Abstract
BACKGROUND AND AIMS
It is unclear whether subcutaneous and visceral fat are differentially correlated to the decline in left ventricular (LV) diastolic function with aging. This study sought to examine the hypothesis that age-related changes in the regional fat distribution account for changes in LV diastolic function and to explore potential mediators of this association.
METHODS AND RESULTS
In this cross-sectional study, we evaluated 843 participants of the Baltimore Longitudinal Study of Aging with echocardiogram, dual-energy x-ray absorptiometry (DEXA), abdominal computed tomography (CT) and blood tests performed at the same visit. LV diastolic function was assessed by parameters of LV relaxation (E/A ratio, Em and Em/Am ratio) and LV filling pressures (E/Em ratio). Total body fat was computed by DEXA, while visceral and subcutaneous fat were determined from abdominal CT. In multivariate models adjusted for demographics, cardiovascular risk factors, antihypertensive medications, physical activity and LV mass, both visceral and subcutaneous fat were associated with LV diastolic dysfunction. When both measures of adiposity were simultaneously included in the same model, only visceral fat was significantly associated with LV diastolic dysfunction. Triglycerides and sex-hormone binding globulin, but not adiponectin and leptin, were found to be significant mediators of the relationship between visceral fat and LV diastolic function, explaining 28 to 47% of the association. Bootstrapping analyses confirmed the significance of these findings.
CONCLUSIONS
Increased visceral adiposity is associated with LV diastolic dysfunction, possibly through a metabolic pathway involving blood lipids and ectopic fat accumulation rather than adipokines.
Keywords: adiposity, diastolic function, triglycerides, sex hormone-binding globulin, adipokines
INTRODUCTION
There is a continuum of cardiac structural and functional changes that occurs with age in healthy humans [1]. Left ventricular (LV) diastolic dysfunction is a hallmark of the aging heart and is associated with the development of heart failure [2]. The mechanistic link between aging and the decline in LV diastolic function has not been fully determined [1].
Visceral adiposity increases with aging more than total and subcutaneous adiposity both in men and women. As a consequence, in very old age the distribution of fat is characterized by more abdominal relative to peripheral storage [3]. This is particularly relevant as accumulation of abdominal fat has more negative metabolic consequences than peripheral fat and is a stronger independent risk factor for cardiovascular events [4]. Emergent theories to explain the detrimental effect of visceral adiposity on cardiovascular health include the secretion of cytokines and adipokines [4, 5] and the overflow of free FAs into the portal circulation which can cause ectopic fat accumulation and have direct lipotoxicity on tissues, including the myocardium [6].
Recently, visceral adiposity estimated by means of waist circumference [7, 8] has been independently associated with LV diastolic dysfunction. However, it remains unclear if visceral and subcutaneous fat contribute equally to the development of LV diastolic dysfunction that occurs with aging, and whether a direct causal link exists between different fat depots and LV diastolic dysfunction. Accordingly, the aims of our study were: 1) to analyze the independent association of total, visceral and subcutaneous body fat with LV diastolic function in a cohort of healthy aging individuals; 2) to investigate the role of possible hormonal and metabolic mediators of the association between visceral adiposity and LV diastolic dysfunction. We firstly hypothesized that, after controlling for known confounders, visceral adiposity would better relate to LV diastolic dysfunction than total and subcutaneous adiposity. Secondly, that in an aging population, disorders in lipid metabolism associated with excess adiposity more than alterations in fat hormones would be responsible for the decline in diastolic function associated with increased visceral adiposity.
METHODS
Study population
The study cohort was a subgroup of the Baltimore Longitudinal Study of Aging, a prospective study of community-dwelling volunteers who undergo approximately 3 days of medical examinations at regular intervals of about 3 years [1]. Participants are enrolled if they are healthy at baseline (e.g., no evidence of diabetes, stroke, heart disease) but remain in the study if disease develops. For the present cross-sectional analysis, we considered 1165 participants who had undergone the following at the same visit: 1) a comprehensive echocardiographic study that included tissue Doppler imaging; 2) a dual-energy x-ray absorptiometry (DEXA) scan, estimating total body fat (TBF); 3) an abdominal CT scan for the estimation of subcutaneous (SAT) and visceral (VAT) adipose tissue; 4) blood tests, including lipids, fat and sex hormones. Of these, we excluded 322 participants with ECG evidence of cardiac conduction disorders or arrhythmias, significant aortic or mitral valve disease, LV ejection fraction <50% or a history of cardiac disease [7]. All participants provided informed consent and the study was approved by the MedStar Research Institute Institutional Review Board.
