Abstract
Background
Left ventricular assist devices (LVADs) have revolutionized management of end stage heart failure (ESHF). However, unexpectedly high rates of gastrointestinal bleeding (GIB) have been described, and etiologies and outcome remain unclear.
Objective
To determine the prevalence, etiology and outcome of GIB in LVAD recipients.
Design
Retrospective case series.
Setting
Tertiary care academic university hospital.
Patients
154 ESHF patients (55.4 yr, 122 M/32F) with LVADs implanted over a 10 year period.
Interventions
None
Main outcome measurements
Overt or occult GIB prompting endoscopic evaluation ≥7 days after LVAD implant.
Results
Over a mean of 0.9 ± 0.1 yrs of follow-up, 29 patients (19%) developed 44 GIB episodes. Patients with GIB were older and anticoagulated before device implant (p ≤ 0.02 for each). GIB was overt (n=31) rather than occult (n=13), and most presented with melena (n=22, 50%); hemodynamic instability was observed in 13.6%. Each bleeding episode required 2.1±0.1 diagnostic or therapeutic procedures, and a source was localized in 71%. Upper endoscopy provided highest diagnostic yield, peptic bleeding (n=14) and vascular malformations (n=8) dominated findings. Endoscopy was safe and well tolerated. Overall mortality was 35%, none directly from GIB.
Limitations
Retrospective design.
Conclusions
Rates of GIB with LVADs are higher than that seen in other patient populations, including those on anticoagulation and antiplatelet therapy. GIB episodes are mostly overt and predominantly from the upper GI tract. Endoscopy is safe in the LVAD population.
INTRODUCTION
Over five million people are affected by congestive heart failure (CHF) in the United States, with a quarter of these developing end stage heart failure (ESHF).(1) Medical therapies have significantly reduced morbidity and mortality, but heart transplantation remains the criterion standard for management.(1, 2) Given the large discrepancy between demand for heart transplants and donor organ supply, mechanical circulatory support (i.e. left ventricular assist devices, LVADs) has evolved rapidly over the past two decades. (3–5). Overall survival after 1 year of LVAD support can be as high as 72%; more than twice that seen with optimal medical therapy.(4) First generation LVADs were based on a pulsatile mechanism, compressed air driving a pump for pressure driven flow. However, poor reliability, high rates of infection and need for extensive surgical dissection during implantation led to development of continuous flow LVADs, which are smaller, do not require a large drive line, and are more reliable than pulsatile LVADs.(6, 7) Thromboembolic complications are countered by antithrombotic therapy, most commonly with a combination of aspirin and warfarin.(4)
The long term implications of lack of pulsatile blood flow on organ function are not well understood. Early animal studies showed that non-pulsatile blood flow adversely affected renal and hepatic perfusion, but limited studies after continuous flow LVAD implantation suggest preservation and even improvement of hepatic and renal function.(8–11) Several investigators have recently reported rates of luminal gastrointestinal bleeding (GIB) as high as 40% in patients after continuous flow LVADs.(12–18) These reports have left many unanswered questions regarding the risk factors for GIB during LVAD support, the role and safety of endoscopy, and outcomes in this population.
The aim of this retrospective study was to determine the prevalence, etiology and outcome of GIB in patients receiving LVAD support, and to assess the safety and efficacy of gastrointestinal endoscopy in this population.
METHODS
Patients
Adult patients (>18 yr) who had either HeartMate XVE (HM XVE, pulsatile flow) or HeartMate II (HM II, continuous flow) LVAD implantation (Thoratec, Pleasanton, CA) for ESHD over a 10 year period (2001–2010) were identified from our institutional database. Patients were excluded if they died in the operating room during LVAD implantation or if only a right ventricular assist device was placed. The review of clinical data for the purpose of this study was approved by the Institutional Review Board at Washington University School of Medicine.
LVAD implantation
LVADs were implanted as a bridge to transplant (BTT) or as destination therapy (DT) in patients deemed not to be transplant candidates. From 2001 to May 2005, HM XVE, a pulsatile LVAD, was used exclusively. After the continuous flow HM II became available, our institution transitioned to this device, which was used exclusively in the last 4 years of the study period.
