Abstract
AtKEAs, homologs of bacterial KefB/KefC, are predicted to encode K+/H+ antiporters in Arabidopsis. The AtKEA family contains six genes forming two subgroups in the cladogram: AtKEA1-3 and AtKEA4-6. AtKEA1 and AtKEA2 have a long N-terminal domain; the full-length AtKEA1 was inactive in yeast. The transport activity was analyzed by expressing the AtKEA genes in yeast mutants lacking multiple ion carriers. AtKEAs conferred resistance to high K+ and hygromycin B but not to salt and Li+ stress. AtKEAs expressed in both the shoot and root of Arabidopsis. The expression of AtKEA1, -3 and -4 was enhanced under low K+ stress, whereas AtKEA2 and AtKEA5 were induced by sorbitol and ABA treatments. However, osmotic induction of AtKEA2 and AtKEA5 was not observed in aba2-3 mutants, suggesting an ABA regulated mechanism for their osmotic response. AtKEAs’ expression may not be regulated by the SOS pathway since their expression was not affected in sos mutants. The GFP tagging analysis showed that AtKEAs distributed diversely in yeast. The Golgi localization of AtKEA3 was demonstrated by both the stably transformed seedlings and the transient expression in protoplasts. Overall, AtKEAs expressed and localized diversely, and may play roles in K+ homeostasis and osmotic adjustment in Arabidopsis.
Introduction
Na+,K+/H+ antiporters are secondary transporters that exist in all kinds of life including bacteria, yeast, plants and animals [1], [2], [3], [4]. They are H+-coupled cotransporters whose biochemical activity is to transfer the Na+ or K+ across a membrane in exchange for protons (H+). Na+,K+/H+ antiporters form a large gene family, and currently there are more than 200 genes that have been annotated as Na+,K+/H+ antiporters [3]. The Na+,K+/H+ antiporter is categorized into the monovalent cation proton antiporter (CPA) gene family [5]. Na+,K+/H+ antiporters are critical for ion homeostasis and pH regulation in cells, and function in diverse cellular processes, including Na+ and K+ movement, salt tolerance, regulation of cell cycle and cell proliferation, vesicle trafficking and fusion, and biogenesis [6], [7], [8].
In the Arabidopsis genome, there are approximately 44 genes that are predicted to encode Na+,K+/H+ antiporters, including 8 AtNHXs, 28 AtCHXs and 6 AtKEAs. The AtNHXs belong to the CPA1 gene family while the AtCHXs and the AtKEAs are members of the CPA2 family [3], [4], [9], [10].
The function, transport activity, and regulatory mechanism of AtNHX transporters have been studied extensively [1], [2], [7], [11], [12]. AtNHXs are involved in the regulation of cellular ion and pH homeostasis, and play an important role in salt tolerance, K+ homeostasis, vesicle trafficking, and plant growth and development [8], [11], [12], [13]. Overexpression of AtNHX1 and SOS1/AtNHX7 reduces cytoplasmic Na+ content and enhances salt tolerance in Arabidopsis [14], [15], [16]. SOS1 activity is regulated by SOS2 kinase [17], [18], [19]. SOS1 is activated by the removal of a C-terminal auto-inhibitory domain upon phosphorylation by the SOS2/SOS3 complex [20]. AtNHX1 may also be regulated by SOS2 kinase [21]. CaM binds and inhibits the Na+/H+ antiport activity of AtNHX1 [22]. AtNHX1 and LeNHX2 have a K+/H+ transport activity and mediate K+ compartmentation in vacuoles [23], [24], [25], [26], [27]. The NHX genes in Ipomea tricolor and Ipomea Nil are involved in vacuolar pH regulation; mutation of a NHX gene in Ipomea Nil abolished the colour change in flowers, a process that is controlled by an increase in vacuolar pH [28], [29]. nhx1nhx2 double knockout mutants showed significantly reduced growth, abnormal stamens and lacked silique formation, indicating that AtNHX1 and AtNHX2 function in cell expansion and flower development [30]. nhx1nhx2 double mutants had reduced ability in creating the vacuolar K+ pool, impaired osmoregulation, and compromised turgor generation for cell expansion, indicating that AtNHX1 and AtNHX2 are essential for active K+ uptake at the tonoplast, turgor regulation, and stomatal function [31]. AtNHX5 and AtNHX6 play an important role in endosomal sorting and stress responses. The nhx5nhx6 double knockout mutants had reduced growth and increased sensitivity to salinity. Vacuolar trafficking was affected in nhx5nhx6 [32].
The function and expression of AtCHX genes are beginning to be explored. AtCHXs regulate K+ and pH homeostasis, and function in controlling membrane trafficking, osmoregulation, and pollen growth and development [4], [10], [33], [34]. Most of the AtCHX genes are preferentially expressed in the male gametophyte and sporophytic tissues, suggesting roles in pollen desiccation at maturation and rehydration on germination [10]. AtCHX13 is localized to the plasma membrane K+ transporter which is responsible for K+ uptake in roots [35]. AtCHX17 is mainly expressed in roots and mediates K+ homeostasis [36]. AtCHX20 is highly expressed in guard cells, and functions in osmoregulation of stomatal opening through K+ movement and pH regulation in guard cells [37]. AtCHX21 is a putative Na+ transporter regulating Na+ homehostasis in xylem and Na+ accumulation in leaves [38]. AtCHX23 functions in the adjustment of pH in the cytosol and possibly in maintaining a high pH level in the chloroplast stroma [39]. In the Atchx21chx23 double mutant, pollen tubes failed to target ovules which resulted in impaired pollen fertility, indicating a role in pollen tube guidance [34]. AtCHX17 and AtCHX21 have K+ transport activities, and are involved in protein sorting [33]. AtCHX20 might be a K+/H+ symporter and AtCHX17 might function as a K+ channel [33].
AtKEAs are homologs of EcKefB and EcKefC, K+ efflux transporters in E. Coli [4].. EcKefB/EcKefC is activated by adducts of glutathione and negatively regulated by glutathione, and function in survival of stress caused by toxic metabolites. The C-terminus of EcKefB/EcKefC has a KTN domain, which is shared by many bacterial potassium channels and transporters, including EcKch, EcTrkA, EcYbal, EcKefB and EcKefC [40]. Binding of the ancillary protein EcKefF and GSH induces conformational changes in EcKefC KTN dimmers and activates the transport activity of EcKefB/EcKefC [41], [42], [43], [44]. EcKefC may function as a K+/H+ antiporter, although it was previously thought to act as a K+ channel [45]. However, the function of the AtKEA gene family remains largely uncharacterized. So far, only AtKEA2 has been characterized experimentally [46]. AtKEA2 was targeted at chloroplasts and expressed highly in aerial parts of Arabidopsis. AtKEA2 conferred resistance to hygromycin B, high K+, and Na+ stress in scnhx1 mutants. AtKEA2 was shown to have cation/H+ antiport activity when measured with reconstituted lipososmes. In another study, AtKEA1and AtKEA3 were detected from chloroplast preparations in Arabidopsis by mass spectrometry [47].
In this report, the function and expression of the AtKEA gene family have been studied. We have used yeast growth, RT-qPCR and GFP labeling techniques to characterize the transport activity, gene expression and cellular localization of the AtKEA family. Our results show that AtKEAs are diversely expressed and distributed in cells, and may function in facilitating K+ homeostasis and osmotic adjustment in Arabidopsis.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana ecotypes Columbia (Col-0), mutants, and transgenic lines were used in this study. Arabidopsis mutant aba2-3 was ordered from ABRC;sos1-1,sos2-1,sos3-1 were gifts from Dr. Jian-Kang Zhu. In the growth chamber, plants were grown on compost (Pindstrup Substrate, Latvia) and subirrigated with tap water. Greenhouse conditions were as follows: 16-h-light / 8-h-dark cycles, light intensity 100 μmol s-1 m-2 photosynthetically active radiation, temperature 22°C. For plate-grown plants, Arabidopsis thaliana seeds were surface sterilized with 20% (v/v) bleach, after cold treatment at 4°C for 3 days, the seeds were germinated on plates with 1/2 strength Murashige and Skoog (MS) medium containing 0.8% agar, pH 5.8. For growth at low potassium, seedlings were cultured on a modified MS medium containing various concentrations of KCl [48].
Bioinformatic analysis
The predicted amino acid sequences of AtKEAs were collected from the GenBank (http://www.ncbi.nlm.nih.gov). Pairwise amino acid sequence were compared using EMBOSS Needle (http://www.ebi.ac.uk/Tools/psa/) [49]. Proteins were compared by multiple alignments using the ClustalX 2.1 method [50]. Phylogenetic analysis was conducted in MEGA 5.02 [51]. The alignment is based on the complete amino acid sequences. Evolutionary distances were computed by the Neighbor Joining method. Bootstrap analysis for each branch was performed 10,000 times.
Yeast Strains, media, and growth conditions
Saccharomyces cerevisiae strains W303-1B (MATα leu2-13 112, ura3-1, trp1-1, his3-11 15, ade2-1, can1-100), ANT3 (ena1-4Δ::HIS3, nha1Δ::LEU2), AXT3 (ena1-4Δ::HIS3, nha1Δ::LEU2, nhx1Δ:: TRP1) and AXT4K (ena1-4Δ::HIS3, nha1Δ::LEU2, nhx1Δ:: TRP1, kha1Δ::KanMX6) were gifts from Dr. Jose M. Pardo [52], [53], [54]. All strains used were derivatives of W303-1B. Untransformed strains were grown at 30°C in YPD medium (1% yeast extract, 2% peptone and 2% glucose). Transformation of yeast cells was performed by the lithium acetate method. After transformation, strains were grown on selective Hartwell's complete (SC) medium or APG medium (10mM arginine, 8mM phosphoric acid, 2 mM MgSO4, 1 mM KCl, 0.2 mM CaCl2, 2% glucose, and trace minerals and vitamins). NaCl, KCl, or hygromycin B was added to the medium. Drop test media contained 20 mM MES, and pH was adjusted to 7.5 with arginine [33] or to acidic pH values with phosphoric acid [55].
Functional expression of AtKEAs in yeast
To clone the full length CDS of AtKEA1, we separated AtKEA1 into two segments, AtKEA1-F1870 and AtKEA1-L1712, respectively, by an EcoRI restriction site in the middle of the gene. Primers KEA1-SmaI F and KEA1-1996R (Table S1) were used to amplify the AtKEA1-F1870 using the PrimeSTARTM HS DNA Polymerase (TaKaRa) by PCR. AtKEA1-L1712 was amplified with primers KEA1-1862F and KEA1-XhoI R (Table S1) by PCR. The PCR product AtKEA1-F1870 was ligated into the yeast expression vector pDR196 at SmaI-EcoRI sites by T4 DNA ligase (promega), resulting in pDR196-AtKEA1-F1870. Then, the PCR product AtKEA1-L1712 was inserted into pDR196-AtKEA1-F1870 at EcoRI-XhoI sites to obtain the full length AtKEA1 (named as pDR196-AtKEA1). The full length CDS of AtKEA1 was verified by sequencing.