Echocardiographic assessment
All transthoracic echocardiograms were performed with the same echocardiographic instrument (HP Sonos-5500, Philips, Andover, MA) by a single diagnostic cardiac sonographer following a standardized protocol and were interpreted by 2 experienced echocardiographers. The LV linear dimensions were measured from a parasternal long-axis view and LV mass and ejection fraction were calculated as previously described [7]. LV mass was indexed to body surface area. From the apical window, a 1- to 2-mm pulsed Doppler sample was placed at the mitral valve tip and the peak velocity of early diastolic mitral inflow (E) and late diastolic mitral inflow (A) were measured. Tissue Doppler systolic, early (Em) and late (Am) diastolic velocities of the medial and lateral mitral annulus were recorded and averaged, as recommended by the American Society of Echocardiography [9]. The following indices of LV diastolic function were used: the ratio between E and A (E/A ratio), Em velocity and the ratio between Em and Am (Em/Am ratio) as indexes of LV relaxation; the ratio between E and Em (E/Em ratio) as an index of LV filling pressures [9].
Adiposity assessment
Total body DEXA was performed using the Prodigy Scanner (General Electric, Madison, WI) and analyzed with version 10.51.006 software. Total body fat mass was used as a measure of general adiposity, and fat percentage was calculated as the total body fat mass in percent of body weight, as used and validated in previous studies [10]. Regional adiposity was evaluated from a 10-mm axial slice at the level between the fourth and the fifth lumbar vertebra obtained using a Somatom Sensation CT scanner (Siemens, Malvern, PA). Subcutaneous and visceral adipose tissue were automatically segmented and quantified as previously described [11].
Laboratory assessment
Fasting blood samples were collected in the morning and hormonal and metabolic parameters of interest were evaluated as potential mediators of the relationship between adiposity and LV diastolic function. Because circulating fatty acids (FAs) and liver fat content quantification are not part of the core protocol of the study, we used triglycerides and SHBG as their substitutes, respectively. Triglycerides are esters derived from glycerol and three FAs, and fasting hypertriglyceridemia has been proposed as a marker of inability to manage and store extra energy in the subcutaneous fat depot [12]. Circulating SHBG is mostly produced by the liver, and has been strongly and inversely correlated with liver fat content in both genders [13]. Leptin and adiponectin are cytokine hormones produced by adipose cells and have gained much attention as mediators of obesity in the regulation of energy homeostasis and lipid metabolism within the heart [4, 5, 14]. Plasma triglycerides were determined by an enzymatic method (ABA-200 ATC Biochromatic Analyzer, Abbott Laboratories). Sex hormone binding globulin (SHBG) was measured using an enzyme-linked immunosorbent assay (DRG Diagnostics, Marburg, Germany) as well as leptin (Linco Research, St Charles, MO). Adiponectin was determined by radioimmunoassay (Linco Research). Intra- and inter-assay variations for these tests in our study have been previously reported [10].
Covariates
Hypertension was defined as mean systolic blood pressure ≥140 mm Hg and/or mean diastolic blood pressure ≥90 mm Hg on three consecutive measurements at the brachial artery right before the echocardiography, or as use of antihypertensive medications. Heart rate was recorded by electrocardiography at the same time of echocardiography. Diabetes mellitus was diagnosed according to the 2011 American Diabetes Association criteria [15] or use of diabetes medications. Hypercholesterolemia was defined as total serum cholesterol ≥ 200 mg/dl or by use of lipid- lowering treatment. Participants who ever smoked >100 cigarettes in their life were considered “ever smoker”. Physical activity was quantified by converting the time spent walking, climbing stairs, or in any moderate to vigorous activity, as assessed by questionnaires, into calories expended per week [16]. Participants were classified as active if reporting ≥1,000 kcal/week of exercise activity [17]. Body mass index was calculated as weight (kg) divided by height-squared (m2), and waist circumference was measured between the lower edge of the rib cage and the iliac crests. Medications were recorded according to the Anatomical Therapeutic Chemical classification system approved by the World Health Organization.