All patients were anticoagulated postoperatively with unfractionated heparin or bivalirudin, and later transitioned to oral warfarin, with a goal international normalized ratio (INR) of 2–3. Aspirin 81 to 325 mg was used universally. Clopidogrel and/or dipyridamole were added at the discretion of the attending cardiologist and cardiac surgeon in select patients. All patients were maintained on intravenous proton pump inhibitor (PPI) until they were transferred out of the intensive care unit (ICU), after which further PPI administration was at the discretion of the treating cardiologist and cardiac surgeon.
Gastrointestinal bleeding
For the purpose of this study, GIB was defined as: a) the development of overt bleeding from the upper or lower GI tract as reported by treating or emergency room physicians, or 2) occult bleeding, with a ≥2 gram/dL drop in hemoglobin from recorded baseline values and hemoccult positive stool with no alternative explanation for anemia. Bleeding events within 7 days after LVAD implantation were deemed procedure related and were excluded. GIB was classified as early (within 8–30 days after LVAD implantation) and late (>30 days). In patients with multiple GIB events, a new episode of GIB was defined as bleeding >30 days from the initial event. A potential source of bleeding on endoscopy was defined as a lesion in the luminal GI tract with stigmata or recent bleeding (active bleeding, adherent clot, non-bleeding visible vessel, pigmented material at lesion base) in patients with overt bleeding, or any luminal GI tract lesion known to cause blood loss in patients with occult bleeding. Hemodynamic instability was defined as clinically significant end organ hypoperfusion requiring emergent blood transfusion and/or vasopressor support.
Endoscopy
All endoscopic procedures were performed in the inpatient endoscopy suite or intensive care unit. Conscious sedation (fentanyl, midazolam) or monitored anesthesia (including propofol) was administered at the discretion of the endoscopist and consulting anesthesiologist, and no set protocol was in place for choosing one method over the other. Heart rate, pulse oximetry and LVAD flow parameters were monitored at frequent intervals during the procedure. When endoscopic therapy was required for control of bleeding, injection (1:10,000 epinephrine), thermal therapy (bipolar or monopolar electrocautery, argon plasma laser), or mechanical therapy with homeostatic clips were utilized. Video capsule endoscopy was performed in select patients when recommended by the GI consult service; in all instances, records of prior imaging were reviewed to document absence of obstructing bowel lesions. Procedure notes on all endoscopic procedures were reviewed as part of data collection for this study.
Radiologic Studies
Radiologic studies were performed when endoscopy was unrevealing or when bleeding was not endoscopically controlled. Radionuclide bleeding studies were performed after IV administration of in vitro Tc-99m labeled red cells. Patients with a positive bleeding study or with rapid bleeding and hemodynamic compromise were referred for visceral angiography, performed under the direction of the attending interventional radiologist.
Data Collection
Demographics, clinical features of CHF, prior GIB, and comorbid medical illnesses were recorded from electronic medical records. With GIB presentations, hemodynamics, units of packed red blood cells transfused, ICU admission, nadir hemoglobin, INR and platelet count were recorded at presentation, discharge, and each subsequent GIB presentation. When applicable, cause of death was determined from our institution’s electronic medical record and LVAD registry.
Statistical analysis
The Shapiro-Wilk test was used to assess normality of data. Normally distributed data are reported as mean ± standard error of the mean, skewed data as median and interquartile range. Grouped continuous variable data were compared using two-tailed Student’s t test. Intergroup comparisons were made using the Chi-squared test and Fisher’s exact test where appropriate. A p value of <0.05 was required for statistical significance in each instance. Bleeding event rate per 100 patient-years was calculated and modeled with a Poisson regression having LVAD type as the independent variable, number of bleeding events as the dependent variable, and log patient years as the offset. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Between 2001 and 2010, 154 patients (55.4±0.87 years old, 79.2% male) underwent LVAD implantation (pulsatile HM XVE in 27.3%, continuous flow HM II in 72.7%). Of these, 29 patients (18.8%) developed 44 episodes of GIB during a mean of 0.9 ±0.1 years (range 0.1–2.8 years) of follow-up, after exclusion of one GIB event in the immediate post-operative period. Patients who developed GIB were older, more likely to have been anticoagulated before LVAD implantation, and more likely to have received an LVAD for DT rather than BTT (p<0.02 for each, Table 1), but PPI use and prior GIB were similar regardless of GIB. Etiology of ESHF (ischemic vs. non-ischemic cardiomyopathy) and indications for LVAD (DT vs. BTT) were similar between HM XVE and HM II devices.
