two other NO- and H2S-releasing NSAIDs, NOSH-naproxen and NOSH-sulindac, Fig. 1.
Figure 1. Structural components of NOSH-naproxen and NOSH-sulindac.
The parent compounds, naproxen and sulindac are shown in the shaded boxes. ADT-OH releases H2S and ONO2 releases NO, both shown in dotted ellipses.
2. Results and discussion
2.1. Chemistry
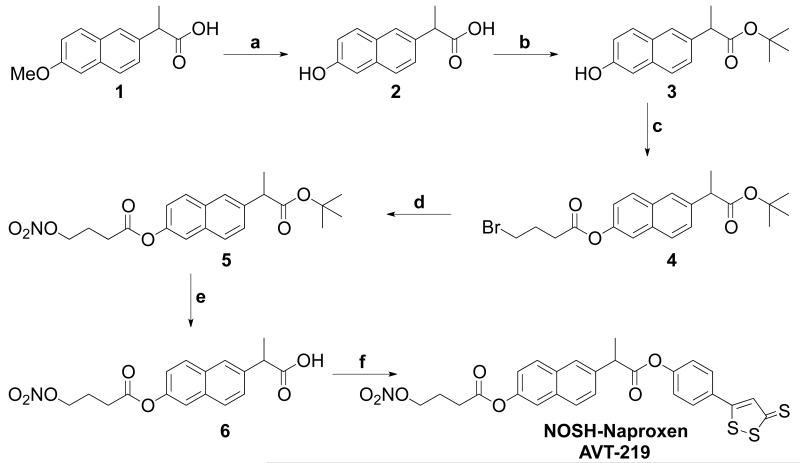
NOSH-naproxen (NOSH-NAP, AVT-219) and NOSH-sulindac (NOSH-SUL, AVT-18A) were prepared as described in schemes 1 and 2, respectively. For the synthesis of NOSH-naproxen, the starting material naproxen (compound 1) was demethylated in the presence of HBr and AcOH to give compound 2. Further, the carboxyl group was protected by a tertiary butyl alcohol to obtain compound 3.9 This was esterified with 4-bromobutyric acid in the presence of DCC/DMAP to compound 4. The bromo moiety in 4 under went nucleophilic displacement with a nitrate group to compound 5. Further, the tertiary butyl group in 5 was deprotected by TFA to give the acid, which was then coupled with ADT-OH in the presence of DCC/DMAP to the final product, NOSH-naproxen, (S)-6-(1-oxo-1-(4-(3-thioxo-3H-1, 2-dithiol-5-yl) phenoxy) propan-2-yl) naphthalen-2-yl 4-(nitrooxy) butanoate) (Scheme-1).
Scheme-1. Synthetic scheme for the synthesis of NOSH-Naproxen (AVT-219).
a. HBr/AcOH, reflux, 4h; b. Tf2O, t-BuOH, NH4OH; c. 4-bromobutyric acid, DCC/DMAP, DCM, rt, 6h; d. AgNO3/acetonitrile, 70°C, 6h; e. TFA/DCM, 30 min; f. ADT-OH, DCC/DMAP, DCM, rt, 6h.
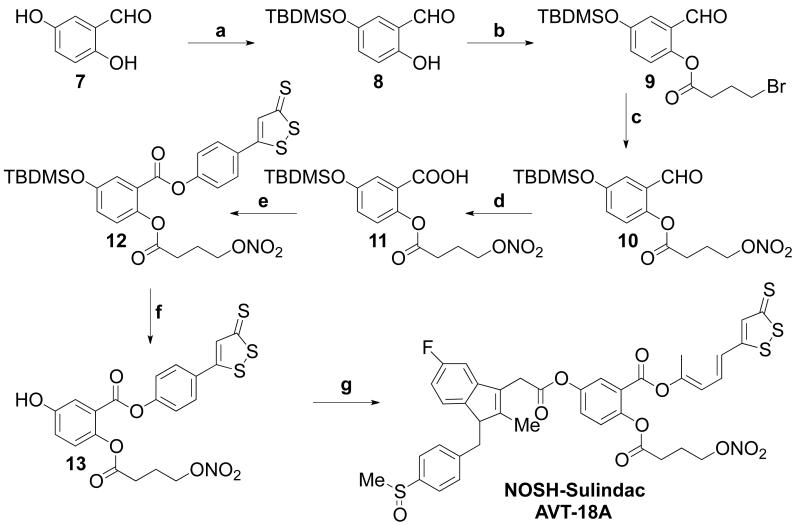
Scheme-2. Synthetic scheme for preparation of NOSH-Sulindac (AVT-18A).
a. TBDMSCl/Imidazole, DCM, 18h; b. 4-bromobutyric acid,DCC/DMAP, DCM, rt, 6h; c. AgNO3/acetonitrile, 70°C, 6h; d. NaH2PO4, H2O2/NaClO2, 0°C, 2h; e. ADT-OH, DCC/DMAP, DCM, rt, 6h; f. TBAF/AcOH, THF, 30 min; g. Sulindac, DCC/DMAP, DCM, rt, 6h.
NOSH-sulindac (AVT-18A) (Z)-4-(3-thioxo-3H-1,2-dithiol-5-yl) phenyl 5-(2-(5-fluoro-2-methyl-1-(4-(methylsulfinyl) benzylidene)-1H-inden-3-yl) acetoxy)-2-((4-(nitrooxy) butanoyl)oxy) benzoate, was synthesized by using 5-hydroxy salicylic acid as the starting material, which underwent selective protection with TBDMSCl to give compound 8.10 This was esterified with 4-bromobutyric acid in the presence of DCC/DMAP to compound 9 which underwent nitration by nucleophilic displacement to afford compound 10. This underwent carboxylation in the presence of NaH2PO4/H2O2, NaClO2 to give compound 11.11 The carboxylic acid in compound 11 was then coupled with ADT-OH to obtain compound 12. This underwent TBDMS deprotection in the presence of TBAF/AcOH to give compound 13, which was then coupled with sulindac in the presence of DCC/DMAP to the final product, NOSH-sulindac (Scheme-II).