Statistical analysis
Continuous variables are presented as mean ±SD or as median (with interquartile ranges), and categorical variables are presented as proportions. To achieve normal distribution, adiposity measures, triglycerides, SHBG, adiponectin and leptin were naturally log-transformed before analysis. Partial Pearson’s correlations coefficients of age with both diastolic and adiposity measures were calculated after accounting for gender, and those between diastolic and adiposity measures after accounting for both age and gender.
Forward stepwise linear regression model was used to test the independent association of TBF, VAT and SAT with LV diastolic function parameters after adjusting for differences in demographic characteristics, cardiovascular risk factors and hemodynamic variables. All adiposity measures were standardized to a mean of 0 and a standard deviation of 1, to allow for comparison of regression coefficients between different fat depots. We also evaluated if the effect of VAT on diastolic parameters was independent of SAT by entering SAT as a covariate in full models. Collinearity was assessed calculating the variance inflation factor (VIF; acceptable collinearity VIF ≤2) [18]. Gender differences in the main study variables were examined using Student’s t tests or chi-square tests as appropriate, and an “adiposity-by-sex” interaction term was included in all previous fully adjusted regression models, to test whether the association between each adiposity measure and LV diastolic function was consistent across gender.
We then examined the mediator effect of proxies of FAs and liver fat content (triglycerides and SHBG, respectively) and adipokines (adiponectin and leptin) in the association between VAT and diastolic dysfunction. Importantly, in cross-sectional studies mediation analysis is considered a causal model that can suggests a direction of influence, based on pre-defined causal associations among the variables posited by the investigator. In brief, the predictor and the mediator are correlated, and the predictor and the outcome are also correlated, and there is an implied causal path that links the three variables, where the predictor causes the outcome “because” the predictor causes the mediator that causes the outcome [19]. As part of the aforementioned conditions necessary for mediation, we firstly confirmed the association of each mediator with both the predictor (VAT) and the outcomes (LV diastolic function parameters). We then used a mediation pathway approach [19] to calculate the percentage decrease of β estimate for VAT after separately adjusting for triglycerides, SHBG, adiponectin and leptin (see Supplemental Figure S1). In order to confirm the role of these variables as potential mediators, we also applied bootstrapping analysis using the SPSS macro developed by Preacher and Hayes [20]. All other statistical analyses were performed using SAS package, version 9.2 (SAS Institute Inc., Cary, NC). For all statistical analyses, a 2-tailed p <0.05 was considered significant.
RESULTS
Characteristics of the study population
The study population consisted of 843 participants (462 women and 381 men, age range 26 to 95 years). Clinical characteristics for the overall study sample are shown in Table 1. With regard to gender differences, women had higher TBF (29±10 vs. 26±9 Kg), body fat percentage (40 vs. 29%) and SAT (308±137 vs. 236±93 cm2), and lower VAT (91±48 vs. 115±58 cm2, p<.0001 for all) as compared to men. Levels of SHBG (89±50 vs. 59±25 mmol/L), leptin (30±29 vs. 11±10 ng/mL) and adiponectin (17±11 vs. 12±11 μg/mL, p<.0001 for all) but not triglycerides (100±50 vs. 104±60 mg/dL, p=ns) were also higher in women than men. Women had higher E/Em ratios (8.6±2.5 vs. 7.9±2.1, p<.0001) and smaller LV mass index (63±19 vs. 74±23 g/m2, p<.0001) compared to men, and similar E/A ratios, Em, Em/Am ratios and LV ejection fraction.
Table 1.