Table 1.
Clinical Characteristics of Study Patients
| All patients | GIB | No GIB | p-value | |
|---|---|---|---|---|
| n=154 | n=29 | n=125 | ||
| Age (± SEM) | 55.4±0.87 years | 61.2±1.5 | 54.1±1.1 | 0.0003 |
| Gender | 122 M/ 32 F | 26 M/ 3 F | 96 M/29 F | |
| Etiology of CHF | NS | |||
| Ischemic | 89 (57%) | 16 (55%) | 73 (57%) | |
| Nonischemic | 65(43%) | 13 (45%) | 52 (43%) | |
| Therapy type | 0.015 | |||
| Bridge to transplant | 105 (68%) | 14 (48%) | 91 (73%) | |
| Destination therapy | 49 (32%) | 15 (52%) | 34 (27%) | |
| Prior GIB | 5 (3%) | 2 (7%) | 3 (2%) | NS |
| Prior anticoagulation | 93 (60%) | 23 (79%) | 69 (56%) | 0.02 |
| Antithrombotic therapy | ||||
| Warfarin | 111 (72.1%) | 20 (69%) | 91 (72.8%) | NS |
| Aspirin | 143 (92.9%) | 28 (97%) | 115 (92.0%) | NS |
| Clopidogrel | 5 (3%) | 1 (3%) | 4 (3%) | NS |
| Dipyridamole | 11 (7.7%) | 2 (6.9%) | 9 (7.2%) | NS |
| PPI use | 113 (73%) | 23 (79%) | 90(73%) | NS |
| Mortality | 55 (35%) | 10 (34%) | 45 (35%) | NS |
GIB: gastrointestinal bleeding; PPI: proton pump inhibitor
GIB events occurred after a mean of 159.3±25.8 days after LVAD implantation. Only 20.4% of GIB episodes occurred in the first month (within 8–30 days). Two-thirds of the GIB events occurred in outpatients and prompted hospital admission; in 14 instances (31.2 %), bleeding started in hospitalized patients. Most episodes of GIB were overt, presenting mainly as melena (Table 2). Bleeding occurred while anticoagulated in 77.3% (Table 3), when median INR was 2.0 (IQR 1.8–2.5). Only 13.6% were hemodynamically unstable, and admission to the ICU was only necessary in 5 instances (11.4%). The median duration of bleeding was 3.5 days (interquartile range, IQR 2–10 days), with mean nadir hemoglobin level of 7.3±0.2 g/dL (decline of 2.9±0.25 g/dL from baseline). Patients were given transfusions of 3.0 (IQR 2–4) units of packed red blood cells per bleeding event.
Table 2.
GI Bleeding Events by LVAD type
| All Patients | HM II Device | |
|---|---|---|
| n=44 | n=39 | |
| Occult | 13 (29.5%) | 11 (28.2%) |
| Overt | 31 (70.5%) | 28 (71.8%) |
| Melena | 22 | 21 |
| Hematochezia | 6 | 5 |
| Hematemesis | 3 | 2 |
| Mode of Localization | ||
| EGD | 20 (45.5%) | 17 (43.6%) |
| Colon | 10 (22.7%) | 9 (23.1%) |
| Deep Enteroscopy | 1 (2.3%) | 1 (2.6%) |
| Not localized | 13 (29.5%) | 12 (30.8%) |
| Final Diagnosis | ||
| Peptic Bleeding | 14 (31.8%) | 11 (28.2%) |
| Vascular Malformations | 12 (27.3%) | 12 (30.8%) |
| Colitis | 3 (6.8%) | 2 (5.1%) |
| Polyps | 2 (4.5%) | 2 (5.1%) |
| No diagnosis made | 13 (29.5%) | 12 (30.8%) |
Table 3.