2.2. Biological evaluation
2.2.1. NOSH-naproxen and NOSH-sulindac are potent inhibitors of cancer cell growth
The cell growth inhibitory effects of NOSH-NAP, NOSH-SUL and their respective parent compounds was evaluated using an MTT assay in four different cancer cell lines of different tissue origin, that being of, colon, breast, pancreas, and leukemia. NOSH-NAP and NOSH-SUL at 24h strongly inhibited the growth of these human cancer cell lines in a concentration dependent manner; the IC50s for cell growth inhibition are shown in Table 1. The data clearly shows that NOSH-NAP and NOSH-SUL are orders of magnitude more potent than their respective parent compounds in all the cell lines. For NOSH-NAP, the IC50s for cell growth inhibition ranged from 80-150 nM, whereas for the parent compound, naproxen, this range was from 2,350,000-2,775,000 nM. The enhanced potency calculated as the ratio of IC50 values (NAP/NOSH-NAP) indicated that NOSH-NAP was 16,000-34,000-fold more potent than NAP (Table 1). Similarly, NOSH-SUL was also significantly more potent than SUL in inhibiting the growth of these cancer cell lines. The IC50s ranging from 89-270 nM, compared to that of SUL, which ranged from 699,000-980,000 nM. This represents an enhanced potency of 2,500-9,650-fold over SUL (Table 1). These data strongly suggest that the effect of these modified NSAIDs on cell growth inhibition is tissue type independent. Further, that NAP which was not as potent as SUL in inhibiting the growth of these cancer cell lines; became significantly more potent once modified. However, since the IC50s for cell growth inhibition in each of the cell line is approximately the same for NOSH-NAP and NOSH-SUL, one may conclude that the enhanced potency must be due to the released NO and H2S.
Table 1. IC50 values at 24 hr for cell growth inhibition in different cancer cell lines.
| Origin/Cell line, IC50, μM | ||||
|---|---|---|---|---|
| Compound | Colon HT-29 |
Leukemia Jurkats |
Breast MCF-7 |
Pancreas BxPC-3 |
| NAP | 2775 ± 188 | 2550 ± 225 | 2385 ±145 | 2450 ± 100 |
| NOSH-NAP | 0.08 ± 0.01* | 0.10 ± 0.01* | 0.12 ± 0.01* | 0.15 ± 0.02* |
|
Potency
enhancement |
~34,000 | ~25,000 | ~20,000 | ~16,000 |
| SUL | 800 ± 90 | 699 ± 75 | 965 ± 65 | 980 ± 85 |
| NOSH-SUL | 0.089 ± 0.01* | 0.27 ± 0.02* | 0.10 ± 0.01* | 0.12 ± 0.01* |
|
Potency
enhancement |
~9,000 | ~2,500 | ~9,650 | ~8,200 |
Colon, leukemia, breast, and pancreas cancer cell lines were treated with various concentrations of naproxen (NAP), NOSH-naproxen (NOSH-NAP), sulindac (SUL), and NOSH-sulindac (NOSH-SUL) as described under Experimental Section/Biology. Cell numbers were determined at 24 h from which IC50 values were calculated. Results are mean ± S.E.M. of five to seven different experiments done in triplicate.
P < 0.001 compared to respective parent compound.
We also evaluated the effects of NOSH-NAP and NOSH-SUL on normal human lung fibroblast cells (IMR-90), a human primary pancreatic epithelial cell line (HPDE, ACBRI 515), and a human mammary epithelial cell line (HMEpC), Table 2. An inspection of the IC50s at 24h clearly showed a differential response compared to the various cancer cell lines. For example, the IC50 for NOSH-NAP in HMEpC cells was 13.8 ± 0.9 μM (Table 2) whereas the IC50 in MCF-7 cells was 0.12 ± 0.01 μM (Table 1). That is NOSH-NAP was approximately 115-fold more potent in the breast cancer cell line. Similarly in the same cell line, NOSH-SUL was about 150-fold more potent, 15.2 ± 0.7 μM (Table 2) vs. 0.1 ± 0.01 μM (Table 1). Comparing the IC50s in the pancreatic normal (HPDE, Table 2) and cancerous (BxPC-3, Table 1) cell lines showed that NOSH-NAP was about 147-fold more potent in the cancer cell line (22.0 ± 1.8 μM vs. 0.15 ± 0.02 μM). For NOSH-SUL the enhanced potency in the same cancer cell line was >200-fold (>25 μM vs. 0.12 ± 0.01 μM). The IC50s for NOSH-NAP and NOSH-SUL in the normal human lung fibroblast cell line (IMP-90) were 20.0 ± 2.5 μM and 21.7 ± 1.7 μM, respectively (Table 2). These IC50s are also considerably higher than the IC50s in the various cancer cell lines (Table 1), the fold difference being about 70-270-fold. These data indicate that NOSH-NAP and NOSH-SUL inhibit cell growth preferentially in cancer cell lines compared with normal cell lines.
Table 2. IC50 values at 24 hr for cell growth inhibition in different human normal cell lines.
| Origin/Cell line, IC50, μM | |||
|---|---|---|---|
| Compound | Lung Fibroblast IMR-90 (CCL186) |
Mammary Epithelial HMEpC |
Pancreatic Epithelial HPDE (ACBRI 515) |
| NOSH-NAP | 20.0 ± 2.5 | 13.8 ± 0.9 | 22.0 ± 1.8 |
| NOSH-SUL | 21.7 ± 1.7 | 15.2 ± 0.7 | > 25 |
Normal human lung fibroblast, mammary epithelial, and pancreatic epithelial cells were treated with NOSH-naproxen (NOSH-NAP) and NOSH-sulindac (NOSH-SUL) as described under Experimental Section/Biology. Cell numbers were determined at 24 h from which IC50 values were calculated. Results are mean ± S.E.M. of three different experiments done in duplicate.
2.2.2. NOSH-naproxen and NOSH-sulindac alter HT-29 colon cancer cell kinetics
Cellular kinetic parameters, that is, proliferation and apoptosis are two determinants of cellular mass. Therefore, we determined the effects of NOSH-NAP and NOSH-SUL at their respective IC50s for cell growth inhibition on these two parameters as a function of time using HT-29 human colon cancer cells.
Cell proliferation
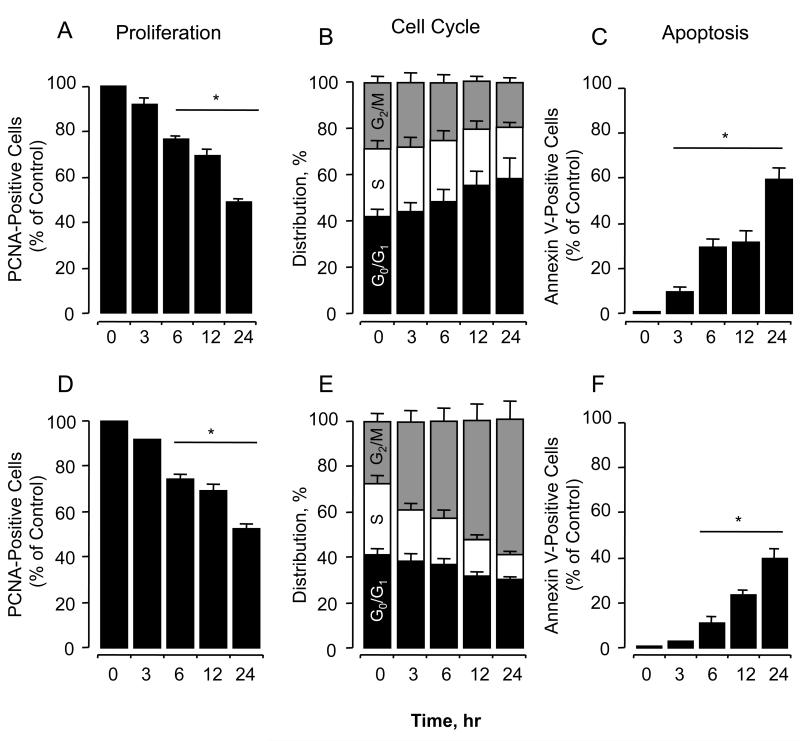
NOSH-NAP and NOSH-SUL reduced PCNA expression in a time-dependant manner. In both cases proliferation was reduced by approximately 50% in 24 hrs, Figs. 2A and 2D respectively.