Clinical, adiposity and echocardiographic characteristics of the study cohort.
| All (n=843) | |
|---|---|
| Age (years) | 67 ±12 |
| Men (%) | 45 |
| Race (%) | |
| White | 61 |
| Black | 31 |
| Other | 8 |
| Body Mass Index (kg/m2) | 26 ±4 |
| Waist circumference (cm) | 90 ±13 |
| Smoking (ever, %) | 44 |
| Physical activity (≥1000 kcal/week, %) | 59 |
| Diabetes (%) | 20 |
| Hypercholesterolemia (%) | 78 |
| Hypertension (%) | 46 |
| Antihypertensive medications (%) | 45 |
| Systolic Blood Pressure (mmHg) | 120 ±16 |
| Heart Rate (beats/min) | 67 ±11 |
| Medications, ATC code (%) | |
| Diuretics, C03 | 15 |
| Beta Blocking Agents, C07 | 14 |
| Calcium channel blockers, C08 | 10 |
| Renin-angiotensin system, C09 | 28 |
| Lipid modifying, C10 | 47 |
| Triglycerides (TG, mg/dL) | 88 (67–120) |
| Sex hormone_binding globulin (SHBG, nmol/L) | 65 (45–94) |
| Leptin (ng/mL) | 14.3 (6.7–28.4) |
| Adiponectin (μg/mL) | 11.8 (6.5–20.1) |
| Total Body Fat Mass (TBF, kg) | 27 ±10 |
| Fat Percentage (%) | 35 |
| Visceral Adipose Tissue area (VAT, cm²) | 102 ±54 |
| Subcutaneous Adipose Tissue area (SAT, cm²) | 275 ±124 |
| E/A ratio | 0.96 ±0.3 |
| Early diastolic mitral annulus velocity (Em, cm/s) | 9.1 ±2.2 |
| E/Em ratio | 8.3 ±2.4 |
| Em/Am ratio | 0.93 ±0.34 |
| LV mass index (g/m2) | 68 ±22 |
| LV ejection fraction (%) | 69 ±9 |
Values are mean ±SD, median (interquartile ranges) or percentages. ATC= Anatomical Therapeutic Chemical classification. Left ventricular (LV) mass was indexed to body surface area.
Correlations between age, adiposity and LV diastolic function
As expected, age had a significant positive correlation with VAT but not TBF or SAT, and it showed strong associations with all diastolic parameters (Table 2). After accounting for age and gender, diastolic parameters were significantly associated with indices of adiposity (except E/Em ratio and TBF), and VAT generally showed higher partial correlation coefficients when compared to SAT (Table 2). No significant differences between genders were found for any of these associations (see Supplemental Table S1).
Table 2.
Pearson correlations between age, adiposity and LV diastolic function.
| Age | E/A ratio | Em | E/Em ratio | Em/Am ratio | |
|---|---|---|---|---|---|
| Age | −0.55* | −0.66* | 0.38* | −0.56* | |
| TBF | −0.02 | −0.18* | −0.20* | 0.06 | −0.22* |
| VAT | 0.35* | −0.17† | −0.21* | 0.07‡ | −0.24* |
| SAT | −0.01 | −0.13† | −0.15* | 0.08‡ | −0.17* |
P<.0001,
P<.001,
P<.05.
Correlations coefficients of age with diastolic parameters and adiposity measures are gender-adjusted. Correlations coefficients between diastolic parameters and adiposity measures are age- and gender-adjusted.
Multivariable models with indexes of adiposity and LV diastolic function
All measures of adiposity were significantly associated with E/A ratio, Em and Em/Am ratio but not with E/Em ratio when separately entered in multivariate regression models adjusted for age, race, gender, smoking, diabetes, hypercholesterolemia, systolic blood pressure, antihypertensive medications, physical activity, heart rate and indexed LV mass (Table 3, Model 1 to 3). However, the standardized regression coefficients were consistently larger for VAT as compared to TBF and SAT. Furthermore, the association between VAT and these diastolic parameters remained significant even after accounting for the differences in SAT (Table 3, Model 4). No significant interaction was found between gender and adiposity measures on LV diastolic function (all p>0.20), suggesting that the nature of association between adiposity and diastolic function parameters is substantially similar in the two sexes.
Table 3.