Management Approach to Anticoagulation During Gastrointestinal Bleeding Episodes
| All patients | HM II device | |
|---|---|---|
| Management Approach | n=34 | n=29 |
| Acute Management | ||
| Fresh frozen plasma | 11 (32.4%) | 8 (27.5%) |
| Vitamin K | 1 (2.9%) | 1 (3.5%) |
| Warfarin held only | 22 (64.7%) | 20 (69.0%) |
| Chronic Management | ||
| Warfarin Continued | 23 (67.7%) | 19 (65.5%) |
| Warfarin Stopped | 11 (32.3%) | 10 (35.5%) |
Endoscopy
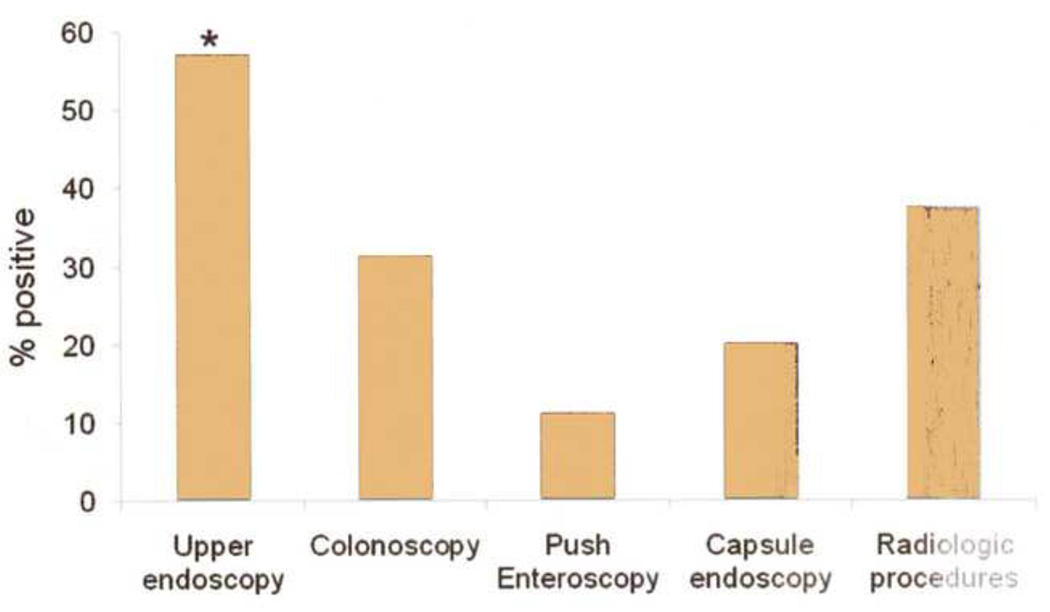
Coagulation parameters were corrected (INR<1.5, PTT< 30) in all patients before endoscopy (Table 3). One patient with obscure occult bleeding declined endoscopy. The time interval to the first endoscopic procedure was 2.04±0.25 days, 1.63±1.5 for overt bleeding and 3.0 ±0.49 for occult bleeding (p=0.01). Monitored anesthesia (involving propofol administration) was administered by ICU staff or an anesthesiologist in 44%; the remainder were administered conscious sedation (fentanyl, midazolam) by the endoscopist. A total of 82 endoscopic procedures and 8 radiologic procedures were performed (mean 2.1± 0.1 procedures per bleeding event). The need for repeat endoscopic procedures was higher (2.7±1.1 endoscopic procedures per bleeding event) when a source for bleeding was identified, compared with 1.8±0.9 procedures per bleeding event when no etiology for GIB could be found (p=0.002). Conscious sedation and monitored anesthesia were well tolerated, with no cardiac or pulmonary complications related to sedation. The sole endoscopic complication was a duodenal ulcer perforation after 3 attempts at hemostasis with monopolar electrocautery. There were no adverse events related to capsule endoscopy. A source for bleeding was localized in 71.5% of bleeding events (Table 2). Upper endoscopy provided highest diagnostic yield with a definitive source in 20 of 35 procedures (Figure 1, p<0.05 compared with colonoscopy and enteroscopy), peptic bleeding (n=14) and vascular malformations (n=5) dominated findings. Testing for Helicobacter pylori (histology, CLO test or serology) was undertaken in all patients with peptic bleeding but only 2 (14.3 %) had evidence of infection. The number of capsule endoscopy procedures performed (5) was limited.
Figure 1.