For AtKEA2 (gene accession AT4G00630.1), a XhoI site in the middle of the gene was chosen to separate the gene into two pieces of A1-G2123 and A2124-A3525 (AtKEA2-F2123 and AtKEA2-L1402, respectively). Primers KEA2-NotI F and KEA2-2196R (Table S1) were used to amplify the AtKEA2-F2123 by PCR. AtKEA2-L1402 was amplified with primers KEA2-2052F and KEA2-XbaI R (Table S1) by PCR. The PCR product AtKEA2-F2123 was ligated into the yeast expression vector pYES2 at NotI-XhoI sites, resulting in pYES2- AtKEA2-F2123. Then, the PCR product AtKEA2-L1402 was inserted into the vector pYES2-AtKEA2-F2123 at XhoI-XbaI sites to obtain the full length AtKEA2 (named as pYES2-AtKEA2). However, after combining, mutations were generated at the region of A1302-A1308 which contains consecutive 7 adenines (As). Either 1 to 2 As were missing or added in the gene sequence. Similar mutations happened when we chose a KpnI site which separated the gene into two pieces of A1-C1359 and A1360-A3525. Thus, the full-length cDNA of AtKEA2 was not cloned in our experiments.
To clone AtsKEA1(short form of AtKEA1 with 1857bp nucleotides), AtKEA3, AtKEA4, AtNHX1 and AtCHX17, gene fragments were amplified by PCR from Arabidopsis cDNA (primers are listed in Table S1). To clone ScNHX1 and ScKHA1, gene fragments were amplified by PCR from the genomic DNA isolated from the Saccharomyces cerevisiae strain BJ3505(primers are listed in Table S1).The PCR fragments were cloned into the same sites of the plasmid pDR196. All gene fragments were verified by sequencing.
The cDNAs of AtsKEA2 (short form of AtKEA2 with 1860bp nucleotides), AtKEA5 and AtKEA6, ordered from ABRC, were cloned in the yeast expression vector pDR196 with the promoter PMA1.
All plasmids were transformed into the yeast strain AXT3 or AXT4K; the empty vector pDR196 was transformed into the same yeast strains as a control. For stress tolerance tests, yeast cells were normalized in water to A600 of 0.4. 4μl aliquots of each 10-fold serial dilution were spotted onto AP plates supplemented with KCl, or YPD plates supplemented with NaCl as indicated, and incubated at 30°C for 3 days. Resistance to hygromycin B was assayed in YPD medium.
Quantitative real-time RT-PCR (RT-qPCR) analysis
14-day-old WT seedlings, growing on 1/2 MS plates, were transferred into the liquid 1/2 MS media without (control) or with 160 mM NaCl, 40 mM LiCl, 320 mM sorbitol or 100 μM ABA, respectively, and maintained for 8 h (Yokoi et al., 2002). The total RNA was isolated using the RNAiso Plus (TaKaRa). The first-strand cDNA was synthesized from the total RNA (1μg ) using the PrimeScript® RT reagent kit with gDNA Eraser (TaKaRa), and was used as templates for PCR amplification. PCR amplification was performed with the CFX96 system (Bio-Rad) using the SYBR® Premix Ex Taq™ (TaKaRa). The Arabidopsis Actin7 gene was used as an internal control, and differences in product levels among the tested samples during the linear amplification phase were used to calculate the differential gene expression [56]. The gene-specific primers are listed in Table S2.
Localization of the AtKEAs-GFP fusion proteins in yeast
To make GFP fusion constructs, we converted the vector pDR196 into a Gateway destination vector pDR196-GFP. GFP was fused at the C-terminal of plant or yeast proteins. Gene fragments of AtKEAs, AtNHX1 and AtCHX17 were amplified by PCR from Arabidopsis cDNAs (primers are listed in Table S3). To make GFP fusion constructs for ScNHX1 and ScKHA1, gene fragments were amplified by PCR from the genomic DNA isolated from the Saccharomyces cerevisiae strain BJ3505 (primers are listed in Table S3). PCR fragments were inserted into the plasmid pDR196-GFP using the Gateway technology (Invitrogen), respectively. Gene fragments were verified by sequencing.
The recombinant plasmids were transformed into the yeast strain W303-1B. Yeast cells grown to logarithmic phase at 30°C in SC-URA medium adjusted to pH 5.8. For FM4-64 staining, yeast cells grown exponentially were harvested and suspended in fresh YPD medium, and then were incubated with FM4-64 dye at a final concentration of 5μM. After incubation for 8h, the cells were washed four times with phosphate buffered saline (PBS) and concentrated by centrifugation. After mixing with 0.6% agarose, the cells were mounted on glass slides and observed by a confocal laser scanning microscope (FV1000, Olympus) [57].
Localization of the RFP-AtKEA3 fusion protein in Arabidopsis protoplasts
Transient expression assay using protoplasts derived from the leaf mesophyll cells of Arabidopsis were performed as described [58]. RFP gene was fused in frame to AtKEA3 at its N-terminus. The AtKEA3 gene was amplified by using the following primers: 5′-AAAAAGCAGGCTTCATGGCAATTAGTACTATGTT-3′ and 5′-AGAAAGCTGGGTCTTAATCTTGAGCTTTATCAG -3′. The PCR fragment was inserted into the plasmid pUBN-RFP [59] using the Gateway technology (Invitrogen). Protoplasts were co-transfected with pUBN-RFP-AtKEA3 and a cis-Golgi marker GFP-AtSYP31. Arabidopsis seedlings of 4 weeks old were used for protoplast isolation. Fluorescence was visualized by a confocal laser scanning microscope (FV1000, Olympus).
Localization of the AtKEA3-GFP fusion protein in stably transformed Arabidopsis seedlings
For the stable transformation assays with Arabidopsis thaliana, the GFP gene was fused in frame to AtKEA3 at its C-terminus. The AtKEA3 gene was amplified by using the following primers: 5′-AAAAAGCAGGCTTCATGGCAATTAGTACTATGTT-3′ and 5′-AGAAAGCTGGGTCATCTTGAGCTTTATCAGC-3′. The PCR fragment was inserted in plasmid pBIB-EGFP using the Gateway technology (Invitrogen), and the resulting construct was transformed into Agrobacterium tumefaciens GV3101. Arabidopsis thaliana (ecotype Columbia) wild-type plants were transformed [60]. The transgenic plants were screened by basta spray; the basta positive seedlings were re-confirmed with PCR amplification of the GFP fragment. GFP fluorescence was visualized under a confocal laser scanning microscope (FV1000, Olympus). The excitation wavelength for EGFP detection was 488 nm. The roots of the transgenic seedlings containing the 35S-AtKEA3-EGFP fusion protein were visualized under the confocal microscope.
The GenBank accession numbers for sequence data used in this article are: AtKEA1 (AEE27335); AtKEA2 (AEE81911); AtKEA3 (AEE82433); AtKEA4 (AEC06899); AtKEA5 (AED96117); AtKEA6 (AED91722); AtNHX1 (AAD16946); AtNHX2 (AAM08403); AtCHX1 (AEE29444); AtCHX17 (AEE84796); EcKefB (ACT40850); EcKefC (CAA40066); ScKHA1 (DAA08706); ScNHX1 (DAA12290).
Results
AtKEAs separate from AtNHXs and AtCHXs in the phylogenetic tree
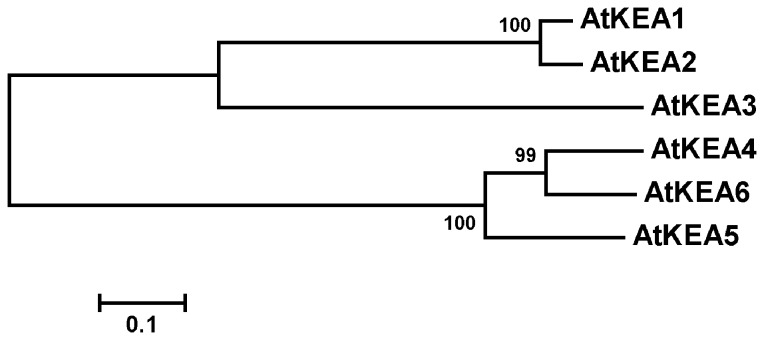
The Arabidopsis AtKEA gene family contains six members, named KEA1 through KEA6 [3], [9]. Based on amino acid sequences, AtKEA are predicted to encode K+/H+ antiporters with 10 transmembrane spanning domains (Table 1 and Figure 1). The AtKEA genes are named in decreasing order of sequence similarity to AtKEA1, except for AtKEA5 which has a higher similarity (22.4%) than AtKEA4 (21.9%) (Table 1 and Figure 2). AtKEA2-3 are 29.9-84.5% similar to AtKEA1, whereas AtKEA4-6 are 75.0-83.4% similar to each other but only 21.9-30.0% similar to the AtKEA1-3 isoforms (Table 1). The AtKEA gene family forms two subgroups in cladogram, AtKEA1-3 and AtNHX4-6 (Figure 2).
Table 1. Amino acid similarity comparison of the six Arabidopsis thaliana family members of AtKEA K+/H+ antiporters.
| AtKEA1 (AEE27335) | AtKEA2 (AEE81911) | AtKEA3 (AEE82433) | AtKEA4 (AEC06899) | AtKEA5 (AED96117) | AtKEA6 (AED91722) | |
|---|---|---|---|---|---|---|
| AtKEA1 (AEE27335) | — | 84.5% | 29.9% | 21.9% | 22.4% | 24.5% |
| AtKEA2 (AEE81911) | — | 31.0% | 23.2% | 22.4% | 24.9% | |
| AtKEA3 (AEE82433) | — | 28.2% | 28.6% | 30.0% | ||
| AtKEA4 (AEC06899) | — | 75.2% | 83.4% | |||
| AtKEA5 (AED96117) | — | 75.0% | ||||
| AtKEA6 (AED91722) | — |
The family members are listed in order of sequence similarity beginning with the prototype AtKEA1. Accession numbers corresponding to the GenBank database are given in parentheses.
