Abstract
In this study we investigated the functional connectivity in 23 Mild TBI (mTBI) patients with and without memory complaints using resting state fMRI in the sub-acute stage of injury as well as a group of control participants. Results indicate that mTBI patients with memory complaints performed significantly worse than patients without memory complaints on tests assessing memory from the Automated Neuropsychological Assessment Metrics (ANAM). Altered functional connectivity was observed between the three groups between the default mode network (DMN) and the nodes of the task positive network (TPN). Altered functional connectivity was also observed between both the TPN and DMN and nodes associated with the Salience Network (SN). Following mTBI there is a reduction in anti-correlated networks for both those with and without memory complaints for the DMN, but only a reduction in the anti-correlated network in mTBI patients with memory complaints for the TPN. Furthermore, an increased functional connectivity between the TPN and SN appears to be associated with reduced performance on memory assessments. Overall the results suggest that a disruption in the segregation of the DMN and the TPN at rest may be mediated through both a direct pathway of increased FC between various nodes of the TPN and DMN, and through an indirect pathway that links the TPN and DMN through nodes of the SN. This disruption between networks may cause a detrimental impact on memory functioning following mTBI, supporting the Default Mode Interference Hypothesis in the context of mTBI related memory deficits.
Keywords: resting state fMRI, mild traumatic brain injury, working memory, default mode network
1. Introduction
Traumatic brain injury (TBI) is one of the most common neurological disorders in the United States with 1.7 million cases each year (Faul et al., 2010) and 75% of these cases are diagnosed as mild injuries as characterized by the Glasgow Coma Scale (GCS) between 13 and 15 (CDC 2003). Individuals with mild TBI (mTBI) often show no apparent injury based on current clinical CT and conventional MR imaging. Furthermore, although diffuse axonal injury (DAI) is common among these patients, it is commonly missed by convention MRI (Iverson et al., 2000, Lee et al., 2008). Regardless of the presentation on CT or conventional MRI, a portion of these individuals suffer from cognitive deficits for months following the initial injury (Kwok et al., 2008). Two of the most common findings following mTBI are impaired information processing represented by increased reaction times on cognitive tasks among TBI patients (Hillary et al., 2010; Johansson et al., 2009) and memory deficits (McAllister et al., 2006; McDowell et al., 1997; Miotto et al., 2010). It is currently unclear as to who among the injured mTBI patients will develop memory deficits and who will continue on to make a full recovery which makes prognosis difficult. Advanced functional neuroimaging techniques including resting state functional MRI (fMRI) may shed some light on the disruptions in large-scale neural networks involved in memory and their association with memory deficits among TBI population.
Multiple fMRI studies on patients with TBI have shown alterations in regional blood oxygenation level dependent (BOLD) responses during experimental tasks designed to probe spatial memory (Slobounov et al., 2010), working memory (Christodoulou et al., 2001, McAllister et al., 2001; Sanchez-Carrion et al., 2008; Turner and Levine, 2008), executive function (Soeda et al., 2005), and sustained attention (Maruishi et al., 2007). Results in regard to increased or decreased activations and cluster size have been contradictory and may be related to study design differences between the studies. The disparities between the studies include variability in severity of injury in sample populations, variability in time since injury, and the wide range in difficulty of tasks chosen between studies. For example, McAllister and colleagues (2001) found that in an auditory N-back task, chronic mTBI patients had increased activations during a moderate working memory load but had decreased activations during a high working memory load suggesting that mTBI populations have differing compensatory mechanisms based on differences in task difficulty or cognitive load. Clearly, the response to any given task is a function of the true nature and extent of injury however ‘small’ and may provide variable BOLD responses leading to disparate conclusions regarding the cognitive effects following TBI.