Diagnostic yield of endoscopic and radiologic procedures performed for the evaluation of gastrointestinal bleeding in patients with LVADs. Upper endoscopy had the highest yield for a source of bleeding, significantly higher than that seen with colonoscopy or enteroscopy. The number of capsule endoscopy (5) and radiologic procedures (8) performed were limited. (*p<0.05 compared to colonoscopy and enteroscopy)
HM II LVAD
Because only the HM II LVAD is currently in use, events specific to this device were separately analyzed (Table 2). There was a trend toward a higher incidence of GIB with continuous flow (HM II) compared with pulsatile (HM XVE) LVADs (34 events/100 patient years with HM II, and 27 events/100 patient yearswith HM XVE, p=0.64). All 10 bleeding events which occurred off anticoagulation were in HM II patients. Proportions of patients with each final diagnosis were not different from the entire cohort; vascular lesions were seen only with HM II. Diagnostic yields from each procedure performed were also comparable (Table 2).
Management
Endoscopic hemostatic therapy was attempted and was initially successful in 9 instances (all cases HM II, all performed for upper gut bleeding lesions). Therapy was performed using a thermal device (heater probe) in 2 instances, argon plasma coagulation in 3, and hemostatic clip in 4 instances; epinephrine injection was used as an adjunct in 3 instances. Six of the patients undergoing endoscopic hemostatic therapy rebled from the same source despite PPI therapy. One patient underwent successful surgery for treatment of a perforated duodenal ulcer. In 5 bleeding episodes, radionuclide red blood cell scans were obtained, and correctly localized the site of bleeding in 60.0%. Visceral angiography was undertaken during three bleeding events, and was negative in all instances. Anticoagulation was resumed at discharge in 23/34 (67.6%) of subjects who were anticoagulated before the onset of GI bleeding (Table 3).
Recurrent GI bleeding
Eleven patients, all with HM2 LVADs, developed 15 recurrent GIB events (38.5% of GIB events in HM 2 group), with a mean of 1.5±0.2 recurrent bleeding events per patient. In 73% of instances, the recurrent GIB event presented with the same symptoms as the initial event. The etiology of bleeding was the same on recurrence in 7 patients, and different in 2 patients (duodenal vascular lesion, ischemic colitis in one, and peptic ulcer, jejunal vascular lesion in the other); an etiology for bleeding could not be established in the remainder (2 patients) with recurrent bleeding. There were no episodes of recurrent GIB in patients supported by HM XVE.
DISCUSSION
In the largest study to date monitoring GIB after LVAD implantation, we report an unusually high rate of GIB during LVAD support in patients with ESHF. The 19% incidence of GIB in LVAD patients is higher than the 1.5–8% incidence typically observed in patients on triple antithrombotic therapy (aspirin, clopidogrel, warfarin or equivalent) for cardiovascular disease.(19) Further, we report that GIB episodes are mostly overt and identified bleeding sources are predominantly from the upper GI tract. Our results indicate that a thorough endoscopic evaluation can identify an etiology for GI bleeding in over 70% of cases, and upper endoscopy has the highest diagnostic value. Finally our experience demonstrates that diagnostic and therapeutic endoscopy, including capsule endoscopy, is safe in the LVAD population.
The high rate of GI bleeding observed in our study is consistent with findings from prior smaller case series. (12, 17, 18) Specifically, Stern et al. reported that an etiology for GIB could not be identified in 65% of cases; however, of only 17 GIB events, none were attributed to PUD [14]. Similarly, Crow et al. report no PUD related bleeds in their 101 patient cohort, despite demonstrating a potential etiology for GIB identified in 75% of patients. Both studies provide few details regarding the nature of GIB events and subsequent work up. In our cohort, an etiology for GIB was identified in 70% of patients, which concurs rates reported with antithrombotic therapy (70–80%) [16, 17, 18]. Further, patients on antithrombotic therapy have upper GI tract bleeding sites >50% of the time, with which our findings are consistent. Finally, our proportions of PUD (32%) are concordant with rates seen with antithrombotic therapy (20–50%) [17, 18]. The high rates of PUD bleeding in these patients has been attributed to nonsteroidal anti-inflammatory drug (NSAID) induced damage to the upper GI tract mucosa coupled with NSAID ± thiopyridine induced platelet inhibition and anticoagulation.(19, 20) We therefore conclude that an upper endoscopic examination is the best initial test in LVAD patients presenting with acute GIB, and provides the highest likelihood of finding a source for the bleed.