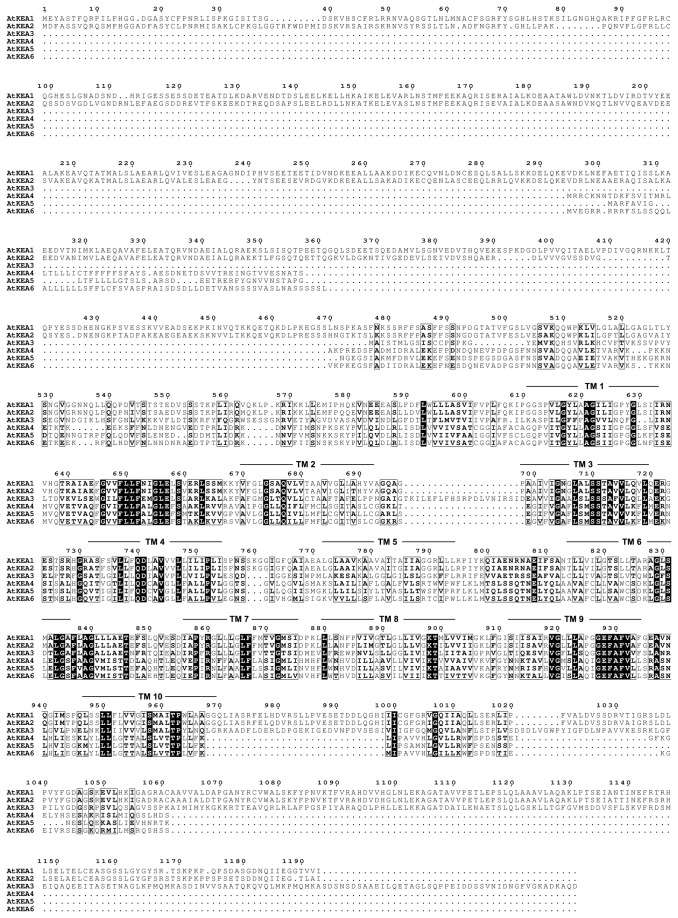
Figure 1. Multiple alignment of the putative amino acid sequences of the AtKEA family.
The predicted amino acid sequences of AtKEA1 (AEE27335), AtKEA2 (AEE81911), AtKEA3 (AEE82433), AtKEA4 (AEC06899), AtKEA5 (AED96117) and AtKEA6 (AED91722) were aligned based on analysis using the ClustalX 2.1 method. Identical or similar residues are blocked as dark or light boxes, respectively. Putative transmembrane domains were analyzed using the TMHMM method and are marked at the approximate TM regions.
Figure 2. Cladogram analysis of the AtKEA family.
Cladogram analysis was conducted using MEGA 5.02. The alignment was based on the predicted amino acid sequences of AtKEA1, AtKEA2, AtKEA3, AtKEA4, AtKEA5 and AtKEA6. Evolutionary distances were computed by the Neighbor Joining method. The scale bar indicates the distance calculated from the multiple alignments.
AtKEA1 and AtKEA2 have a long N-terminal domain, which contains 610 and 590 amino acids, respectively (Figure 1). The N-terminal regions of AtKEA1 and AtKEA2 have been predicted to carry a chloroplast transit peptide [46]. The chloroplast localization of AtKEA1 has been identified by a mass spectrometry assay [47]. The localization of AtKEA2 in chloroplasts has been visualized in seedlings transformed with the GFP tagged AtKEA2 [46]. In addition, AtKEA1 and AtKEA2 have a long C-terminal tail, containing 224 and 222 amino acids, respectively (Figure 1), suggesting that AtKEA1 and AtKEA2 may have a distinct regulatory mechanism.
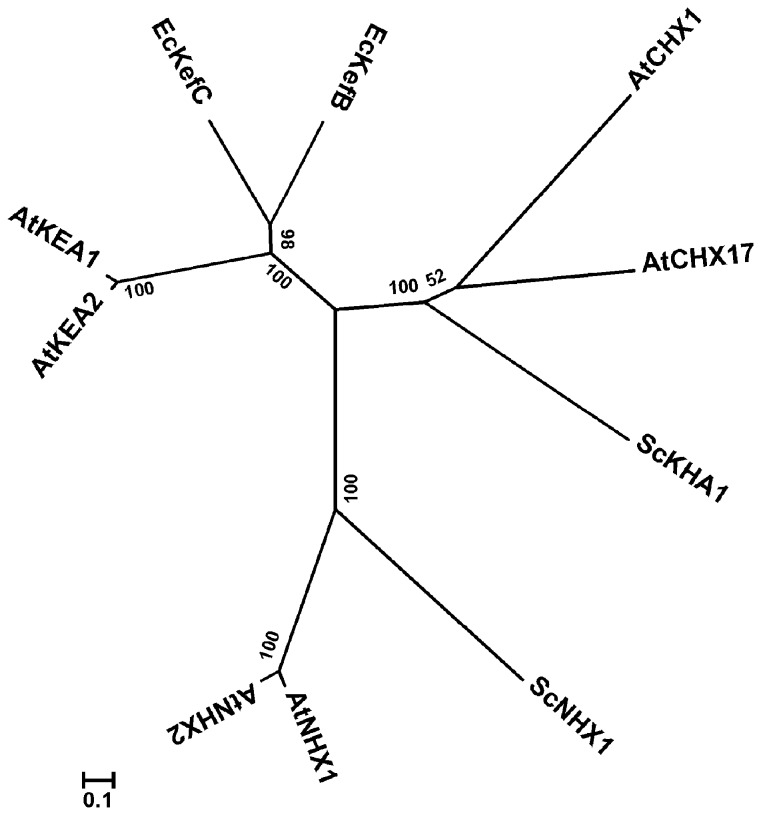
Phylogenetic analysis shows that AtKEAs form a cluster with their E. coli orthologs EcKefB/EcKefC, separating clearly from the clusters of AtNHXs and AtCHXs with their yeast orthologs (Figure 3). Thus, AtKEAs may function distinctly from either AtNHXs or AtCHXs.
Figure 3. Phylogenetic analysis of AtKEAs, AtNHXs and AtCHXs.
Phylogenetic analysis was conducted using MEGA 5.02. The alignment is based on the predicted amino acid sequences. Evolutionary distances were computed by the Neighbor Joining method. The scale bar indicates the distance calculated from the multiple alignment. The accession numbers and sources of the Na+,K+/H+ antiporters are as follows: EcKefB(ACT40850), EcKefC(CAA40066), Escherichia coli; ScKHA1(DAA08706), ScNHX1(DAA12290), Saccharomyces cerevisiae; AtKEA1, AtKEA2, AtNHX1(AAD16946), AtNHX2(AAM08403), AtCHX1 (AEE29444), AtCHX17 (AEE84796), Arabidopsis thaliana.
The full-length AtKEA1 protein is inactive in yeast
Protein organization analysis showed that AtKEA1 and AtKEA2 are comprised of a soluble N-terminal domain, a Na_H exchange domain and a C-terminal KTN domain [4], [46]. The long N-terminal domains of AtKEA1 and AtKEA2 were missed in early gene annotation. The cDNA sequences of the short version AtKEA1 and AtKEA2, AtsKEA1 and AtsKEA2 , lacking the N-terminal domains but containing the Na_H exchange domains, have been cloned in yeast by Dr. John Ward lab.
We attempted to clone the full-length cDNAs of AtKEA1 and AtKEA2 genes. Since the direct amplification from the Arabidopsis cDNA preparation was not successful, we used a two-step strategy. We separated the gene into two pieces by choosing a restriction enzyme site in the middle of the gene; the two pieces were cloned separately. We then combined them to get the full-length cDNA. For AtKEA1, an EcoRI site was chosen to separate the gene into two pieces of A1-C1870 and T1871-A3582, and we successfully cloned the full-length cDNA. However, using the same strategy, we did not clone the full length AtKEA2 gene. Aranda-Sicilia et al. (2012) also failed to clone the full-length AtKEA2 in their study.
The activity of the full-length AtKEA1 was tested in yeast. The full-length AtKEA1 did not confer resistance to high K+ and hygromicin B (50 ug/ml) in yeast growth compared with AtsKEA1 (Figure S1). Moreover, the full-length AtKEA1 did not confer resistance to high K+ at pH 4.5 relative to AtsKEA1 (Figure S2). Thus, the full-length AtKEA1 is inactive in yeast, whereas the AtsKEA1 is functional, suggesting that the N-terminal end of AtKEA1 may be an autoinhibitory domain controlling the transport activity.
The subcellular localization of the full-length AtKEA1 was detected by expressing AtKEA1-GFP in yeast cells (Figure S3). The GFP fluorescence did not appear at clear cellular structures; instead, the proteins formed structureless clumps in yeast cells, suggesting that the full-length AtKEA1 did not distribute properly in yeast cells.
AtKEAs specifically mediate K+ transport in yeast
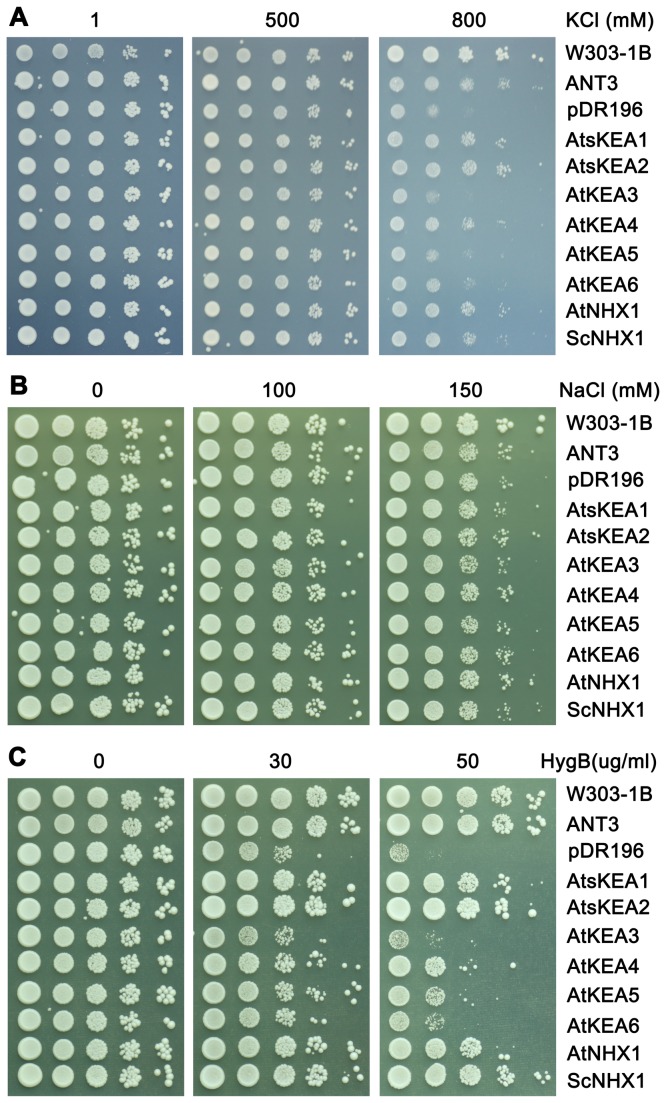
To test the function of AtKEAs, the coding sequences of AtKEA1-6 were cloned in the yeast expression vector pDR196 and introduced into a Saccharomyces cerevisiae strain AXT3. Strain AXT3 lacks the functional plasma membrane Na+-ATPases (ScENA1-4), plasma membrane Na+,K+/H+ antiporter ScNHA1, and vacuolar Na+,K+/H+ antiporter ScNHX1. Thus, it is sensitive to salt and to high K+. The transformed yeast was grown on Arg phosphate (AP) medium with high levels of KCl or NaCl (Figure 4). AXT3 mutants failed to grow in the medium containing 800 mM KCl while the nhx1-positive strains (W303-1B and ANT3) grew vigorously (Figure 4A). Expression of AtsKEA1 and AtsKEA2, AtKEA4, -5 and -6 recovered tolerance to high K+, similar to the AXT3 strains expressing ScNHX1 or AtNHX1 (Figure 4A). However, the recovery capacities among the AtKEA family were different. AtsKEA1 and AtsKEA2 had the highest effect, whereas AtKEA3 had no effect (Figure 4A). Interestingly, although AtKEA genes were well expressed in AXT3 mutants as detected with GFP tagged proteins (Figure S5), yeast growth was not improved in salt stress (Figure 4B), suggesting that AtKEAs do not confer tolerance to salt stress. In addition, AtKEAs did not confer Li+ tolerance (data not shown). AXT3 mutants were shown to be sensitive to hygromicin B (50 μg/ml), and ScNHX1 and AtNHX1 enhanced tolerance to hygromicin B (Figure 4C). While all AtKEA genes conferred resistance to the drug hygromicin B, AtsKEA1 and AtsKEA2 had the most effect (Figure 4C), suggesting their roles in endosomal compartments. These results suggest that AtKEAs specifically facilitate K+ homeostasis, which is dissimilar to AtNHXs. However, AtKEAs are similar to both AtNHXs and AtCHXs in that all three function in endosomal trafficking.