Figure 2. Effect of NOSH-NAP and NOSH-SUL on HT-29 colon cancer cell kinetics.
NOSH-NAP and NOSH-SUL inhibited proliferation by altering cell cycle progression and inducing apoptosis. Cells were treated with vehicle, or NOSH-NAP (panels A-C) or NOSH-SUL (panels D-F) at their respective IC50 for cell growth inhibition (80 and 90 nM) and analyzed at the indicated times. Panels A and D, proliferation by PCNA antigen expression; Panels B and E), cell cycle phases by PI staining and flow cytometry; Panels C and F), apoptosis by Annexin V staining and flow cytometry. Results are mean ± SEM for 3 different experiments performed in duplicate, *P < 0.05, compared to control.
Cell cycle
Cell cycle progression as measured by DNA content of treated cells using flow cytometry was also affected by NOSH-NAP and NOSH-SUL. Cells treated with NOSH-NAP at its IC50 accumulated progressively in G0/G1 phase of the cell cycle as a function of time (Fig. 2B). The cell populations in the different phases were altered in the following manner compared to control: G0/G1 increased from 42.2 ± 4% to 58.2 ± 7.6%; S phase was reduced from 29.2 ± 3.3% to 22.3 ± 2.2 %, and G2/M reduced from 28.7 ± 2.1 to 19.5 ± 2.1%; suggesting a G0/G1 cell cycle block. This mode of cell cycle arrest has been reported for NOSH-aspirin in HT-29 colon cancer cells12 and for the parent compound aspirin, in different cancer cell lines.13, 14 A different pattern emerged for NOSH-SUL, G0/G1 phase of the cell cycle decreased from 40.9 ± 3.1% to 29.6 ± 2 % as a function of time, S phase was reduced from 31.7 ± 3.7% to 10.6 ± 2.6 %, while the G2/M increased from 27.7 ± 4.2% to 59.8 ± 8.1 % (Fig. 2E), suggesting a G2/M block. This mode of cell cycle arrest has been reported for the parent drug sulindac in SW480 human colon cancer cells.15
Cell death
NOSH-NAP and NOSH-SUL increased the proportion of cells undergoing apoptosis in a time-dependant manner as determined by the Annexin V-FITC staining and flow cytometry. NOSH-NAP treatment of the cells resulted in 9.2 ± 2.6%, 25.3 ± 3.3%, 31.9 ± 4.7%, and 59.2 ± 5.6% of the cells in early apoptotic phase, at 3, 6, 12, and 24 hrs, respectively, compared to untreated control (Fig. 2C). NOSH-SUL treatment of the cells also increased the population of cells undergoing apoptosis but to a lesser extent (Fig. 2F). At 3, 6, 12, and 24 hrs and percentage of cells undergoing apoptosis were, 2.8 ± 0.4, 10.1 ± 2.4, 23.2 ± 2.2, and 39.4 ± 4.8, respectively. Therefore, NOSH-NAP and NOSH-SUL inhibit proliferation of HT-29 colon cancer cells by a combined induction of G0/G1 or G2/M arrest and apoptosis.
2.2.3. Effects of NO- and H2S-releasing groups on cell growth
In order to determine equivalency of NOSH-NAP to the sum of its parts, we carried out a series of reconstitution and structure-activity studies in HT-29 cells, looking at cell growth inhibition using naproxen, the exogenous NO donor SNAP, and ADT-OH which releases H2S. We examined the combinations of naproxen plus SNAP, naproxen plus ADT-OH, and naproxen plus SNAP and ADT-OH, and the intact NOSH-NAP. For the combinations, various concentrations of naproxen were combined with different fixed concentrations of SNAP, ADT-OH, or SNAP and ADT-OH. Such simulation of intact NOSH-NAP using naproxen plus SNAP and ADT-OH represents a fairly close approximation to the intact NOSH-NAP. The growth inhibition curves of HT-29 cells were analyzed with these combinations; the respective IC50s of naproxen in these were evaluated for a possible shift. Table 2 shows that various combinations had a synergistic effect in terms of cell growth inhibition, but the respective IC50s of naproxen in the combinations were far higher than those of NOSH-NAP. In particular, the combination of naproxen plus SNAP and ADT-OH should have given an IC50 for cell growth inhibition comparable to that of NOSH-NAP. However, the combination gave an IC50 of 780 ± 55 μM, whereas that for NOSH-NAP was 0.09 ± 0.004 μM. That is, the intact molecule was approximately 8000-fold more potent than the combination, or the sum of the parts does not equal the whole clearly indicative of a strong synergistic effect. These findings indicate that the combined molecular components cannot completely account for the biological activity of intact NOSH-NAP and that these constituents may only, in part, contribute to its activity. Our group has also reported this phenomenon for NOSH-aspirin.12
2.2.4. NOSH-naproxen and NOSH-sulindac have potent anti-inflammatory properties
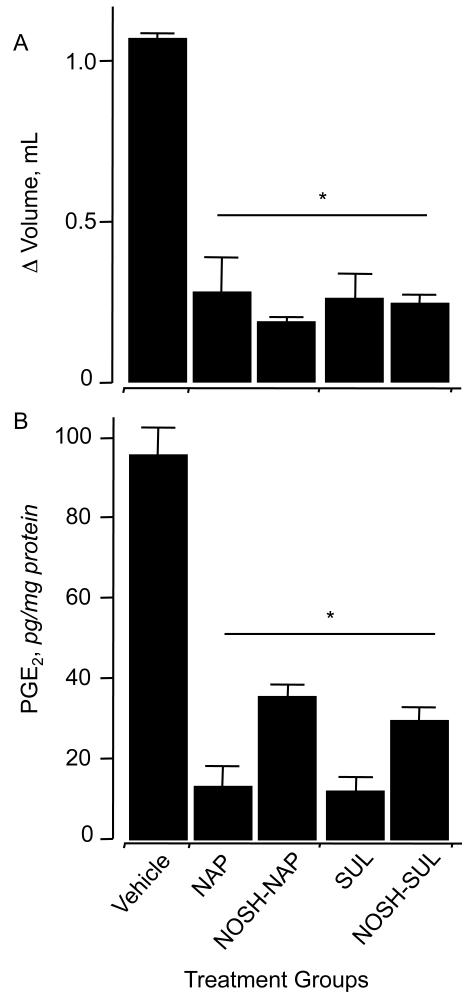
NSAIDs are generally used for treatment of inflammatory conditions. Therefore, we wanted to compare the COX-dependent anti-inflammatory activities of NOSH-NAP and NOSH-SUL to that of their respective parent compound. This was done by using the rat paw edema model, as previously reported.16 After inducing inflammation in rat’s paw with carrageenan, at 4 hrs animals receiving vehicle showed an increase in paw volume of 1.1 ± 0.1 mL. In contrast, the increase in paw volume for the animals receiving naproxen and sulindac was 0.25 ± 0.16 mL and 0.22 ± 0.14 mL, respectively. Animals receiving NOSH-NAP and NOSH-SUL exhibited potent anti-inflammatory properties, the increase in paw volume being 0.15 ± 0.02 mL and 0.21 ± 0.01 mLin NOSH-NAP and NOSH-SUL treated animals, respectively (Fig. 3A).