Multivariate analysis showing the relationship between adiposity and LV diastolic function.
| E/A ratio
|
Em
|
E/Em ratio
|
Em/Am ratio
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Model 1 | ||||||||||||
| TBF | −0.034 | 0.009 | <.001 | −0.191 | 0.060 | 0.002 | 0.065 | 0.081 | 0.43 | −0.046 | 0.010 | <.0001 |
|
| ||||||||||||
| Model 2 | ||||||||||||
| VAT | −0.035 | 0.010 | <.001 | −0.245 | 0.066 | <.001 | 0.085 | 0.089 | 0.34 | −0.053 | 0.011 | <.0001 |
|
| ||||||||||||
| Model 3 | ||||||||||||
| SAT | −0.026 | 0.009 | 0.004 | −0.138 | 0.061 | 0.02 | 0.103 | 0.082 | 0.21 | −0.038 | 0.010 | <.001 |
|
| ||||||||||||
| Model 4 | ||||||||||||
| VAT | −0.028 | 0.011 | 0.01 | −0.228 | 0.076 | 0.003 | 0.037 | 0.104 | 0.73 | −0.044 | 0.013 | <.001 |
| SAT | −0.012 | 0.010 | 0.23 | −0.031 | 0.070 | 0.66 | 0.086 | 0.095 | 0.37 | −0.017 | 0.011 | 0.14 |
All adiposity measures were naturally log-transformed and then standardized to a mean of 0 and a SD of 1 to facilitate comparison of regression coefficients between different fat depots. Models were adjusted for age, race, gender, smoking, diabetes, hypercholesterolemia, systolic blood pressure, antihypertensive medications, physical activity, heart rate and LV mass index. Variance inflation factor was < 2 in all models. SE= standard error; β= standardized coefficients. See Table 1 for other abbreviations.
Mediators of the relationship between visceral adiposity and LV diastolic function
We further evaluated the role of triglycerides, SHBG, adiponectin and leptin as possible mediators of the association between VAT and E/A ratio, Em and Em/Am ratio. We firstly confirmed the association of each potential mediator with both the predictor of interest (VAT) and the outcomes (E/A ratio, Em and Em/Am ratio) after adjusting for age and gender (see Supplemental Table S2). Then, when added to previous multivariate regression model, triglycerides explained 22%, 27%, and 11%, while SHBG explained 38%, 17%, and 22%, of the association between VAT and each of E/A ratio, Em, and Em/Am ratio, respectively. When triglycerides and SHBG were entered together in the same models, these percentages increased to 47%, 40% and 28%, respectively, and the association of VAT with E/A ratio and Em was no longer significant (Figure 1, see Supplemental Table S3 for details). Adiponectin and leptin both explained a smaller part of the associations between VAT and diastolic parameters (ranging from 3% to 13%, Figure 1). Bootstrapping analysis confirmed SHBG and triglycerides as significant mediators of the association of VAT with E/A ratio (point estimate for SHBG = −0.0114, bias corrected 95%CI=−0.0213/−0.0043) and Em (point estimate for triglycerides = −0.058, bias corrected 95%CI=−0.1353/−0.0002), respectively. Neither adiponectin nor leptin were found to be significant mediators in bootstrapping analysis, not even their combination explained the association between VAT and diastolic dysfunction (Figure 1).
Figure 1. Potential mediators of the association between VAT and LV diastolic function.
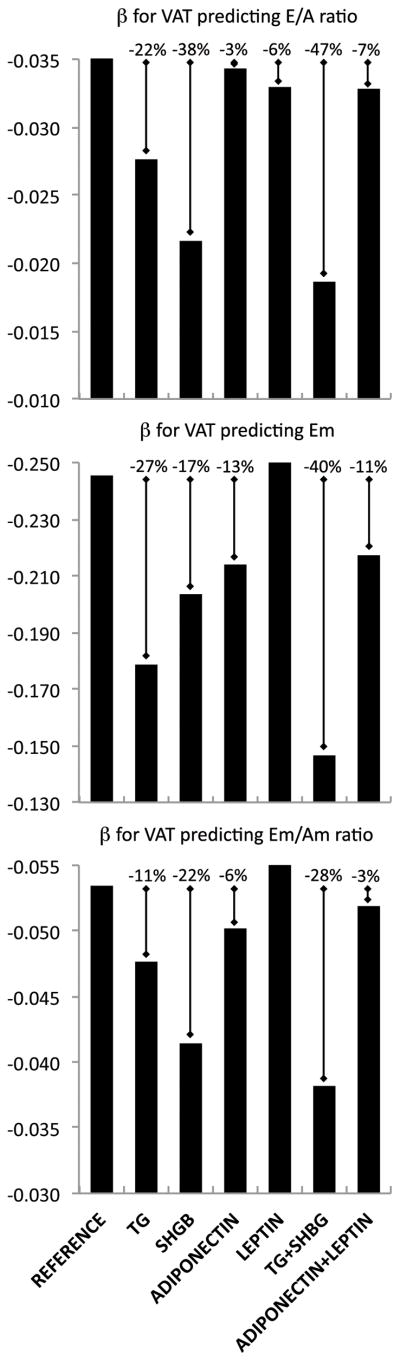
The association of VAT with E/A ratio, Em and Em/Am ratio was significantly reduced after separate and simultaneous adjustment for triglycerides (TG) and sex hormone-binding globulin (SHBG), as compared to adiponectin and leptin. Detailed regression coefficients are provided in Supplemental Table S3. Other abbreviations as in Table 1.