Figure 4. AtKEAs mediate K+ transport and confer resistance to hygromycin B in yeast mutants.
The cDNAs of AtKEAs, AtNHX1 and ScNHX1 were subcloned into the yeast expression vector pDR196 and transformed into the AXT3 mutant (ena1-4 nha1 nhx1). Yeast cells were normalized in water to A600 of 0.4. Aliquots (4μL) from normalized yeast cultures or 10-fold serial dilutions were spotted onto AP plates containing different concentrations of KCl (A), or YPD plates with different concentrations of NaCl (B), or hygromycin B (C). The strains were grown at 30°C for 3 days.
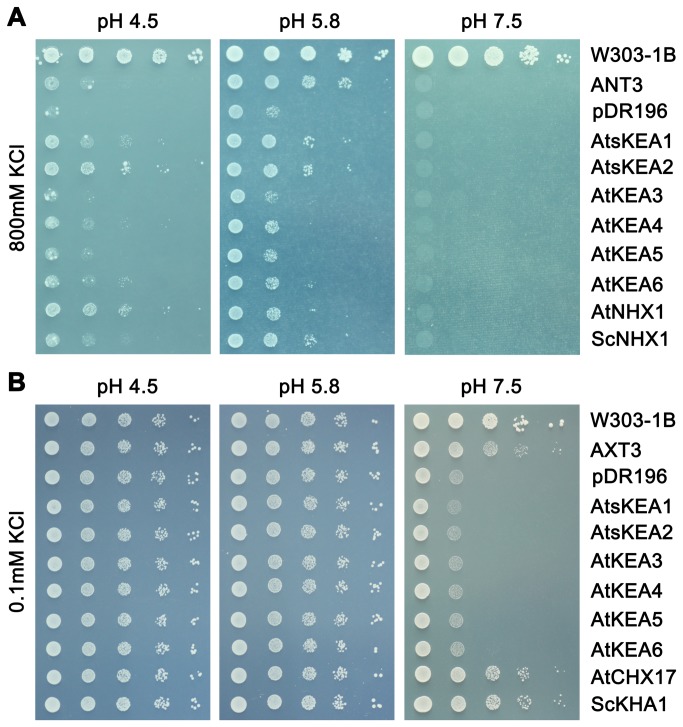
AtKEAs have strict pH requirements in mediating K+ transport in yeast
AtKEA genes (except AtKEA3) recovered AXT3 mutant growth at 800 mM KCl at an external pH of 5.8 (Figure 5A). However, the recovery capacity of the AtKEA family was significantly reduced when pH was dropped to 4.5 under 800 mM KCl, while AtNHX1 and ScNHX1 were still active under the same conditions. Furthermore, AtKEAs completely lost their functions at pH 7.5 at 800 mM KCl (Figure 5A). Thus, AtKEAs require a specific pH in mediating K+ transport in yeast; either alkaline or more acidic conditions will affect their functions. AtKEAs were further tested in the yeast mutant strain AXT4K, generated by deleting kha1 in the AXT3 mutant background. AXT4 mutants failed to grow at low K+ at pH 7.5, while the kha1-positive strains (W303-1B and AXT3) grew dynamically (Figure 5B). However, expression of AtKEAs failed to rescue AXT4K growth at low K+ at pH 7.5, while ScKHA1 and AtCHX17 enhanced yeast growth (Figure 5B). These results indicate that AtKEAs, AtNHXs and AtCHXs may have different modes of action in facilitating K+ homeostasis. AtKEAs function at high K+ at pH 5.8 while AtNHXs function at high K+ in acidic environments and AtCHXs at low K+ under alkaline conditions.
Figure 5. AtKEAs have strict pH requirements in mediating K+ transport in yeast.
(A) The cDNAs of AtKEAs, AtNHX1and ScNHX1 were subcloned into the yeast expression vector pDR196 and transformed into strain AXT3 (ena1-4 nha1 nhx1). Strains were spotted onto AP plates containing 800mM KCl at pH 4.5, 5.8 or 7.5. (B) The cDNAs of AtKEAs, AtCHX17 and ScKHA1 were subcloned into the yeast expression vector pDR196 and transformed into strain AXT4K (ena1-4 nha1 nhx1 kha1). Strains were spotted onto AP plates containing 0.1mM KCl at pH 4.5, 5.8 or 7.5. Cells were normalized in water to A600 of 0.4. Aliquots (4μL) from normalized yeast cultures or 10-fold serial dilutions were spotted onto AP plates. The strains were grown at 30°C for 3 days.
AtKEAs express in both the shoot and root of Arabidopsis
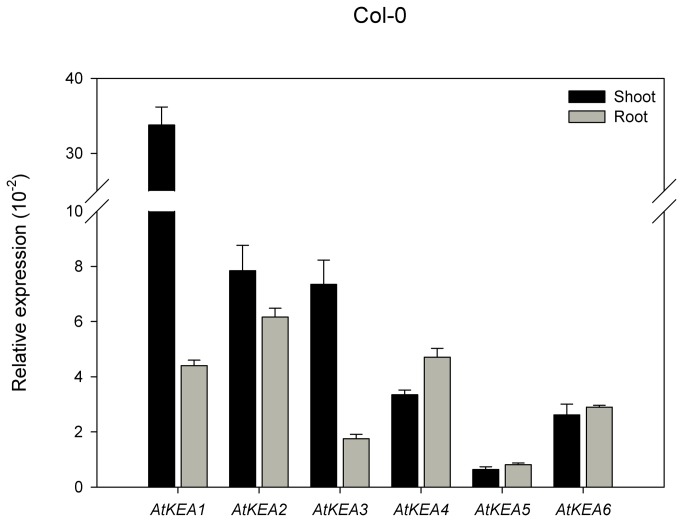
The transcript abundances of AtKEA genes were analyzed by RT-qPCR using gene-specific primers. Shoots and roots were isolated from 14-day-old WT seedlings growing on 1/2 MS medium. The transcripts of AtKEA genes were detected in shoots and roots (Figure 6). AtKEA2, -4, -5 and -6 had almost equal expression in shoots and roots; AtKEA1 and AtKEA3, however, showed a relatively higher expression in shoots than in roots, suggesting their dominant roles in shoots. AtKEA5 had the lowest expression in both shoots and roots. The differential expression indicates that AtKEA genes have diversified roles in Arabidopsis.
Figure 6. The expression of AtKEAs in shoots and roots of Arabidopsis seedlings.
Fourteen-day-old Arabidopsis seedlings (Col-0) were used. The relative transcript abundance of AtKEAs in shoots and roots were analyzed by RT-qPCR. Actin7 gene was used as an internal positive control. Error bars represent SE (n =3).
AtKEAs respond differently to low K+ stress
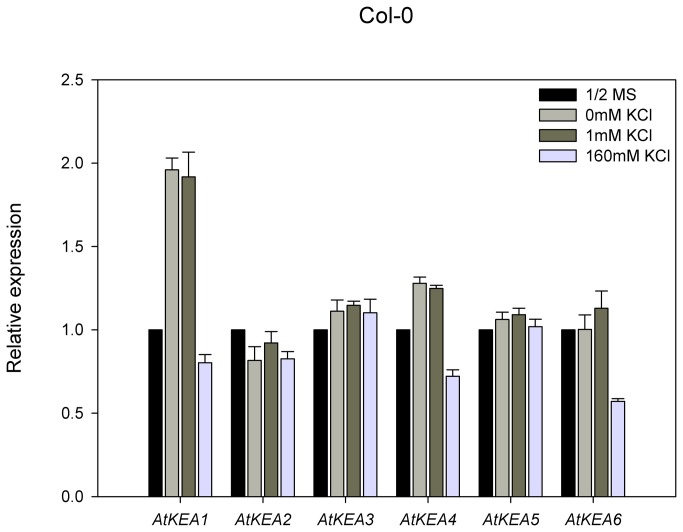
Since yeast growth assays have shown that AtKEAs mediate K+ transport (Figure 4A and 5A), we tested whether AtKEA expression was induced by K+. Interestingly, AtKEA1 expression was enhanced significantly under low K+ stress (0 and 1 mM K+) compared to the untreated control (Figure 7). The expression of AtKEA3 and AtKEA4 was also enhanced under low K+ stress, but AtKEA2, -5, and -6 was not. In contrast, gene expressions were significantly reduced for AtKEA1, -2, -4 and -6 at high K+ (160 mM) (Figure 7). The differential expression in response to low K+ stress suggests that AtKEA1, -3,and -4 are involved in K+ acquisition under low K+ conditions in Arabidopsis, whereas AtKEA2, -5 and -6 may have different functions.
Figure 7. The expression of AtKEAs responds differently to low K+ treatment.
Fourteen-day -old Arabidopsis seedlings (Col-0) were cultured on the 1/2 MS medium or modified MS without K+ (0 mM K+) or supplemented with 1mM or 160mM KCl for 8h. Actin7 gene was used as an internal positive control. The transcript levels of AtKEAs in the untreated control seedlings were set as 1.0. Error bars represent SE (n=3).