Figure 3. Anti-inflammatory properties of NOSH-NAP and NOSH-SUL.
Rat paw edema was induced by carrageenan injection. Panel A, all agents caused a significant reduction in paw volume at 4hr. Results are mean ± S.E.M. of three rats in each group, *P < 0.05 versus vehicle treated rats. Panel B, all agents caused a significant reduction in PGE 2 levels in the paw exudate. Results are mean ± S.E.M. for three rats in each group, *P < 0.01versus vehicle
Prostaglandins (PGE2) are the main product of cyclooxygenase-mediated arachidonic acid metabolism.1 Comparison of PGE2 content of paw exudates from control, naproxen, sulindac, NOSH-NAP, and NOSH-SUL-treated animals showed a clear and significant COX inhibition by all these agents (Fig. 3B). Naproxen and sulindac caused a considerable decrease in PGE2 levels from 95 ± 7 pg/mg in the control groups to 15 ± 4 pg/mg and 12 ± 3 pg/mg, respectively. Treatment with NOSH-NAP and NOSH-SUL reduced PGE2 levels to 35 ± 3 and 29 ± 2 pg/mg respectively.
2.2.5. NOSH-NSAIDs release both NO and H2S
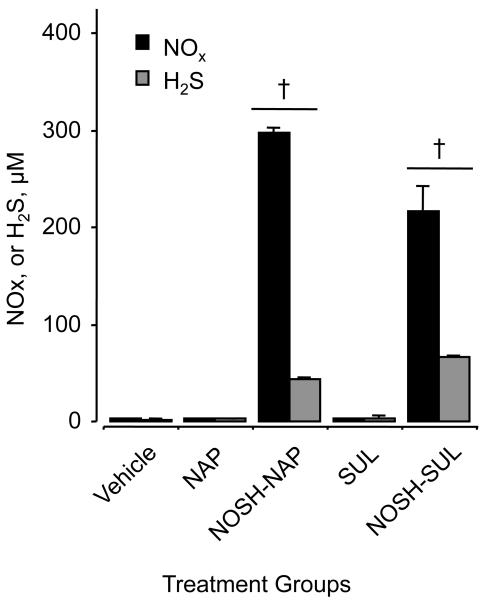
NOSH-naproxen and NOSH-sulindac were designed to release both NO and H2S. In order to show that indeed this was the case in vivo, blood was collected from vehicle-, NSAID and NOSH-NSAID-treated animals at the end of the carrageenan-induced edema studies. Figure 4 shows that indeed both NO and H2S were significantly higher in the NOSH-NSAID-treated animals. The serum concentrations of H2S as determined by the methylene blue method described in section 4.2.6 are not accurate, as this method is associated with considerable artifact.17 However, what our data shows are not absolute H2S levels in the serum, but that there appears to be an increase in some form of sulfide species (H2S + HS− + and S2− + others) that must be due to the administered NOSH-NSAIDs.
Figure 4. NO and H2S levels in vivo after NOSH-NAP and NOSH-SUL administration.
Plasma concentration of NO x and H 2S was quantified as detailed in Section 4.2.6. Results are mean ± S.E.M. of three rats in each group. †P < 0.001 versus vehicle and parent NSAID-treated animals.
3. Conclusions
In the present study, we described the synthesis of NOSH-NAP and NOSH-SUL designed to release both NO and H2S. These NOSH compounds inhibited the growth of several cancer cell lines arising from a variety of tissue types such as colon, breast, pancreas, and T cell leukemia. NOSH-NAP and NOSH-SUL inhibited the growth of these cancer lines with IC50s ranging from 80-270 nM at 24hr. These NOSH compounds were more potent than their respective parent counterparts, with enhanced potency ranging from about 2,500 to greater than 34,000-fold. Our data indicate that the effect of these NOSH compounds may be tissue-type independent since both NOSH-NAP and NOSH-SUL were effective against adenomatous, epithelial, and lymphocytic cancer cell lines. The cell growth inhibitory effects of the NOSH-NSAIDs were associated with cell cycle arrest, inhibition of proliferation and induction of apoptosis. NOSH-NAP and NOSH-SUL also showed strong anti-inflammatory properties that were comparable to that of their respective parent NSAIDs as demonstrated by measuring the in vivo carrageenan-induced rat paw oedema, and direct measurement of cyclooxygenase-dependant production of PGE2. It appears that these agents have strong antineoplastic potential.
4. Experimental section
4.1. Chemistry
All moisture-sensitive reactions were performed under an argon atmosphere using oven-dried glassware and anhydrous solvents as previously reported.4 Anhydrous solvents were freshly distilled from sodium benzophenone ketyl, for THF and DCM was distilled from calcium hydride. Extracts were dried over anhydrous Na2SO4 and filtered prior to removal of all volatiles under reduced pressure. Unless otherwise noted, commercially available materials were used without purification. Silica gel chromatography was performed using 100-200 mesh silica gel (Natland). Thin layer chromatography was performed using precoated 250 plates (Analtech). Nuclear magnetic resonance (NMR) splitting patterns are described as singlet (s), doublet (d), triplet (t), quartet (q); the value of chemical shifts (δ) are given in ppm relative to residual solvent (chloroform δ=7.27 for 1HNMR), and coupling constants (J) are given in hertz (Hz). The mass spectra were recorded on AB SCIEX 4000 QTRAP LC-MS/MS instrument (EI).
4.1.1. Synthesis of (7-hydroxynaphthalen-1-yl) acetic acid (2)
To the solution of Naproxen (5g, 23 mmol) in AcOH (50 mL) was added HBr (47%, 25 mL) and the mixture was refluxed for 4h. The whole reaction mixture was then removed under reduced pressure then washed with water. The obtained precipitate was filtered and washed with petroleum ether, then recrystallized from toluene to give (7-hydroxynaphthalen-1-yl) acetic acid 2 (3.52 g, 75%).