DISCUSSION
We investigated the relationship between different fat depots and LV diastolic function in a healthy sample from the Baltimore Longitudinal Study of Aging. Comparing measures of total, subcutaneous and visceral fat derived by DEXA and abdominal CT scan, we observed a stronger association between VAT and LV diastolic dysfunction as compared to SAT and TBF. Our findings also suggest that the effect of VAT on LV diastolic function may be mediated by excess of blood and liver lipids more than by fat hormones released by adipocytes.
Relationship between adiposity and LV diastolic function
Normal aging in the absence of detectable diseases is associated with redistribution in the pattern of adiposity, due to a disproportionate increase in VAT as opposed to SAT. Women have a more peripheral subcutaneous distribution of fat in early adulthood, but greater parity and menopause induce a more visceral distribution of fat with increasing age [3]. These observations were confirmed in our older cohort, where in both genders VAT was the only fat depot displaying a significant positive correlation with age. A decrease in LV diastolic performance with advancing age has been regarded as part of “normative” aging [21] and has been, in part, explained to be a byproduct of the prolonged exposure to a number of risk factors [1], including abdominal adiposity [7, 8]. To date, the independent relationship between visceral adiposity and LV diastolic dysfunction has not been fully evaluated in a healthy aging population. In a younger sample of participants from the Framingham Heart Study (542 women and 455 men, mean age 60 years) who underwent cardiac magnetic resonance and abdominal volumetric CT, VAT was associated with increased LA dimension and LV mass in both men and women, without significant gender interaction [22]. Unfortunately, the study did not report any details for the effect of VAT or SAT on LV diastolic function parameters. In a cohort of African Americans from the Jackson Heart Study (including 924 women and 477 men, mean age 59 year), Liu and colleagues found a significant association between VAT and E/A ratio in women (β −0.03, SE 0.01, p= 0.03) but not in men (β −0.03, SE 0.01, p= 0.06, probably due to a sample size effect) [23]. No interaction between gender and VAT was reported. Other important limitations of this study included missing adjustment for LV mass, lack of tissue Doppler measures of diastolic function and the time gap between echocardiography and abdominal volumetric CT measures (ranging from 3 to 9 years) [23]. Finally, approximately 20% of the sample in both studies had prevalent cardiovascular disease [22, 23].
We extended these previous findings in an older population free from cardiovascular disease, analyzing multiple echocardiographic parameters expressive of LV relaxation. In our analysis, VAT displayed stronger association with diastolic parameters than SAT and TBF, and this association was also independent of SAT, further emphasizing the specific pathogenetic role of visceral as compared to subcutaneous fat. In accordance with previous studies, we found no significant interaction between gender and VAT. The low prevalence of subjects with elevated LV filling pressures (E/Em ratio ≥12, accounting for less than 6% of our sample) and pseudonormal and restrictive filling patterns in our population (see reference [7]) may explain why we observed no significant association of E/Em ratio with any fat depot after multivariate adjustments.