The expression of AtKEA2 and AtKEA5 is induced by osmotic stress and is dependent on ABA signaling
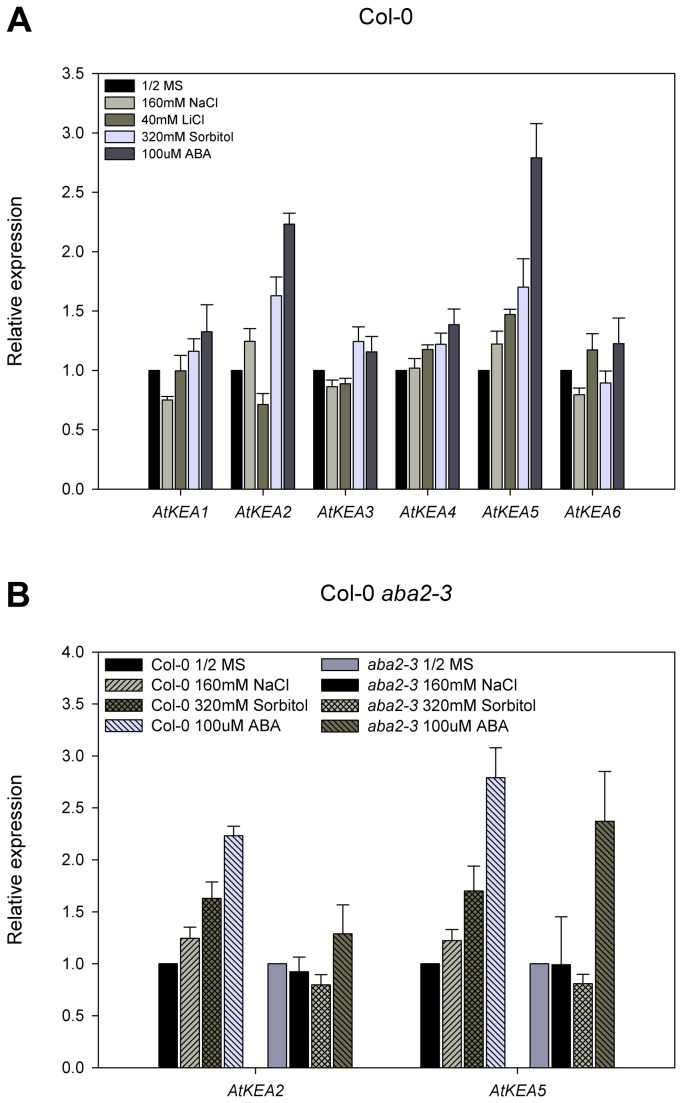
AtKEA expression was further tested under Na+, Li+ and sorbitol stresses. Interestingly, the expression of AtKEA2 and AtKEA5 was strongly induced under 320 mM sorbitol and 100 μM ABA treatments compared to their untreated controls, indicating that these two genes were osmotic responsive and were ABA-dependent (Figure 8A). AtKEA2 and AtKEA5 were also induced by 160 mM NaCl, which was iso-osmotic to 320 mM sorbitol (Figure 8A), suggesting that AtKEA2 and AtKEA5 are responsive to osmotic stress other than ionic stress. In addition, the expression of AtKEA4 and AtKEA5 were induced by Li+ stress compared to their untreated controls, while no induction was observed for AtKEA1, -2, -3 and -6 (Figure 8A).
Figure 8. The expression of AtKEA2 and AtKEA5 is strongly enhanced under osmotic and ABA treatments.
Arabidopsis seedlings (Col-0, aba2-3) were grown on 1/2MS-agar media for 14 days. (A) Col-0 were continued culturing on the fresh 1/2 MS medium without or supplemented with 160 mM NaCl, 40 mM LiCl, 320 mM Sortibol, or 100 μM ABA, respectively, for 8h. (B) Col-0 and aba2-3 were continued culturing on the fresh 1/2 MS medium without or supplemented with 160 mM NaCl, 320 mM Sortibol or 100μM ABA, respectively, for 8h. Actin7 gene was used as an internal positive control. The transcript levels of AtKEAs in the untreated control seedlings were set as 1.0. Error bars represent SE (n =3).
ABA regulation of AtKEA2 and AtKEA5 expression was further tested with aba2-3 mutant. The aba2-3 mutant is deficient in ABA synthesis but is responsive to exogenously applied ABA [61]. Under iso-osmotic NaCl and sorbitol treatments, while the expression of AtKEA2 and AtKEA5 was induced in WT plants, it was not induced in aba2-3 mutants compared to the untreated control (Figure 8B). However, ABA treatments strongly induced the expression of both AtKEA2 and AtKEA5 in aba2-3 mutants relative to their untreated controls (Figure 8B). These results suggest that the induction of AtKEA2 and AtKEA5 by osmotic stress is dependent on ABA signaling.
AtKEAs are not regulated by the SOS pathway
The Arabidopsis thaliana Salt Overly Sensitive (SOS) pathway controls ion homeostasis in plants [62], [63]. The SOS pathway is composed of SNF-like kinase SOS2 and Ca2+-binding protein SOS3. SOS3 perceives the ion stress signals and activates SOS2. SOS2 in turn activates SOS1, a plasma membrane Na+/H+ antiporter in Arabidopsis [15], [17], [62]. In an attempt to understand whether AtKEA expression is regulated by the SOS pathway, we tested AtKEA expression in sos mutants by RT-qPCR (Figure 9). Interestingly, the expression of the AtKEA family in sos1, sos2, or sos3 mutants was not affected by salt stress compared to their WT plants with salt stress, suggesting that AtKEAs were not controlled by the SOS pathway under salt stress. On the contrary, AtKEA5 expression in sos1and sos2 mutants was consistently high compared to either the WT plants with salt stress or their untreated controls. Similarly, AtKEA2 expression in sos1and sos2 mutants was high relative to their untreated controls (Figure 9A, 9B). These results suggest that AtKEA2 and AtKEA5 may be regulated negatively by the SOS pathway. Or, the induction of AtKEA2 and AtKEA5 is caused by the osmotic stress generated by the accumulation of salt in sos mutants.
Figure 9. The expression of AtKEAs is not controlled by the SOS signaling pathway.
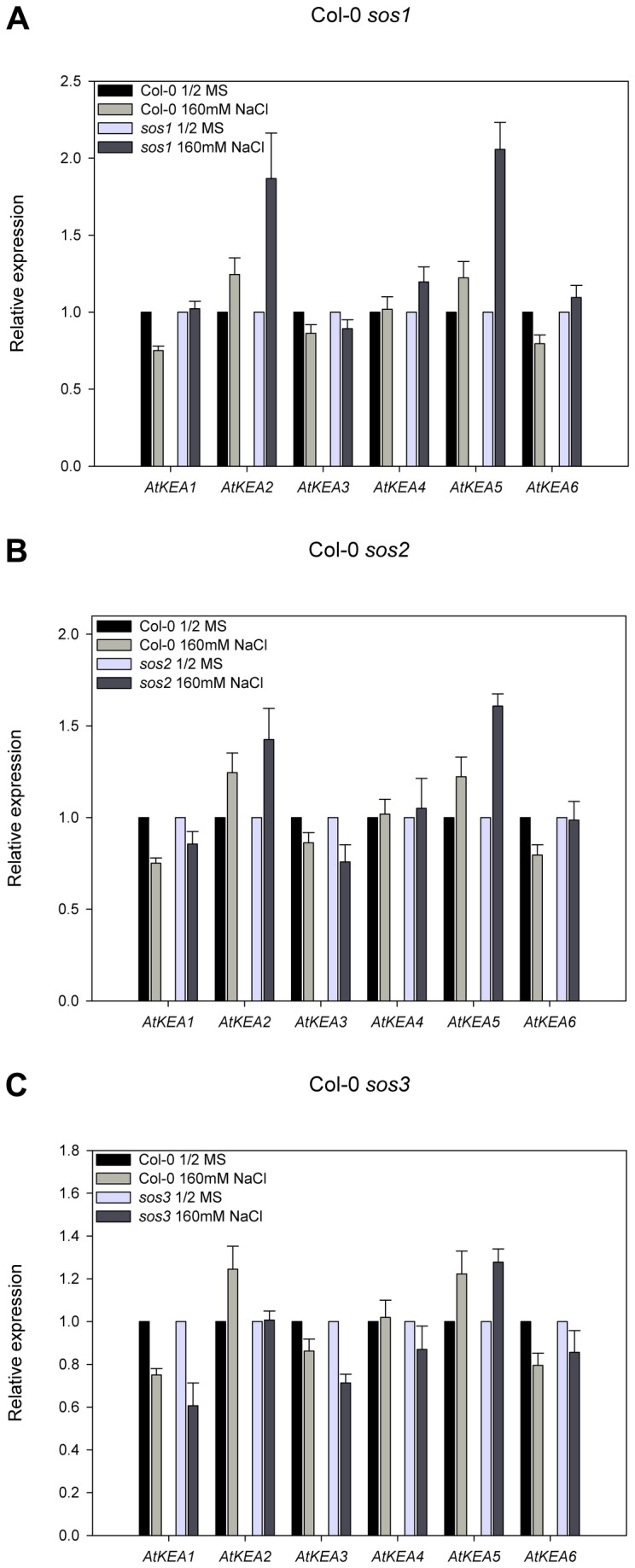
Arabidopsis seedlings (Col-0, sos1-1, sos2-1 or sos3-1) were grown on 1/2MS-agar media for fourteen days. Seedlings were continued culturing on the fresh 1/2 MS medium without or supplemented with 160 mM NaCl, respectively, for 8h. (A) Col-0 and sos1-1; (B) Col-0 and sos2-1 ; (C) Col-0 and sos3-1. Actin7 gene was used as an internal positive control. The transcript levels of AtKEAs in the untreated control seedlings were set as 1.0. Error bars represent SE (n =3).
AtKEAs distribute diversely in yeast cells
AtKEAs fused with GFP at the C-terminals retained resistance to hygromicin B in yeast (Figure S4). In addition, the GFP-tagged AtKEAs distributed properly in the transport activity test strains AXT3 and shared the same pattern as that in wild-type yeast (W303-1B) (Figure S5, Figure 10). These results demonstrate that the AtKEAs tagged with GFP at their C-terminals retained activity in yeast cells.
Figure 10. The diversified distribution of AtKEAs in yeast.