1H NMR (CDCl3, 500 MHz): 7.64 (d, J = 8.8 Hz, 1H), 7.62 (s, 1H), 7.58 (d, J = 8.3 Hz, 1H), 7.35 (dd, J = 8.8, 1.46 Hz, 1H), 7.09 (s, 1H), 7.08 (dd, J = 8.8, 1.45 Hz,1H), 3.77 (q, J = 7.3 Hz, 1H), 1.52 (d, J = 7.3 Hz, 3H). ESIMS: m/z 217 (M++1).
4.1.2. Synthesis of compound 3
To the solution of compound 2 (2.39 g, 11.05 mmol) in dry THF (100 mL) at 0°C was added trifluoro acetic anhydride (13.9 ml. 9.2 g, 66.32 mmol) drop wise and stirred for 4h at same temperature. tert-Butanol (30 mL) was added drop wise at 0oC and stirred at room temperature for overnight. At 0°C NH4OH (35 % in water, 6 mL) was added drop wise, and then stirred at room temperature for 30 min. After that volatiles were evaporated under reduced pressure. The crude product was triturated with boiling DCM and the crystalline solid thus obtained was removed by filtration. The filtrate was washed with saturated aqueous NaHCO3 and dried over Na2SO4. The organic layer was removed under reduced pressure to give the tert-butyl ester 3 (2.46 g, 82%) as a white solid.
1H NMR (CDCl3,500 MHz): 7.67 (d, J = 8.8 Hz, 1H), 7.62 (s, 1H), 7.59 (d, J = 8.3 Hz, 1H), 7.37 (d, J = 8.8, 1H), 7.09 (s, 1H), 7.04 (dd, J = 8.8, 1.45 Hz,1H), 3.72 (q, J = 7.3 Hz, 1H), 1.50 (d, J = 7.3 Hz, 3H), 1.38 (s, 9H). ESIMS: m/z 273 (M++1).
4.1.3. Synthesis of compound 4
To the solution of 4-bromobutyric acid (614 mg, 3.67 mmol) in dry DCM were added DCC (757 mg, 3.67 mmol) and DMAP and followed by addition of compound 3 (1.0 g, 3.67 mmol). The whole reaction mixture was stirred for overnight at room temperature. After completion of the reaction DCU was filtered off and the solvent was removed under the reduced pressure to obtain the crude product. It was further purified by column chromatography by using hexane: ethyl acetate (8:2) as a eluent to obtain the compound 4 (1.05 g, 65 %).
1H NMR (CDCl3,500 MHz): 7.83 (d, J = 8.8 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.74 (s, 1H), 7.54 (d, J = 1.45, 1H), 7.47 (dd, J = 8.8, 1.45 Hz, 1H), 7.22 (dd, J = 8.8, 1.45 Hz, 1H), 3.79 (q, J = 7.2 Hz, 1H), 3.58 (t, J = 6.8 Hz, 2H), 2.84 (t, J = 6.8 Hz, 2H), 2.34 (q, J = 6.8 Hz, 2H), 1.54 (d, J = 7.3 Hz, 3H), 1.40 (s, 9H). ESIMS: m/z 421 (M++1).
4.1.4. Synthesis of compound 5
To the solution of compound 4 (925 mg, 2.19 mmol) in acetonitrile was added AgNO3 (747 mg, 4.39 mmol) under dark conditions (protecting from light). The whole reaction mixture was heated at 70°C for 6h. Reaction mixture was filtered through celite and concentrated, further it was purified by silica gel column chromatography by using hexane: ethyl acetate (8:2) as a solvent system to obtain the compound 5 (575.5 mg, 65%).
1H NMR (CDCl3,500 MHz): 7.81 (d, J = 8.8 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.76 (s, 1H), 7.53 (s, 1H), 7.46 (d, J = 8.8 Hz, 1H), 7.20 (dd, J = 8.8, 1.45 Hz,1H), 4.62 (t, J = 6.8 Hz, 2H), 3.76 (q, J = 7.2 Hz, 1H), 2.78 (t, J = 6.8 Hz, 2H), 2.22 (q, J = 6.8 Hz, 2H), 1.53 (d, J = 7.3 Hz, 3H), 1.38 (s, 9H). ESIMS: m/z 426 (M++Na).
4.1.5. Synthesis of compound 6
To the solution of compound 5 (550 mg, 1.36 mmol) in dry DCM (5 mL) was added trifluoro acetic acid TFA (5 mL) at 0°C then stirred at room temperature for 30 min. The volatiles were evaporated and the crude product was washed with water and extracted into DCM. The organic layers were dried on Na2SO4 and concentrated under reduced pressure. The solid product was used for further reaction without further purification (260.0 mg, 55 %).
1H NMR (CDCl3,500 MHz): 7.82 (d, J = 8.8 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.76 (s, 1H), 7.53 (s, 1H), 7.48 (d, J = 8.8 Hz, 1H), 7.21 (dd, J = 8.8, 1.45 Hz, 1H), 4.61 (t, J = 6.8 Hz, 2H), 3.91 (q, J = 7.2 Hz, 1H), 2.78 (t, J = 6.8 Hz, 2H), 2.22 (q, J = 6.8 Hz, 2H), 1.60 (d, J = 7.3 Hz, 3H). ESIMS: m/z 370 (M++Na).
4.1.6. Synthesis of NOSH-naproxen (AVT-219)
To the solution of compound 6 (250.0 mg, 0.72 mmol) in dichloromethane was added DCC (148.0 mg, 0.72 mmol), DMAP (12.4 mg, 0.07 mmol) at 0°C under argon atmosphere. Then added ADT-OH ((5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione) (162.0 mg, 0.72 mmol) and the whole reaction mixture was stirred at room temperature for 6h. After completion of the reaction as checked by TLC, filtered off and water was added then extracted into dichloromethane (2x25 ml). Organic solvent was removed under reduced pressure to get the crude product. Further it was purified by column chromatography by using hexane: ethyl acetate (7:3) as a eluent to afford pure NOSH-naproxen, (224.0 mg, 56 % yield).
1H NMR (CDCl3,500 MHz): 7.86 (d, J = 8.8 Hz, 1H), 7.85 (s, 1H), 7.83 (d, J = 8.8 Hz, 1H), 7.63 (d, J = 8.8 Hz, 2H), 7.57 (d, J = 1.5 Hz, 1H), 7.55 (dd, J = 8.8 Hz, 1.5 Hz, 1H),7.37 (s, 1H),7.26 (dd, J = 8.6 Hz, 1.4 Hz, 1H), 7.12 (d, J = 8.8 Hz, 2H), 4.52 (t, J = 7.2 Hz, 2H), 4.15 (q, J = 7.3 Hz, 1H), 2.56 (t, J = 7.2 Hz, 2H), 2.42 (m, 2H), 1.54 (d, J = 7.3 Hz, 3H). ESIMS: m/z 556 (M++1), 578 (M++Na).