More importantly, task evoked functional BOLD techniques have limited clinical utility during the acute stage on a TBI patient. A proposed solution is the use of resting state BOLD techniques which are both independent of cognitive load as well as easily administered during all stages of TBI. The ability of resting state fMRI to acquire functional information in the acute stage of TBI when task evoked functional BOLD techniques may be limited is of great clinical value. Although, resting state functional connectivity does not directly address structural connections within the brain, it does have great promise in addressing the questions regarding connectivity between spatially disparate regions and their interactions by determining efficiency and strength of numerous large-scale neuronal networks in the absence of a task (Sporns et al., 2011; van den Heuvel and Hulshoff Pol 2010).
The default mode network (DMN) is perhaps the most widely studied resting state networks (RSN). The Default Mode Network (DMN) is a widely studied RSN and consists of regions that are consistently “turned off’ during task related activities while remaining more active during rest (Raichle et al., 2001). It represents internally directed, self-reflective processes (Gusnard et al., 2001) and includes lateral parietal cortex, posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), medial temporal lobe (MTL), and medial frontal cortex (Greicius et al., 2003; Fox et al., 2005). On the other hand, a network that is often activated during goal directed behaviors such as working memory is the executive network, which includes the bilateral dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (Esposito et al., 2007). Due to the variations in labeling task positive networks across various studies, for simplicity we will refer to this network as the task positive network (TPN). Ideally the DMN and the TPN are anti-correlated at rest (Fox et al., 2005) and interaction between the two networks is proposed to be crucial for cognitive functioning (Hampson et al., 2010). Termed the Default Mode Interference Hypothesis, it has been suggested that the failure of the TPN to adequately deactivate the DMN during a goal directed task could lead to lapses in attention and therefore diminished performance ability (Sonuga Barke et al., 2007; Weissman et al., 2006). An understanding of the differences in resting state memory related networks among mTBI patients who present with memory deficits and those who do not, may provide valuable insights into causes of persistent memory deficits and information regarding possible mechanisms for compensation.
TBI has been shown to alter functional connectivity in multiple resting state networks including interhemispheric functional connectivity (Marqez de la Plata et al., 2011, Slobounov et al., 2011), the motor network (Kasahara et al., 2010), TPN (Hillary et al., 2011, Mayer et al., 2011), and the DMN (Hillary et al., 2011, Mayer et al., 2011, Johnson et al., 2012, Zhou et al 2012). A recent study on severe TBI population found that TBI patients with low sustained attention had reduced deactivations of the DMN during a task over the course of the experiment compared to TBI patients with high sustained attention (Bonnelle et al., 2011), suggesting an imbalance between the DMN and TPN in TBI populations. Although a general understanding of the disruption in networks in the chronic stage of injury is emerging from these studies, to our knowledge no study has investigated the changes in memory related resting state connectivity among mTBI patients that present with memory problems in comparison to those who do not at the sub-acute stage of injury which is the motivation of this pilot study.
Since the DMN and the TPN are often associated with cognitive performance in both healthy control subjects and patient populations, we believe that resting state functional connectivity may be a sensitive measure to help distinguish the changes in memory related networks that contribute to persistent memory deficits in mTBI patients. In this pilot study we examined a group of mTBI patients with and without memory complaints in the sub-acute stage of injury to primarily (a) determine if mTBI patients with memory complaints exhibit reduced functional connectivity within the DMN and the TPN?; (b) determine whether mTBI patients who report memory complaints exhibit more robust functional connectivity (or a reduction in anti-correlations) between nodes of the DMN and TPN compared to those who do not?; and (c) whether an association exists between individual memory performance and the strength of functional connectivity between the DMN and TPN.
2. Results
2.1. Participants
Twenty-three mTBI patients (39.5+/−16.4yrs, 11M:12F) were prospectively recruited from the Adam Cowley Shock Trauma Center at the University of Maryland Medical Center as part of a larger imaging protocol using a combination of advanced MR imaging, and neuropsychological assessments including the Automated Neuropsychological Assessment Metrics (ANAM) (Kane et al., 2006). Fourteen neurologically intact subjects (37.1+/−14.3yrs, 8M:6F) served as a control population. Patient demographic information for all participants is shown in Table 1. There was no difference in GCS (p = 0.85), time since injury (p = 0.88) or incidence of positive head CT (p = 0.40) between the two mTBI groups. There was no significant difference in gender (p = 0.10) between the three groups. However, there was a non-significant trend in difference in mean years of education years (p = 0.064) and age (p=0.070) across the three groups. To address the possible difference in education and age between the three groups, partial correlations between the cognitive results and functional connectivity measures were computed to correct for the effect of education and age.