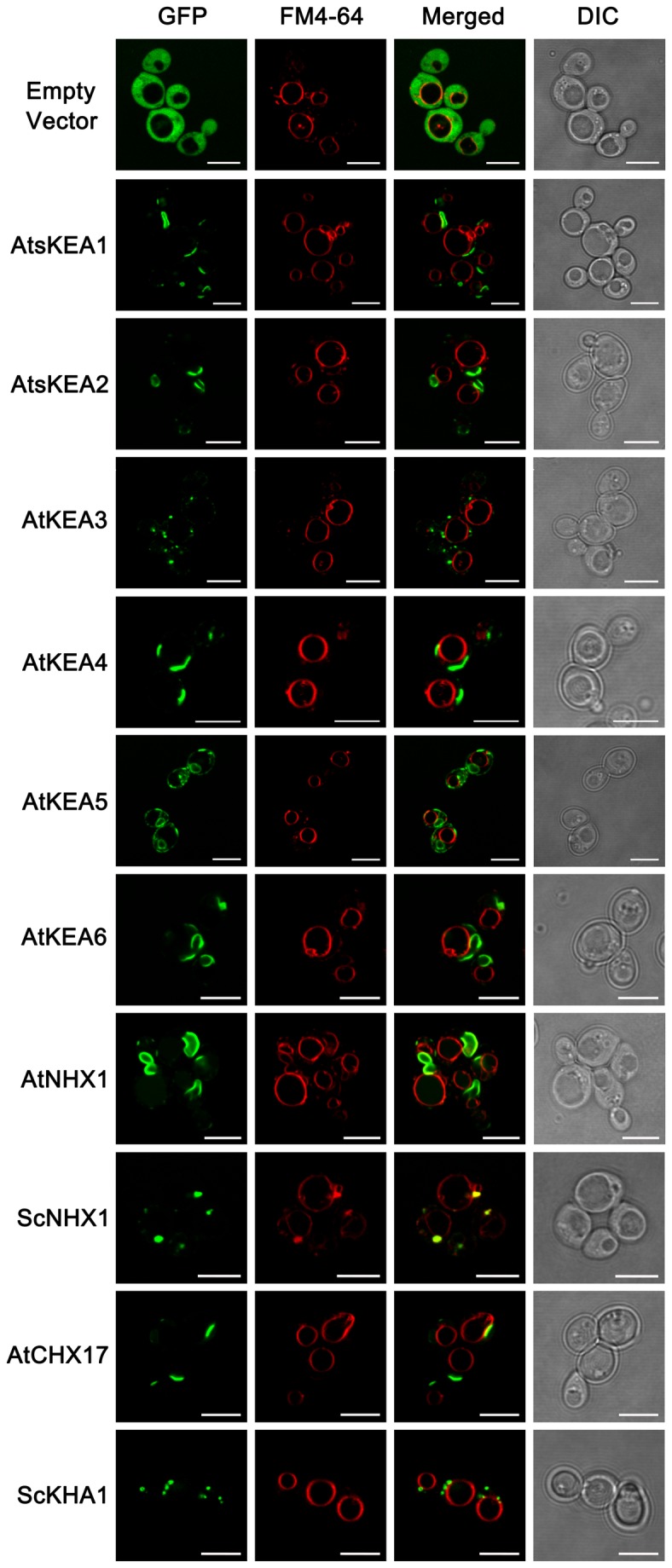
Wild-type (W303-1B) yeast strains harboring pDR196-GFP, pDR196-AtKEAs-GFP, pDR196-AtNHX1-GFP, pDR196-AtCHX17-GFP, pDR196-ScNHX1-GFP, and pDR196-ScKHA1-GFP (fused with GFP at the C terminus), respectively, were grown to logarithmic phase in SC-URA medium (pH 5.8) and were stained with FM4-64 dye. The subcellular localizations of the GFP-tagged proteins (green) and FM4-64 fluorescence (red) were observed under the Laser Scanning Confocal Microscope. Bars, 5µm.
In wild-type yeast (W303-1B), AtsKEA1-GFP, AtsKEA2-GFP and AtKEA6-GFP had similar distribution patterns; the fluorescent signals appeared at a structure at or near the plasma membrane and a membrane structure in the cytosol. These structures were not overlapped with FM4-64 (Figure 10). The distribution patterns of AtsKEA1-GFP, AtsKEA2-GFP and AtKEA6-GFP were similar to AtNHX1 (Figure 10). However, AtKEA3-GFP shared similar patterns with ScKHA1; the fluorescent signals appeared at punctate structures (Figure 10). ScKHA1 has been determined to be localized to Golgi [64], [65], indicating that AtKEA3-GFP is localized to Golgi in the yeast cells. AtKEA4-GFP shared similar patterns with AtCHX17; the fluorescent signals appeared at a membrane structure in the cytosol that was not overlapped with FM4-64 (Figure 10). AtKEA5-GFP signals appeared at both punctate structures and a membrane structure in the cytosol; neither structure was overlapped with FM4-64 (Figure 10). These patterns suggest that AtKEAs have different distributions in yeast cells.
AtKEA3 is localized to Golgi in Arabidopsis
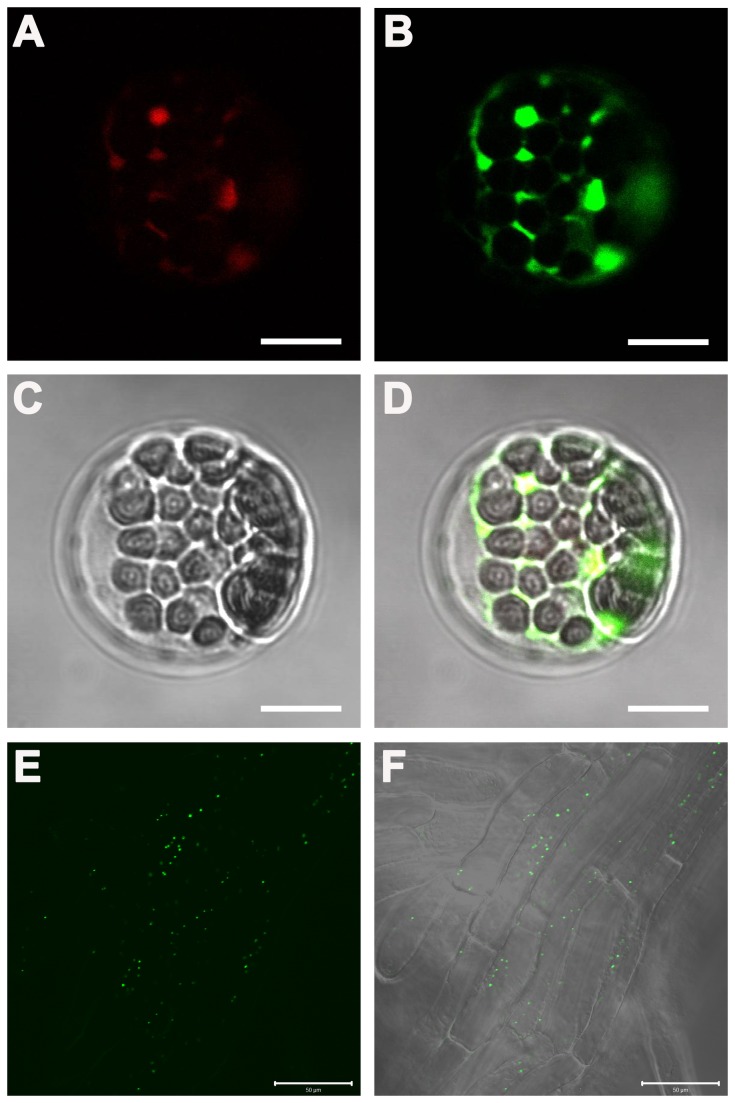
We visualized the subcellular localization of AtKEA3 by transient expression in Arabidopsis protoplasts. Surprisingly, RFP-AtKEA3 fluorescence appeared on punctate structures in the cytosol but not in chloroplasts (Figure 11A). RFP-AtKEA3 fluorescent signals were co-localized extensively with the Golgi marker GFP-AtSYP31, suggesting that AtKEA3 is localized to Golgi (Figure 11B, D).
Figure 11. AtKEA3 is localized to Golgi in Arabidopsis.
(A), (B), (C) and (D): The transient expression of AtKEA3 in Arabidopsis protoplasts. A cis-Golgi marker GFP-AtSYP31 and RFP-AtKEA3 were co-transformed in Arabidopsis (Col-0) protoplasts. (A): RFP-AtKEA3 fluorescence image; (B): GFP-AtSYP31 fluorescence image; (C): Transmission image; (D): Overlay of the fluorescence and transmission images. Bars, 10µm. (E) and (F): The localization of AtKEA3 in root cells of the stably transformed Arabidopsis seedlings. (E): GFP fluorescence image; (F): Overlay of the fluorescence and transmission images. Images were observed under the Laser Scanning Confocal Microscope. Bars, 50µm.
To verify the subcellular localization of AtKEA3, we generated stably transformed Arabidopsis seedlings expressing cauliflower mosaic virus 35S-driven AtKEA3-GFP. In consistent with the transient expression assay, AtKEA3-GFP fluorescent signals were visualized at the punctate structures within the cells (Figure 11E, F). These punctate structures are the typical structures of Golgi bodies [66], [67], indicating that AtKEA3 is localized to the Golgi membranes. However, no fluorescent signals appeared in chloroplasts (Figure 11E, F). These studies from both yeast and plants suggest that AtKEA3 is localized to Golgi.
Discussion
AtKEAs may facilitate K+ homeostasis and play diversified roles in Arabidopsis
In this report, we presented the first experimental characterization of the novel AtKEA gene family, putative K+/H+ antiporters in Arabidopsis. We showed that AtKEAs (except AtKEA3) conferred resistance to high K+ stress in yeast mutants (Figure 4, 5), and the expression of AtKEA1, -3, and -4 were induced under low K+ stress (Figure 7). A previous study also showed that AtKEA2 had K+ transport activity in a reconstituted liposome assay [46]. Therefore, similar to their bacterial homologs EcKefB/EcKefC, AtKEAs may encode K+/H+ transporters that function in facilitate K+ homeostasis in plants.
Studies have shown that members of the CPA2 family have various catalytic modes [10]. For example, although EcKefB and EcKefC were thought to function as K+/H+ antiporters, they share structural similarities with K+ channels and act like ligand-gated K+ efflux channels [68], [69]. In addition, while the AtCHX family was predicted to encode cation/H+ exchangers, studies have shown that AtCHX20 might be a K+/H+ symporter and AtCHX17 might function as a K+ channel [33]. Currently, the catalytic mechanisms for the ion transport activities of AtKEAs remain unclear. The observations that AtKEAs have diversified capacities in conferring resistance to ion, hygromycin B, and pH stresses in yeast (as discussed below) suggest that AtKEAs may have diversified action modes.
Our results suggest that the members of the AtKEA family may function diversely in plants (1). AtKEAs had various capacities in conferring resistance to high K+ and hygromycin B in yeast growth (Figure 4); (2) AtKEAs diversely enhanced yeast growth at acidic condition (pH 4.5) (Figure 5A); (3) AtKEAs displayed a diversified patterns of expression in shoots and roots, and were induced differently under low K+ or osmotic stress (Figures 6, 7, 8). (4) AtKEAs localized diversely in yeast cells (Figure 10). These diversified patterns of K+ transport, expression and localization may imply that AtKEAs play diversified roles in different cellular processes under different growth and developmental or environmental conditions.
AtKEA2 and AtKEA5 function in osmotic adjustment regulated by the ABA signaling pathway
We showed that the expression of AtKEA2 and AtKEA5 were strongly induced by sorbitol treatment (Figure 8A), implying that AtKEA2 and AtKEA5 may function in osmotic adjustment in plants. The response of AtKEA2 and AtKEA5 to osmotic stresses may be regulated by the ABA signaling pathway, since we found that the expression of AtKEA2 and AtKEA5 was induced by ABA treatment (Figure 8A). This is supported by the assay with the ABA deficient mutant aba2-3 in which AtKEA2 and AtKEA5 were not induced by osmotic stresses (Figure 8B), indicating requirement of the ABA signal for the osmotic response. Indeed, AtKEA2 and AtKEA5 contain ABA responsive elements (ABRE) TACGGTC and TACGTGTC, respectively, in their promoter regions (1.5 kb upstream of the translation start codon). However, AtKEA2 and AtKEA5 were not controlled by the SOS pathway since gene expression was not altered in sos mutants (Figure 9). An earlier study showed that AtNHX1 and AtNHX2 were regulated by the ABA signaling pathway in response to osmotic stress. Similarly, AtNHX1 and AtNHX2 were not regulated by the SOS pathway [70]. Therefore, AtKEA2 and AtKEA5 may share a similar mechanism to AtNHX1 and AtNHX2 in response to osmotic stress.