4.1.7. Synthesis of compound 9
To the solution of 4-bromobutyric acid (994 mg, 5.95 mmol) in dry DCM were added DCC (1.22 mg, 5.95 mmol) and DMAP (102 mg, 0.595 mmol) and followed by addition of compound 8 (1.5 g, 5.95 mmol). The whole reaction mixture was stirred for overnight at room temperature. After completion of the reaction DCU was filtered off and the solvent was removed under the reduced pressure to obtain the crude product. It was further purified by column chromatography by using hexane: ethyl acetate (9:1) as a eluent to obtain the compound 9 (1.54 g, 65 %).
1H NMR (CDCl3,500 MHz): 9.99 (s, 1H), 7.26 (d, J = 2.4 Hz, 1H), 7.07 (dd, J = 8.2, 2.4 Hz, 1H), 7.05 (d, J = 8.2 Hz, 1H), 3.52 (t, J = 6.6 Hz, 2H), 2.84 (t, J = 6.6 Hz, 2H), 2.31 (q, J = 6.6 Hz, 2H), 0.98 (s, 9H), 0.21 (s, 6H). ESIMS: m/z 403 (M++1).
4.1.8. Synthesis of compound 10
To the solution of compound 4 (1.5 g, 3.75 mmol) in acetonitrile was added AgNO3 (1.27 g, 7.5 mmol) under dark conditions (protecting from light). The whole reaction mixture was heated at 70°C for 6h. Reaction mixture was filtered through celite and concentrated, further it was purified by silica gel column chromatography by using hexane: ethyl acetate (9:1) as a solvent to obtain the compound 10 (0.79 g, 55%).
1H NMR (CDCl3,500 MHz): 9.97 (s, 1H), 7.27 (d, J = 2.4 Hz, 1H), 7.07 (dd, J = 8.8, 3.2 Hz, 1H), 7.05 (d, J = 8.3 Hz, 1H), 4.62 (t, J = 6.5 Hz, 2H), 2.80 (t, J = 6.5 Hz, 2H), 2.22 (q, J = 6.3 Hz, 2H), 1.00 (s, 9H), 0.23 (s, 6H). ESIMS: m/z 384 (M++Na), 406 (M++Na).
4.1.9. Synthesis of compound 11
To the solution of compound 10 (0.75 g, 19.58 mmol) in CH3CN (40 mL) kept 0°C were added a solution of KH2PO4 (2.0 g) in H2O (15 mL) and 30% H2O2 (2.19 mL, 19.58 mmol) and drop wise added a solution of 80% NaClO2 in H2O (15 mL). After 2h stirring at same temperature Na2SO3 was added to destroy the excess of H2O2. After acidification with 6M HCl the mixture was diluted with H2O (100 mL) and extracted twice with DCM (100mL). The organic layer was dried, filtered and concentrated under reduced pressure to obtain the compound 11 (531.0 mg, 68 %).
1H NMR (CDCl3,500 MHz): 7.54 (d, J = 2.9 Hz, 1H), 7.08 (dd, J = 8.8, 2.9 Hz, 1H), 7.05 (d, J = 8.8 Hz, 1H), 4.62 (t, J = 6.5 Hz, 2H), 2.77 (t, J = 6.5 Hz, 2H), 2.20 (q, J = 6.3 Hz, 2H), 1.00 (s, 9H), 0.24 (s, 6H). ESIMS: m/z 400 (M++Na), 422 (M++Na).
4.1.10. Synthesis of compound 12
To the solution of compound 11 (500.0 mg, 1.25 mmol) in dichloromethane was added DCC (258.0 mg, 1.25 mmol), DMAP (21.55 mg, 0.125 mmol) at 0°C under argon atmosphere. Then added ADT-OH ((5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione) (283.0 mg, 1.25 mmol) and the whole reaction mixture was stirred at room temperature for 6h. After completion of the reaction as checked by TLC, filtered off and water was added then extracted into dichloromethane (2 × 25 ml). Organic solvent was removed under reduced pressure to get the crude product. Further it was purified by column chromatography by using hexane: ethyl acetate (8:2) as a eluent to afford compound 12 (484.0 mg, 62 % yield).
1H NMR (CDCl3,500 MHz): 7.73 (d, J = 8.8 Hz, 2H), 7.63 (d, J = 2.93 Hz, 1H), 7.42 (s, 1 H), 7.31 (d, J = 8.8 Hz, 2H), 7.12 (dd, J = 8.8, 2.99 Hz, 1H), 7.05 (d, J = 8.8 Hz, 1H), 4.52 (t, J = 6.8 Hz, 2H), 2.72 (t, J = 6.8 Hz, 2H), 2.14 (q, J = 6.8 Hz, 2H), 1.00 (s, 9H), 0.24 (s, 6H). ESIMS: m/z 608 (M++1), 631 (M++Na).
4.1.11. Synthesis of compound 13
A solution of tetrabutylammonium fluoride (1 mL, 1.0 mmol) and acetic acid (1 mL) in THF (5 mL) was added to the compound 12 (450.0 mg, 0.722 mmol) and stirred it for 30 min. After completion of the reaction as checked by TLC, volatiles were removed under reduced pressure. Furthur thus obtained crude product was purified by column chromatography by using hexane: ethyl acetate (7:3) as a eluent to get the pure compound 13 (249.0 mg, 72 %).
1H NMR (CDCl3,500 MHz): 7.71 (d, J = 8.8 Hz, 2H), 7.70 (d, J = 1.28 Hz, 1H), 7.42 (s, 1 H), 7.27 (d, J = 8.8 Hz, 2H), 7.19 (dd, J = 8.8, 3.0 Hz, 1H), 7.01 (d, J = 8.8 Hz, 1H), 4.54 (t, J = 6.8 Hz, 2H), 2.73 (t, J = 6.8 Hz, 2H), 2.15 (q, J = 6.8 Hz, 2H). ESIMS: m/z 494 (M++1), 516 (M++Na).
4.1.12. Synthesis of NOSH-sulindac (AVT-18A)
To the solution of sulindac (acid form, 144.0 mg, 0.405 mmol) in dichloromethane was added DCC (83.0 mg, 0.405 mmol), DMAP (12.4 mg, 0.07 mmol) at 0°C under argon atmosphere. Then added compound 13 (200.0 mg, 0.405 mmol) and the whole reaction mixture was stirred at room temperature for 6h. After completion of the reaction as checked by TLC, filtered off and water was added then extracted into dichloromethane (2x25 ml). Organic solvent was removed under reduced pressure to get the crude product. Further it was purified by column chromatography by using 5 % DCM/MeOH as a eluent to afford pure NOSH-sulindac, (216.0 mg, 63 % yield).