Our finding of the lack of protection afforded by PPI use to GIB in our patient population is surprising, because data from randomized controlled trials suggest that the addition of PPI to antiplatelet therapy decreases the risk of GIB by more than 60% in individuals who are at average risk and by as much as 89% in those with history of GIB.(21, 22) Further, our rate of Helicobacter pylori positivity was extremely low. Taken together, these findings lead us to speculate that non-traditional factors likely have a significant role in the pathogenesis of ulceration and GIB in patients being supported by LVADs. We note that higher rates of GIB have been reported with continuous flow LVADs compared with pulsatile devices; a similar trend can be seen in our data.(12, 17) This observation may suggest alterations in GI mucosal and vascular biology with continuous flow that may lower mucosal protection against gastric acid. It is also possible that autoregulation of intestinal blood flow is compromised with lack of pulsatile flow, similar to that observed in normotensive critically ill patients with stress ulcers, 50% of whom have evidence of splanchnic hypoperfusion despite normal blood pressure.(23) This hypothesis is also consistent with animal studies by Yasue et al. suggesting that ischemic mucosal damage in the stomach occurs even in the absence of gastric acid.(24)
Other investigators have proposed that the high rate of bleeding in continuous flow LVADs is from a defect in thrombogenesis brought on by nonpulsatile blood flow.(25) Patients can develop decreased levels of high molecular weight von Willebrand factor (vWF), which are critical for platelet adhesion. Levels can be low enough to support the diagnosis of acquired vWF syndrome.(25, 26), potentially from high shear forces encountered in the continuous flow LVAD that resolve after transplant.(25). This insult, combined with pharmacologically induced platelet dysfunction, could significantly increase the risk of GIB in patients with pre-existing gastrointestinal pathology, similar to Heyde’s syndrome in patients with aortic stenosis.(27, 28) Our study was not geared to test these concepts, but we propose that impaired mucosal perfusion combined with an acquired bleeding diathesis may be responsible for the increased GIB risk in continuous flow LVADs. Given the observed lack of protection afforded by PPIs, adjunctive mucosal protective agents such as sucralfate or misoprostol need to be investigated. Furthermore, given the fact that an acquired vWF deficiency may contribute to bleeding during LVAD support, the current approach of using antiplatelet and anticoagulation therapy may need to be reconsidered in favor of vitamin K antagonists or oral direct thrombin inhibitors.
Our study does have some limitations. The retrospective nature of the study may have led us to underestimate the true incidence of GIB. Second, the pulsatile and continuous flow LVAD groups were not balanced. Despite this we observed a trend toward a higher rate of GIB in the HM II group. Third, the workup of GIB was not standardized; a source could have been identified if a more aggressive endoscopic workup was pursued, as can be the case with obscure GIB in the general population.(29) However, given the relatively slow and benign course of GIB in most patients in our series, the conservative approach of observation and support with iron or transfusion therapy appears to be adequate for clinical management when GI bleeding remains obscure after bidirectional endoscopy. Finally, we did not assess splanchnic perfusion or vWF activity and can only speculate on the pathogenesis of GIB during LVAD support.
In conclusion, we report that the rate of GIB after LVAD implantation is significantly higher than that seen after anticoagulation or antiplatelet therapy for cardiovascular conditions. The majority of GIB episodes during LVAD support are overt and from the upper GI tract, peptic erosions and ulcers dominating the etiology when a bleeding source was identified. A thorough endoscopic evaluation can identify an etiology for GIB in over 70% of cases; with upper endoscopy (followed by push enteroscopy, if negative) offering the highest upfront diagnostic yield. Further prospective studies are needed in this population to elucidate the mechanisms leading to GIB, as well as evaluate novel therapeutic approaches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362(3):228–238. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1035–1046. doi: 10.1111/j.1600-6143.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 4.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg. 2010;25(4):490–494. doi: 10.1111/j.1540-8191.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 7.Caccamo M, Eckman P, John R. Current State of Ventricular Assist Devices. Curr Heart Fail Rep. 2011 doi: 10.1007/s11897-011-0050-z. [DOI] [PubMed] [Google Scholar]