AtKEA2 has been visualized to be localized in chloroplasts, implying its critical roles in chloroplasts [46]. The chloroplast is a K+ pool in cells which contain around 100-200 mM K+. K+ is important for the electrical balance across the thylakoid membranes and H+ homeostasis in the stroma [71], [72]. In addition, as the major inorganic osmolyte in plant cells, K+ maintains the structural and volume integrity of chloroplasts in response to light, osmotic stress or water deficit stresses [73]. Thus, our findings regarding its role in K+ transport and osmotic adjustment suggest that AtKEA2 may function in facilitating electrical balance and pH hometasis in chloroplasts and maintaining chloroplast structural integrity under osmotic stress.
AtKEA3 functions in Golgi
Our results suggest that AtKEA3 is localized to Golgi. First, in yeast cells, the GFP-tagged AtKEA3 appeared at punctuate structures that are similar to the yeast ScKHA1, a Golgi localized K+/H+ antiporter (Figure 10). Second, in transiently expressed Arabidopsis protoplasts, the RFP-tagged AtKEA3 was well merged with the Golgi marker AtSYP31 (Figure 11A, B, C, D). Third, in stably transformed Arabidopsis seedlings, the GFP-tagged AtKEA3 was visualized at the typical punctate structures of Golgi bodies spreading within the cells (Figure 11E, F). Thus, the chloroplast localization of AtKEA3 reported by mass spectroscopy might be caused by the contamination with Golgi membranes in the preparation of chloroplasts [47].
The plant Golgi apparatus is an important organelle for polysaccharide synthesis and peptide glycosylation modifications. The Golgi also functions in the trafficking of proteins, lipids, and complex carbohydrates to the cell wall and other organelles [67]. Thus, Golgi localization of AtKEA3 indicates that AtKEA3 may play important roles in the polysaccharide synthesis, peptide modifications, and membrane trafficking processes occurring in the Golgi. This is supported by the result from the hygromycin B assay (Figure 4C), which showed that AtKEA3 conferred resistance to hygromycin B in yeast growth. The Alterations in sensitivity to the cationic drug hygromycin B reflect the changes in membrane potential and membrane trafficking. Indeed, controlling membrane trafficking is an emerging role of Na+,K+/H+ antiporters [8]. It has been shown that yeast ScNhx1p is required for trafficking out of the late endosome [6], [74] [75], and vacuole fusion [57]. Human NHE8 is essential for maintaining endosomal structure and for the regulation of protein sorting [76]. In addition, AtCHX17 and AtCHX21 are involved in protein sorting [33]. Arabidopsis nhx5nhx6 mutants were defects in vacuolar trafficking [32]. Therefore, AtKEAs may have the same function as the yeast, animal, and plant NHXs and Arabidopsis AtCHX and play important roles in membrane trafficking.
Supporting Information
The full-length AtKEA1 is inactive in K+ transport in yeast.
(TIF)
The full-length AtKEA1 does not transport K+ at acidic pH in yeast.
(TIF)
The full length AtKEA1is not properly distributed in yeast cells.
(TIF)
AtKEAs fused with GFP at the C-terminus retained activity.
(TIF)
AtKEAs fused with GFP at the C-terminus are properly distributed in yeast cells.
(TIF)
Materials and methods of supporting figures.
(DOC)
Primers for the plasmid constructs used in functional expression in yeast.
(DOC)
Primers for RT-qPCR.
(DOC)
Primers for the plasmid constructs used in localization of GFP fusion proteins in yeast.
(DOC)
Acknowledgments
We thank Dr. John Ward for helpful discussions and suggestions, Dr. Jose M. Pardo for sending the yeast strains W303-1B, ANT3, AXT3 and AXT4K, Dr. Jian-Kang Zhu for sending the seeds of sos mutants, Dr. Rutilio Fratti for sending the yeast strain BJ3505, and Dr.Jia Li for providing pBIB plasmid.
Funding Statement
This work was supported by the National Basic Research Program of China (973 project, 2013CB429904 to QSQ), the National Natural Science Foundation of China (NSFC) (31070222 to QSQ), and the Fundamental Research Funds for the Central Universities (lzujbky-2013-k10). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12: 431-434. doi: 10.1016/S0955-0674(00)00112-5. PubMed: 10873827. [DOI] [PubMed] [Google Scholar]
- 2. Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465: 140-151. doi: 10.1016/S0005-2736(00)00135-8. PubMed: 10748251. [DOI] [PubMed] [Google Scholar]
- 3. Brett CL, Donowitz M, Rao R (2005) Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol 288: C223-C239. doi: 10.1152/ajpcell.00360.2004. PubMed: 15643048. [DOI] [PubMed] [Google Scholar]
- 4. Chanroj S, Wang G, Venema K, Zhang MW, Delwiche CF et al. (2012) Conserved and diversified gene families of monovalent cation/h(+) antiporters from algae to flowering plants. Front - Plant Sci 3: 25 PubMed: 22639643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang AB, Lin R, Keith Studley W, Tran CV, Saier MH Jr. (2004) Phylogeny as a guide to structure and function of membrane transport proteins. Mol Membr Biol 21: 171-181. doi: 10.1080/09687680410001720830. PubMed: 15204625. [DOI] [PubMed] [Google Scholar]
- 6. Brett CL, Tukaye DN, Mukherjee S, Rao R (2005) The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell 16: 1396-1405. doi: 10.1091/mbc.E04-11-0999. PubMed: 15635088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63: 5727-5740. doi: 10.1093/jxb/ers250. PubMed: 22991159. [DOI] [PubMed] [Google Scholar]
- 8. Qiu QS (2012) Plant and yeast NHX antiporters: roles in membrane trafficking. J Integr Plant Biol 54: 66-72. doi: 10.1111/j.1744-7909.2012.01097.x. PubMed: 22222113. [DOI] [PubMed] [Google Scholar]
- 9. Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K et al. (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646-1667. doi: 10.1104/pp.126.4.1646. PubMed: 11500563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sze H, Padmanaban S, Cellier F, Honys D, Cheng NH et al. (2004) Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol 136: 2532-2547. doi: 10.1104/pp.104.046003. PubMed: 15347787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57: 1181-1199. doi: 10.1093/jxb/erj114. PubMed: 16513813. [DOI] [PubMed] [Google Scholar]
- 12. Rodríguez-Rosales MP, Gálvez FJ, Huertas R, Aranda MN, Baghour M et al. (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4: 265-276. doi: 10.4161/psb.4.4.7919. PubMed: 19794841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang X, Leidi EO, Pardo JM (2010) How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal Behav 5: 792-795. doi: 10.4161/psb.5.7.11767. PubMed: 20495345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256-1258. doi: 10.1126/science.285.5431.1256. PubMed: 10455050. [DOI] [PubMed] [Google Scholar]
- 15. Shi H, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci U S A 97: 6896-6901. doi: 10.1073/pnas.120170197. PubMed: 10823923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi H, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21: 81-85. PubMed: 12469134. [DOI] [PubMed] [Google Scholar]
- 17. Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci U S A 99: 8436-8441. doi: 10.1073/pnas.122224699. PubMed: 12034882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu QS, Barkla BJ, Vera-Estrella R, Zhu JK, Schumaker KS (2003) Na+/H+ exchange activity in the plasma membrane of Arabidopsis. Plant Physiol 132: 1041-1052. doi: 10.1104/pp.102.010421. PubMed: 12805632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo Q, Sahoo SP, Wang PR, Milot DP, Ippolito MC et al. (2004) A novel peroxisome proliferator-activated receptor alpha/gamma dual agonist demonstrates favorable effects on lipid homeostasis. Endocrinology 145: 1640-1648. PubMed: 14701675. [DOI] [PubMed] [Google Scholar]
- 20. Quintero FJ, Martinez-Atienza J, Villalta I, Jiang X, Kim WY et al. (2011) Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc Natl Acad Sci U S A 108: 2611-2616. doi: 10.1073/pnas.1018921108. PubMed: 21262798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiu QS, Guo Y, Quintero FJ, Pardo JM, Schumaker KS et al. (2004) Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J Biol Chem 279: 207-215. PubMed: 14570921. [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi T, Aharon GS, Sottosanto JB, Blumwald E (2005) Vacuolar Na+/H+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a Ca2+- and pH-dependent manner. Proc Natl Acad Sci U S A 102: 16107-16112. doi: 10.1073/pnas.0504437102. PubMed: 16249341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19: 765-768. doi: 10.1038/90824. PubMed: 11479571. [DOI] [PubMed] [Google Scholar]
- 24. Venema K, Quintero FJ, Pardo JM, Donaire JP (2002) The Arabidopsis Na+/H+ exchanger AtNHX1 catalyzes low affinity Na+ and K+ transport in reconstituted liposomes. J Biol Chem 277: 2413-2418. doi: 10.1074/jbc.M105043200. PubMed: 11707435. [DOI] [PubMed] [Google Scholar]
- 25. Apse MP, Sottosanto JB, Blumwald E (2003) Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J 36: 229-239. doi: 10.1046/j.1365-313X.2003.01871.x. PubMed: 14535887. [DOI] [PubMed] [Google Scholar]
- 26. Rodriguez-Rosales MP, Jiang X, Gálvez FJ, Aranda MN, Cubero B et al. (2008) Overexpression of the tomato K+/H+ antiporter LeNHX2 confers salt tolerance by improving potassium compartmentalization. New Phytol 179: 366-377. doi: 10.1111/j.1469-8137.2008.02461.x. PubMed: 19086176. [DOI] [PubMed] [Google Scholar]
- 27. Leidi EO, Barragán V, Rubio L, El-Hamdaoui A, Ruiz MT et al. (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61: 495-506. doi: 10.1111/j.1365-313X.2009.04073.x. PubMed: 19912566. [DOI] [PubMed] [Google Scholar]
- 28. Fukada-Tanaka S, Inagaki Y, Yamaguchi T, Saito N, Iida S (2000) Colour-enhancing protein in blue petals. Nature 407: 581. doi: 10.1038/35036686. PubMed: 11034195. [DOI] [PubMed] [Google Scholar]
- 29. Yoshida K, Kawachi M, Mori M, Maeshima M, Kondo M et al. (2005) The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of Morning Glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol 46: 407-415. doi: 10.1093/pcp/pci057. PubMed: 15695444. [DOI] [PubMed] [Google Scholar]