1H NMR (CDCl3, 500 MHz): 7.95 (d, J = 1.8 Hz, 1H), 7.64-7.74 (m, 6H), 7.40 (s, 1H), 7.39 (d, J = 1.42 Hz, 1H), 7.27 (d, J = 8.8 Hz, 2H), 7.20 (m, 3H), 6.97 (dd, J = 7.8 Hz, 1.5 Hz, 1H), 6.58 (t, J = 7.8 Hz, 1H), 4.50 (t, J = 8.8 Hz, 2H), 3.83 (s, 2H), 2.80 (s, 3H), 2.73 (t, J = 8.8 Hz, 2H), 2.28 (s, 3H), 2.11 (m, 2H). ESIMS: m/z 832 (M++1), 854 (M++Na).
4.2. Biology
4.2.1. Cell culture
HT-29, human colon adenocarcinoma, BxPC-3 human pancreatic cancer, MCF-7 (estrogen receptor positive) breast cancer, and Jurkat T human leukemia cell lines were obtained from American Type Tissue Collection (Manassas, VA). All cells lines were grown as monolayers except for the Jurkat T cells which were grown in suspension. The pancreatic and breast cancer cells were grown in Dulbecco’s modified Eagle’s medium, the Jurkat T cells were grown in RPMI 1640 medium, and the colon HT-29 cells were grown in McCoy 5A. All media were supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA) penicillin (50 U/ml), and streptomycin (50 μg/ml) (Invitrogen, Carlsbad, CA). Normal human lung fibroblast cells IMR-90 (CCL186) were obtained from the American Type Tissue Collection (Manassas, VA) and maintained in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine and penicillin/streptomycin (100 units/mL). Human primary pancreatic epithelial cells (ACBRI 515) was obtained from Cell Systems Corporation (Kirkland, WA) and maintained in Complete Serum-Free Medium (CSC Catalog SF-4K0-500D) and with Attachment Factor (CSC Catalog 4Z0-200) as suggested by manufacturer. Normal human mammary epithelial (HMEpC) cell line was obtained from Promo Cell, (Heidelberg, Germany) and was cultured in ready-to-use mammary epithelium cell growth medium as suggested by manufacturer. Cells were seeded on culture dishes at a density of 25×103 cells/cm2 and incubated at 37°C in 5% CO2 and 90% relative humidity. Single cell suspensions were obtained by trypsinization (0.05% trypsin/EDTA), and cells were counted using a hemocytometer. Viability was determined by the trypan blue dye exclusion method.
4.2.2. MTT Assay
Cell growth inhibitory effect of NOSH-NAP, NOSH-SUL and their respective parent NSAID were measured using a colorimetric MTT assay kit (Roche, Indianapolis, IN). HT-29, BxPC-3, MCF-7, and Jurkat T cells were plated in 96-well plates at a density of 50,000 cells/well. The cells were incubated for 24 h with different concentrations of test compounds, after which 10μL of MTT dye (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide), was added to each well, and the plates were incubated for 2 hours at 37°C. The media was then aspirated, and 100 μL of the solubilization solution (10% SDS in 0.01 M HCl) was added to solubilize the formant crystals. The absorbance of the plates was determined on an ELISA reader at 570 nm. Since Jurkat T cells are non-adherent, they were added (30,000 cells per well) to serially diluted NOSH-NSAIDs in V-bottom shaped 96-well plates and incubated for 24 hrs in a humidified 5% CO2 incubator at 37°C. After 24 hrs of incubation, 20 μL of the MTT solution (1.1 mg/mL) was added to each well and the plates were incubated for an additional 4 h. The colored formazan crystals produced from the MTT was then dissolved by adding 150 μL of dimethyl sulfoxide (DMSO). The optical density (OD) of the solutions were measured as indicated above.
4.2.3. Cell kinetics parameters
For proliferation and cell cycle analysis, HT-29 cells (1×106 cells/mL) were treated for 24 hrs with various concentrations of NOSH-NAP and NOSH-SUL. PCNA was determined using an ELISA Kit (Calbiochem, La Jolla, CA), in accordance with the manufacturers protocol as previously reported.18 For cell cycle analysis, the treated cells (0.5×106) were harvested by trypsinization, centrifugation and then fixed in 70% ethanol for 10 min at −20°C, pelleted (5000 rpm ×10 min at 4°C), resuspended and incubated in PBS containing 1% FBS/0.5% NP-40 on ice for 5 min. After washing and addition of propidium iodide (40 μg/mL) to stain for DNA and 200 μg/mL RNase type IIA, cell were analyzed by flow cytometry. Cell cycle phase distributions of control and treated cells were obtained using a Coulter Profile XL equipped with a single argon ion laser. For each subset, >10,000 events were analyzed. All parameters were collected in list mode files. Data were analyzed on a Coulter XL Elite Work station using the Software programs MultigraphTM and MulticycleTM. The percentage of cells in G0/G1, G2/M, and S phases was determined form DNA content histograms.
To evaluate apoptosis, control and treated cells (0.5×106 cells/mL) were washed with and resuspended in 1X Binding Buffer (Annexin V binding buffer, 0.1 M Hepes/NaOH (pH 7.4), 1.4 M NaCl, 25mM CaCl2) from BD BioSciences (San Diego, CA). Annexin V-FITC and Propidium iodide were then added and the cells incubated at room temperature for 5–15 min in the dark. Cells were then transferred to FACS tubes and analysed by flow cytometry as described above using Flow Jo software.
4.2.4. Inflammatory edema model
Our institutional animal care and research committee approved the experimental procedure described here. Male Wistar rats (3 per group) weighing 180–200 g were obtained from Charles River Laboratories International (Wilmington, MA). The rats were fed standard laboratory chow and water. On the day of the experiments, rats were given by gavage equimolar doses of naproxen (80 mg/kg), NOSH-naproxen (188 mg/kg); sulindac (200 mg/kg), NOSH-sulindac (467 mg/kg); or the vehicle, 0.5% carboxymethylcellulose solution. One hour post drug administration, freshly prepared, 100 μL of 1% carrageenan, Sigma Chemicals (St. Louis, MO) was subcutaneously injected into the plantar surface of the right hind paw of the rats following the protocol described by Winter et al 19. Paw volume was measured using a water displacement plethysmometer (Model 520, IITC/Life Sciences Instruments, Woodland Hills, CA) before carrageenan injection and at 4 hr post injection. This time point was chosen because previous studies had indicted it to be optimum where greatest volume changes were observed 16. The paw volume measured just prior to carrageenan injection was used as the control volume. Data are expressed as the change in paw volume (mL) at 4hr.