- 8.Sezai A, Shiono M, Orime Y, Nakata K, Hata M, Yamada H, et al. Renal circulation and cellular metabolism during left ventricular assisted circulation: comparison study of pulsatile and nonpulsatile assists. Artif Organs. 1997;21(7):830–835. doi: 10.1111/j.1525-1594.1997.tb03752.x. [DOI] [PubMed] [Google Scholar]
- 9.Radovancevic B, Vrtovec B, de Kort E, Radovancevic R, Gregoric ID, Frazier OH. End-organ function in patients on long-term circulatory support with continuous- or pulsatile-flow assist devices. J Heart Lung Transplant. 2007;26(8):815–818. doi: 10.1016/j.healun.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Saito S, Westaby S, Piggot D, Dudnikov S, Robson D, Catarino PA, et al. End-organ function during chronic nonpulsatile circulation. Ann Thorac Surg. 2002;74(4):1080–1085. doi: 10.1016/s0003-4975(02)03846-8. [DOI] [PubMed] [Google Scholar]
- 11.Kamdar F, Boyle A, Liao K, Colvin-adams M, Joyce L, John R. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28(4):352–359. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Crow S, John R, Boyle A, Shumway S, Liao K, Colvin-Adams M, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137(1):208–215. doi: 10.1016/j.jtcvs.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui MA, Slaughter MS, Silva RG. Gastrointestinal Complications in Patients Supported with Ventricular Assist Devices. Gastroenterology. 2009;136(5):S1210. [Google Scholar]
- 14.Young SD, Meyer MM, Azzouz M. The Role of Endoscopy in the Evaluation of Gastrointestinal Bleeding in Patients with Ventricular Assist Devices. Gastrointest Endosc. 2009;69(5):S1556. [Google Scholar]
- 15.Maso NO, Reid BB, Harmstan G, Jones J, Stoker S, Budge D, et al. Characterization of Gastrointestinal Bleeding in HeartMate II Left Ventricular Assist Device Patients. The Journal of Heart and Lung Transplantation. 2010;29(2):S175. [Google Scholar]
- 16.Muthiah K, Hayward C, Macdonald P, E. Kotlyar P, Spratt EG, Jansz P, et al. Gastrointestinal Bleeding Due to Angiodysplasia in Patients With Continuous Flow Left Ventricular Assist Device. Heart, Lung and Circulation. 2010;19:S70. [Google Scholar]
- 17.Stern DR, Kazam J, Edwards P, Maybaum S, Bello RA, D'Alessandro DA, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25(3):352–356. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 18.Schaffer JM, Arnaoutakis GJ, Allen JG, Weiss ES, Patel ND, Russell SD, et al. Bleeding complications and blood product utilization with left ventricular assist device implantation. Ann Thorac Surg. 91(3):740–747. doi: 10.1016/j.athoracsur.2010.11.007. discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 19.Barada K, Abdul-Baki H, El H, II, Hashash JG, Green PH. Gastrointestinal bleeding in the setting of anticoagulation and antiplatelet therapy. J Clin Gastroenterol. 2009;43(1):5–12. doi: 10.1097/MCG.0b013e31811edd13. [DOI] [PubMed] [Google Scholar]
- 20.Tanigawa T, Watanabe T, Nadatani Y, Otani K, Machida H, Okazaki H, et al. Gastrointestinal bleeding after percutaneous coronary intervention. Digestion. 2011;83(3):153–160. doi: 10.1159/000321813. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363(20):1909–1917. doi: 10.1056/NEJMoa1007964. [DOI] [PubMed] [Google Scholar]
- 22.Lai KC, Lam SK, Chu KM, Wong BC, Hui WM, Hu WH, et al. Lansoprazole for the prevention of recurrences of ulcer complications from long-term low-dose aspirin use. N Engl J Med. 2002;346(26):2033–2038. doi: 10.1056/NEJMoa012877. [DOI] [PubMed] [Google Scholar]
- 23.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135(1):41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Yasue N, Guth PH. Role of exogenous acid and retransfusion in hemorrhagic shockinduced gastric lesions in the rat. Gastroenterology. 1988;94(5 Pt 1):1135–1143. doi: 10.1016/0016-5085(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 25.Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56(15):1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Geisen U, Heilmann C, Beyersdorf F, Benk C, Berchtold-Herz M, Schlensak C, et al. Non-surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33(4):679–684. doi: 10.1016/j.ejcts.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 27.Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349(4):343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 28.Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: is acquired von Willebrand's disease the link? Lancet. 1992;340(8810):35–37. doi: 10.1016/0140-6736(92)92434-h. [DOI] [PubMed] [Google Scholar]
- 29.Fisher L, Lee Krinsky M, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, et al. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc. 2010;72(3):471–479. doi: 10.1016/j.gie.2010.04.032. [DOI] [PubMed] [Google Scholar]