Mediators of the association between adiposity and diastolic dysfunction
Endocrine and metabolic effects of adipose tissue are still under investigation, as well as their potential contribution to the decline in cardiovascular function with aging [5, 6]. In our population, we found a significant role for triglycerides and SHBG (considered as surrogates for circulating FAs [12] and liver fat content [13], respectively) over adipokines as mediators of the relationship between VAT and LV diastolic dysfunction. We speculate that myocardial triglyceride accumulation may represent a potential mechanism by which the age-related increase in VAT and consequent overflow of free FAs to the viscera determines LV diastolic dysfunction. With aging, triglyceride accumulation in the myocardium is independently associated with LV diastolic dysfunction [24] and it results from a combination of decreased FAs utilization and increased FAs exposure [25]. As a consequence of the progressive inability of subcutaneous adipocytes to act as lipid storage sites, FAs are released directly into the portal circulation and accumulated in non-subcutaneous tissues, including the liver and the myocardium [3]. Non-alcoholic fatty liver disease is increasingly reported as an independent risk factor for cardiac dysfunction [26]. Animal studies have shown that cardiac dysfunction is mainly determined by the accumulation of lipids in the myocardium in obese-induced rats [27], and that therapeutic interventions causing a decrease in FAs, visceral adiposity and hepatic steatosis were also responsible for a reduction of cardiac hypertrophy and improvement of cardiac function [28]. While such evidences are still lacking in humans, our finding that VAT was no longer a significant correlate of LV diastolic dysfunction after adjusting for SHBG and triglycerides is in support of a metabolic connection between the viscera and the heart, involving lipids and liver fat accumulation.
Leptin has been shown to be better correlated with TBF and SAT than with VAT, and adiponectin to better reflect visceral than total adiposity [4]. These relationships were confirmed in our analysis, as well as the positive and negative association of adiponectin and leptin respectively with LV diastolic function (see Supplemental Table S2). However, these associations were no longer significant after multivariate adjustment, and in mediation analysis both adipokines (considered separately and together) did not appear to significantly modify the relationship between VAT and LV diastolic function. Clinical and experimental data on leptin and adiponectin effects on the heart are not entirely consistent [5, 14], and their prognostic significance is controversial, especially in elderly adults and people with chronic diseases [14, 29]. Hyperleptinemia may confer antisteatosis protection to the myocardium by increasing FAs oxidation in early stages of obesity, but this effect may be lost with the progression of leptin resistance that occurs in late stage obesity, leading to lipotoxicity that promotes cardiomyocyte contractile dysfunction and apoptosis [5]. We hypothesize that in our aging population leptin and adiponectin may have lost any potential protective effect on the myocardium (anti-steatotic and anti-hypertrophic effect, respectively), which meanwhile is exposed to an increasing amount of lipids.
Strengths and limitations
Strengths of this study include the large sample of healthy aging individuals free from cardiac diseases, the contemporaneous evaluation of cardiac function and fat indices, and the ability of the CT software to remove food residues in the intestines from abdominal fat quantification. The first and main limitation of this study is the cross-sectional design, which precludes us from making strong statements about causality, as this would require prospective design. Secondly, methodological issues may have influenced our results, as the differentiation between mediators and confounders was based on a specific pre-defined causal framework posited by the Authors. With this respect, bootstrapping analysis used to confirm statistically significant variables also does not allow per se to distinguish between mediators and confounders. Thirdly, to limit radiation exposure, abdominal adiposity has been assessed in the study with a single-slice image. However, a strong correlation has been shown between single-slice measurements of VAT and SAT with volumetric measurements [30]. Fourthly, biplane left atrial volume, deceleration time of mitral E velocity and isovolumic relaxation time were not available on all subjects, and therefore were not considered. Finally, although at present we can only speculate that myocardial triglyceride accumulation is a potential mechanism by which VAT may contribute to the age-related decline in LV diastolic function, cardiac and liver magnetic resonance spectroscopy is currently beginning in some study participants so that we can evaluate this possibility in future analysis.