4.2.5. Determination of PGE2 in rat paw exudates
Rats were euthanized by asphyxiation in a CO2 chamber. After cutting each hind paw at the level of the calcaneus bone, exudates (oedema fluid) and some tissue were collected, weighed and placed in a test tube containing 5 mL of 0.1 M phosphate buffer (pH7.4), 1 mM EDTA, and 10 μM indomethacin. The mixture was homogenized and centrifuged for 10 min at 12,000 r.p.m. at 4°C. PGE2 content in supernatant was determined in duplicate by an enzyme immunoassay kit following the protocol described by the manufacturer (Cayman Chemical, Ann Arbor, MI) and previously reported by us.16
4.2.6. Determination of plasma NO and H2S levels
The Griess method was used to estimate plasma NO levels indirectly as the concentration of nitrate (NO −3) and nitrite (NO −2) using an assay kit from Cayman Chemical (Ann Arbor, MI) and following the manufacturer’s protocol. Plasma was filtered using a 10 KD molecular weight cut-off filter from Millipore (Bedford, MA) before each analysis, to reduce background absorbance due to the presence of hemoglobin. After centrifugation for 10 min at 3000 rpm, samples (40 μL/well) were mixed with 10μL nitrate reductase mixture and incubated for 3 h after which Griess reagents 1 and 2 (50 μL each) were added. Absorbance was read after 10 min at 540nm using a plate reader. The concentration of nitrate/nitrite was calculated graphically from a calibration curve prepared from NaNO2 standard solution, and it is expressed as micromolar nitrate. H2S levels were measured as previously described.4, 20, 21Aliquots (100 μL) of rat plasma were mixed with distilled water (100 μL), Zinc acetate (1% w/v, 250 μL), trichloroacetic acid (10% w/v, 250 μL), N, N-dimethyl-p-phenylenediamine sulfate (133 μL, 20 μM) in 7.2M HCl and FeCl3 (133 μl, 30 μM) in 7.2M HCl. The absorbance of the resulting mixture (300 μL) was determined after 15 min using a 96-well microplate reader at 670 nm. All samples were assayed in duplicate and H2S levels were calculated against a calibration curve of NaHS (1-250 μM). This method overestimates H2S levels as it measures free H2S, HS− (hydrosulfide anion), and S2− (sulfide).22 Therefore, our results presented here indicate the sum total of these species.
4.2.7. Statistical analysis
In vitro: data are presented as mean ± SEM for 3-7 different sets of plates done in triplicate. In vivo: 3 animals were used per treatment group. Comparison between treatment groups was performed by one-factor analysis of variance (ANOVA) followed by Turkey’s test for multiple comparisons. P < 0.05 was regarded as statistically significant.
Supplementary Material
Table 3.
IC50 values for cell growth inhibition by various components of NOSH-naproxen or other agents that release NO or H2S alone or in combination.
| Compound | IC50 at 24h, μM |
|---|---|
| Naproxen | 2600 ± 150 |
| SNAP | 508 ± 35 |
| ADT-OH | 35 ± 4 |
| Naproxen + SNAP | 900 ± 57 |
| Naproxen + ADT-OH | 300 ± 65 |
| Naproxen + SNAP + ADT-OH | 780 ± 55 |
| NOSH-naproxen | 0.09 ± 0.004† |
Cells were treated with various concentrations of the test agents shown above, cell numbers were determined at 24h from which IC50 values were calculated. Results are mean ± SEM of three different experiments performed in duplicate.
P<0.001 compared to all other treatment groups.
The reconstitution experiments at concentrations that provide virtually identical concentrations of “NOSH-naproxen”, NO and H2S in the media show that the IC50 of the intact NOSH-naproxen molecule were not obtained, the sum of the parts does not equal the whole.
5. Acknowledgements
Supported in part by NIH grant R24 DA018055. The funding agency had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
6. Notes and references
- 1.Kashfi K. Adv Pharmacol. 2009;57:31–89. doi: 10.1016/S1054-3589(08)57002-5. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe MM, Lichtenstein DR, Singh G. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 3.Wallace JL, Vong L. Curr Opin Investig Drugs. 2008;9:1151–1156. [PubMed] [Google Scholar]
- 4.Kodela R, Chattopadhyay M, Kashfi K. ACS Med Chem Lett. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallace JL, Reuter B, Cicala C, McKnight W, Grisham M, Cirino G. Eur J Pharmacol. 1994;257:249–255. doi: 10.1016/0014-2999(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 6.del Soldato P, Sorrentino R, Pinto A. Trends Pharmacol Sci. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- 7.Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace JL. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 8.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 9.del Amo V, McGlone AP, Soriano JM, Davis AP. Tetrahedron. 2009;65:6370–6381. [Google Scholar]
- 10.Tasler S, Baumgartner R, Ammendola A, Schachtner J, Wieber T, Blisse M, Rath S, Zaja M, Klahn P, Quotschalla U, Ney P. Bioorg Med Chem Lett. 2010;20:6108–6115. doi: 10.1016/j.bmcl.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Lazzarato L, Donnola M, Rolando B, Marini E, Cena C, Coruzzi G, Guaita E, Morini G, Fruttero R, Gasco A, Biondi S, Ongini E. J Med Chem. 2008;51:1894–1903. doi: 10.1021/jm701104f. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay M, Kodela R, Olson KR, Kashfi K. Biochemical and biophysical research communications. 2012;419:523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 13.De Luna-Bertos E, Ramos-Torrecillas J, Garcia-Martinez O, Diaz-Rodriguez L, Ruiz C. TheScientificWorldJournal. 2012;2012:834246. doi: 10.1100/2012/834246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raza H, John A, Benedict S. Eur J Pharmacol. 2011;668:15–24. doi: 10.1016/j.ejphar.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Xiao D, Deguchi A, Gundersen GG, Oehlen B, Arnold L, Weinstein IB. Molecular cancer therapeutics. 2006;5:60–67. doi: 10.1158/1535-7163.MCT-05-0260. [DOI] [PubMed] [Google Scholar]
- 16.Chattopadhyay M, Velazquez CA, Pruski A, Nia KV, Abdellatif KR, Keefer LK, Kashfi K. J Pharmacol Exp Ther. 2010;335:443–450. doi: 10.1124/jpet.110.171017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson KR. Biochim Biophys Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Kodela R, Chattopadhyay M, Nath N, Cieciura LZ, Pospishill L, Boring D, Crowell JA, Kashfi K. Bioorg Med Chem Lett. 2011;21:7146–7150. doi: 10.1016/j.bmcl.2011.09.075. [DOI] [PubMed] [Google Scholar]
- 19.Winter CA, Risley EA, Nuss GW. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Free Radic Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, Chua JH, Yew WS, Sivaraman J, Moore PK, Tan CH, Deng LW. J Mol Biol. 2010;396:708–718. doi: 10.1016/j.jmb.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 22.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.