- 30. Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K et al. (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23: 3482-3497. doi: 10.1105/tpc.111.089581. PubMed: 21954467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A et al. (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24: 1127-1142. doi: 10.1105/tpc.111.095273. PubMed: 22438021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z et al. (2011) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23: 224-239. doi: 10.1105/tpc.110.079426. PubMed: 21278129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chanroj S, Lu Y, Padmanaban S, Nanatani K, Uozumi N et al. (2011) Plant-specific cation/H+ exchanger 17 and its homologs are endomembrane K+ transporters with roles in protein sorting. J Biol Chem 286: 33931-33941. doi: 10.1074/jbc.M111.252650. PubMed: 21795714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N et al. (2011) Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23: 81-93. doi: 10.1105/tpc.110.080499. PubMed: 21239645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao J, Cheng NH, Motes CM, Blancaflor EB, Moore M et al. (2008) AtCHX13 is a plasma membrane K+ transporter. Plant Physiol 148: 796-807. doi: 10.1104/pp.108.124248. PubMed: 18676662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cellier F, Conéjéro G, Ricaud L, Luu DT, Lepetit M et al. (2004) Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J 39: 834-846. doi: 10.1111/j.1365-313X.2004.02177.x. PubMed: 15341627. [DOI] [PubMed] [Google Scholar]
- 37. Padmanaban S, Chanroj S, Kwak JM, Li X, Ward JM et al. (2007) Participation of endomembrane cation/H+ exchanger AtCHX20 in osmoregulation of guard cells. Plant Physiol 144: 82-93. doi: 10.1104/pp.106.092155. PubMed: 17337534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall D, Evans AR, Newbury HJ, Pritchard J (2006) Functional analysis of CHX21: a putative sodium transporter in Arabidopsis. J Exp Bot 57: 1201-1210. doi: 10.1093/jxb/erj092. PubMed: 16513816. [DOI] [PubMed] [Google Scholar]
- 39. Song CP, Guo Y, Qiu Q, Lambert G, Galbraith DW et al. (2004) A probable Na+(K+)/H+ exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci U S A 101: 10211-10216. doi: 10.1073/pnas.0403709101. PubMed: 15220473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choe S (2002) Potassium channel structures. Nat Rev Neurosci 3: 115-121. doi: 10.1038/nrn727. PubMed: 11836519. [DOI] [PubMed] [Google Scholar]
- 41. Miller S, Ness LS, Wood CM, Fox BC, Booth IR (2000) Identification of an ancillary protein, YabF, required for activity of the KefC glutathione-gated potassium efflux system in Escherichia coli. J Bacteriol 182: 6536-6540. doi: 10.1128/JB.182.22.6536-6540.2000. PubMed: 11053405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Roosild TP, Miller S, Booth IR, Choe S (2002) A mechanism of regulating transmembrane potassium flux through a ligand-mediated conformational switch. Cell 109: 781-791. doi: 10.1016/S0092-8674(02)00768-7. PubMed: 12086676. [DOI] [PubMed] [Google Scholar]
- 43. Roosild TP, Castronovo S, Miller S, Li C, Rasmussen T et al. (2009) KTN (RCK) domains regulate K+ channels and transporters by controlling the dimer-hinge conformation. Structure 17: 893-903. doi: 10.1016/j.str.2009.03.018. PubMed: 19523906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roosild TP, Castronovo S, Healy J, Miller S, Pliotas C et al. (2010) Mechanism of ligand-gated potassium efflux in bacterial pathogens. Proc Natl Acad Sci U S A 107: 19784-19789. doi: 10.1073/pnas.1012716107. PubMed: 21041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fujisawa M, Ito M, Krulwich TA (2007) Three two-component transporters with channel-like properties have monovalent cation/proton antiport activity. Proc Natl Acad Sci U S A 104: 13289-13294. doi: 10.1073/pnas.0703709104. PubMed: 17679694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aranda-Sicilia MN, Cagnac O, Chanroj S, Sze H, Rodríguez-Rosales MP et al. (2012) Arabidopsis KEA2, a homolog of bacterial KefC, encodes a K(+)/H(+) antiporter with a chloroplast transit peptide. Biochim Biophys Acta 1818: 2362-2371. doi: 10.1016/j.bbamem.2012.04.011. PubMed: 22551943. [DOI] [PubMed] [Google Scholar]
- 47. Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O et al. (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLOS ONE 3: e1994. doi: 10.1371/journal.pone.0001994. PubMed: 18431481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pandey GK, Cheong YH, Kim BG, Grant JJ, Li L et al. (2007) CIPK9: a calcium sensor-interacting protein kinase required for low-potassium tolerance in Arabidopsis. Cell Res 17: 411-421. doi: 10.1038/cr.2007.39. PubMed: 17486125. [DOI] [PubMed] [Google Scholar]
- 49. Campanella JJ, Bitincka L, Smalley J (2003) MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics 4: 29. doi: 10.1186/1471-2105-4-29. PubMed: 12854978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947-2948. doi: 10.1093/bioinformatics/btm404. PubMed: 17846036. [DOI] [PubMed] [Google Scholar]
- 51. Tamura K, Peterson D, Peterson N, Stecher G, Nei M et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731-2739. doi: 10.1093/molbev/msr121. PubMed: 21546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R (1989) A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58: 409-419. doi: 10.1016/0092-8674(89)90855-6. PubMed: 2546682. [DOI] [PubMed] [Google Scholar]
- 53. Quintero FJ, Blatt MR, Pardo JM (2000) Functional conservation between yeast and plant endosomal Na(+)/H(+) antiporters. FEBS Lett 471: 224-228. doi: 10.1016/S0014-5793(00)01412-5. PubMed: 10767428. [DOI] [PubMed] [Google Scholar]
- 54. Maresova L, Sychrova H (2005) Physiological characterization of Saccharomyces cerevisiae kha1 deletion mutants. Mol Microbiol 55: 588-600. PubMed: 15659172. [DOI] [PubMed] [Google Scholar]
- 55. Mitsui K, Koshimura Y, Yoshikawa Y, Matsushita M, Kanazawa H (2011) The endosomal Na(+)/H(+) exchanger contributes to multivesicular body formation by regulating the recruitment of ESCRT-0 Vps27p to the endosomal membrane. J Biol Chem 286: 37625-37638. doi: 10.1074/jbc.M111.260612. PubMed: 21896492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mei Y, Jia WJ, Chu YJ, Xue HW (2012) Arabidopsis phosphatidylinositol monophosphate 5-kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Res 22: 581-597. doi: 10.1038/cr.2011.150. PubMed: 21894193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qiu QS, Fratti RA (2010) The Na+/H+ exchanger Nhx1p regulates the initiation of Saccharomyces cerevisiae vacuole fusion. J Cell Sci 123: 3266-3275. doi: 10.1242/jcs.067637. PubMed: 20826459. [DOI] [PubMed] [Google Scholar]
- 58. Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565-1572. doi: 10.1038/nprot.2007.199. PubMed: 17585298. [DOI] [PubMed] [Google Scholar]
- 59. Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K et al. (2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64: 355-365. doi: 10.1111/j.1365-313X.2010.04322.x. PubMed: 20735773. [DOI] [PubMed] [Google Scholar]
- 60. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735-743. doi: 10.1046/j.1365-313x.1998.00343.x. PubMed: 10069079. [DOI] [PubMed] [Google Scholar]
- 61. Cheng WH, Endo A, Zhou L, Penney J, Chen HC et al. (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14: 2723-2743. doi: 10.1105/tpc.006494. PubMed: 12417697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4: 401-406. doi: 10.1016/S1369-5266(00)00192-8. PubMed: 11597497. [DOI] [PubMed] [Google Scholar]
- 63. Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247-273. doi: 10.1146/annurev.arplant.53.091401.143329. PubMed: 12221975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Flis K, Hinzpeter A, Edelman A, Kurlandzka A (2005) The functioning of mammalian ClC-2 chloride channel in Saccharomyces cerevisiae cells requires an increased level of Kha1p. Biochem J 390: 655-664. doi: 10.1042/BJ20050480. PubMed: 15926887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maresova L, Sychrova H (2006) Arabidopsis thaliana CHX17 gene complements the kha1 deletion phenotypes in Saccharomyces cerevisiae. Yeast 23: 1167-1171. doi: 10.1002/yea.1424. PubMed: 17133624. [DOI] [PubMed] [Google Scholar]
- 66. daSilva LL, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C et al. (2004) Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16: 1753-1771. doi: 10.1105/tpc.022673. PubMed: 15208385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parsons HT, Christiansen K, Knierim B, Carroll A, Ito J et al. (2012) Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol 159: 12-26. doi: 10.1104/pp.111.193151. PubMed: 22430844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ferguson GP, Nikolaev Y, McLaggan D, Maclean M, Booth IR (1997) Survival during exposure to the electrophilic reagent N-ethylmaleimide in Escherichia coli: role of KefB and KefC potassium channels. J Bacteriol 179: 1007-1012. PubMed: 9023177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miller S, Douglas RM, Carter P, Booth IR (1997) Mutations in the glutathione-gated KefC K+ efflux system of Escherichia coli that cause constitutive activation. J Biol Chem 272: 24942-24947. doi: 10.1074/jbc.272.40.24942. PubMed: 9312097. [DOI] [PubMed] [Google Scholar]
- 70. Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA et al. (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30: 529-539. doi: 10.1046/j.1365-313X.2002.01309.x. PubMed: 12047628. [DOI] [PubMed] [Google Scholar]
- 71. Fang Z, Mi F, Berkowitz GA (1995) Molecular and Physiological Analysis of a Thylakoid K+ Channel Protein. Plant Physiol 108: 1725-1734. PubMed: 12228576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang X, Berkowitz GA, Peters JS (1993) K+-conducting ion channel of the chloroplast inner envelope: functional reconstitution into liposomes. Proc Natl Acad Sci U S A 90: 4981-4985. doi: 10.1073/pnas.90.11.4981. PubMed: 11607404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Robinson SP (1985) Osmotic adjustment by intact isolated chloroplasts in response to osmotic stress and its effect on photosynthesis and chloroplast volume. Plant Physiol 79: 996-1002. doi: 10.1104/pp.79.4.996. PubMed: 16664560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bowers K, Levi BP, Patel FI, Stevens TH (2000) The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol Biol Cell 11: 4277-4294. doi: 10.1091/mbc.11.12.4277. PubMed: 11102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ali R, Brett CL, Mukherjee S, Rao R (2004) Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J Biol Chem 279: 4498-4506. PubMed: 14610088. [DOI] [PubMed] [Google Scholar]
- 76. Lawrence SP, Bright NA, Luzio JP, Bowers K (2010) The sodium/proton exchanger NHE8 regulates late endosomal morphology and function. Mol Biol Cell 21: 3540-3551. doi: 10.1091/mbc.E09-12-1053. PubMed: 20719963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full-length AtKEA1 is inactive in K+ transport in yeast.
(TIF)
The full-length AtKEA1 does not transport K+ at acidic pH in yeast.
(TIF)
The full length AtKEA1is not properly distributed in yeast cells.
(TIF)
AtKEAs fused with GFP at the C-terminus retained activity.
(TIF)
AtKEAs fused with GFP at the C-terminus are properly distributed in yeast cells.
(TIF)
Materials and methods of supporting figures.
(DOC)
Primers for the plasmid constructs used in functional expression in yeast.
(DOC)
Primers for RT-qPCR.
(DOC)
Primers for the plasmid constructs used in localization of GFP fusion proteins in yeast.
(DOC)