CONCLUSIONS
Increased visceral adiposity is associated with LV diastolic dysfunction and might explain some of the decline in LV diastolic function that accompanies aging in healthy individuals. The phenomenon may be mediated by a metabolic pathway involving blood lipids and ectopic fat accumulation rather than adipokines. Further studies are needed to confirm these potential interactions and to assess whether decreasing visceral adiposity and therefore improving lipid metabolism may slow the decline in LV diastolic function associated with aging.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–9. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 3.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing research reviews. 2009;8:339–48. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, et al. Assessing adiposity: a scientific statement from the american heart association. Circulation. 2011;124:1996–2019. doi: 10.1161/CIR.0b013e318233bc6a. [DOI] [PubMed] [Google Scholar]
- 5.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–59. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 6.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Annals of internal medicine. 2006;144:517–24. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 7.Canepa M, Strait JB, Abramov D, Milaneschi Y, AlGhatrif M, Moni M, et al. Contribution of central adiposity to left ventricular diastolic function (from the Baltimore Longitudinal Study of Aging) Am J Cardiol. 2012;109:1171–8. doi: 10.1016/j.amjcard.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libhaber CD, Norton GR, Majane OH, Libhaber E, Essop MR, Brooksbank R, et al. Contribution of central and general adiposity to abnormal left ventricular diastolic function in a community sample with a high prevalence of obesity. Am J Cardiol. 2009;104:1527–33. doi: 10.1016/j.amjcard.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Windham BG, Griswold ME, Farasat SM, Ling SM, Carlson O, Egan JM, et al. Influence of leptin, adiponectin, and resistin on the association between abdominal adiposity and arterial stiffness. American journal of hypertension. 2010;23:501–7. doi: 10.1038/ajh.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makrogiannis S, Ramachandran R, Chee C, Ferrucci L. Automated Abdominal Fat Quantification and Food Residue Removal in CT. (Abstract) Mathematical Methods in Biomedical Image Analysis (MMBIA), IEEE Workshop on; 2012 Jan 9–10; pp. 81–86. [DOI] [Google Scholar]
- 12.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 13.Peter A, Kantartzis K, Machann J, Schick F, Staiger H, Machicao F, et al. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59:3167–73. doi: 10.2337/db10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pischon T. Use of obesity biomarkers in cardiovascular epidemiology. Disease markers. 2009;26:247–63. doi: 10.3233/DMA-2009-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnosis and classification of diabetes mellitus. Diabetes care. 2011;34 (Suppl 1):S62–9. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–82. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 17.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Medicine and science in sports and exercise. 2001;33:S459–71. doi: 10.1097/00005768-200106001-00016. discussion S93–4. [DOI] [PubMed] [Google Scholar]
- 18.Slinker BK, Glantz SA. Multiple linear regression: accounting for multiple simultaneous determinants of a continuous dependent variable. Circulation. 2008;117:1732–7. doi: 10.1161/CIRCULATIONAHA.106.654376. [DOI] [PubMed] [Google Scholar]
- 19.Babyak MA. Understanding confounding and mediation. Evidence-based mental health. 2009;12:68–71. doi: 10.1136/ebmh.12.3.68. [DOI] [PubMed] [Google Scholar]
- 20.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 21.Fleg JL, Shapiro EP, O’Connor F, Taube J, Goldberg AP, Lakatta EG. Left ventricular diastolic filling performance in older male athletes. JAMA. 1995;273:1371–5. [PubMed] [Google Scholar]
- 22.Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–91. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Fox CS, Hickson DA, May WL, Ding J, Carr JJ, et al. Pericardial fat and echocardiographic measures of cardiac abnormalities: the Jackson Heart Study. Diabetes care. 2011;34:341–6. doi: 10.2337/dc10-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Meer RW, Rijzewijk LJ, Diamant M, Hammer S, Schar M, Bax JJ, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J. 2008;29:1516–22. doi: 10.1093/eurheartj/ehn207. [DOI] [PubMed] [Google Scholar]
- 25.Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, et al. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–9. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 26.Hallsworth K, Hollingsworth KG, Thoma C, Jakovljevic D, Macgowan GA, Anstee QM, et al. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mells JE, Fu PP, Sharma S, Olson D, Cheng L, Handy JA, et al. Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302:G225–35. doi: 10.1152/ajpgi.00274.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer JR, Palmas W, Teresi J, Weinstock R, Shea S, Luchsinger JA. Adiponectin and all-cause mortality in elderly people with type 2 diabetes. Diabetes care. 2012;35:1858–63. doi: 10.2337/dc11-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010;34:781–7. doi: 10.1038/ijo.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