Table 1.
Demographics of all patient participants and control subjects
| Patient ID | N | GCS | Age | Sex (Males) | Education | Days Post Injury | Positive Head CT |
|---|---|---|---|---|---|---|---|
| MTBI Memory Problems | 13 | 14.9 +/−0.3 | 44.8+/−16.7 | 7 | 13.1 +/−2.7 | 36.0 +/− 7.9 | 4 |
| MTBI No Memory Problems | 10 | 14.9 +/− 0.3 | 32.5 +/−13.9 | 9 | 15.6 +/−2.8 | 35.5 +/− 8/4 | 1 |
| Control | 14 | NA | 37.1 +/−14.3 | 8 | 14.1 +/−2.0 | NA | NA |
| p-value | NA | 0.85 | 0.070 | 0.10 | 0.064 | 0.88 | 0.40 |
2.2 Neuropsychological Assessment
All three groups performed similarly on Mini Mental State Exam (MMSE) and no significant differences were found (p = 0.368) suggesting that the overall level of cognitive functioning was similar across the groups. There were also no significant differences in memory scores from the ANAM between the control group and either of the mTBI groups (all p > 0.05). MTBI patients with memory complaints performed significantly worse than the control group only on the Delayed Recall Subtest of the MACE (p=0.032). However, MTBI patients with memory complaints scored worse on the Glasgow Outcome Scale Extended (GOSE) (Teasdale et al., 1998) than those without memory complaints although the difference was only marginally significant (p = 0.062). As predicted, compared to those without memory complaints, mTBI patients who reported memory complaints performed significantly worse on the total score of the Military Acute Concussion Evaluation (MACE) (McCrea et al., 2000) (p = 0.034), and on the Match to Sample (MTS) (p = 0.023), and Code Substitution Delayed (CSD) (p = 0.018) subtests of the ANAM (Fig. 1). This is in concordance with the self-reported memory complaints provided by the patients on the Modified Rivermead Post-Concussion Symptoms Questionnaire (RPQ) (Savola and Hillbom 2003). Based on the International Classification of Disease tenth revision (ICD10) symptom criteria for post concussive disorder (PCD), all 13 of the patients with memory complaints qualified as having PCD while only one of the patients without memory complaints qualified as having PCD.
Figure 1.
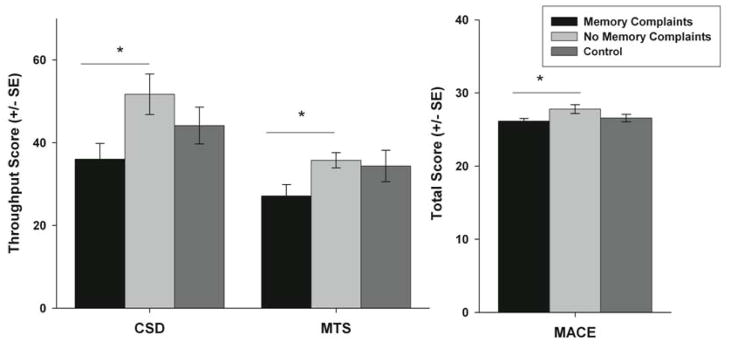
Cognitive scores from the two subtests of the ANAM and from the MACE. For the ANAM the throughput score for code substitution delay (CSD) and match to sample (MTS) represents summary score that incorporates information from the reaction time as well as accuracy. * p < 0.05.
2.3 Resting State Voxel-based Analysis
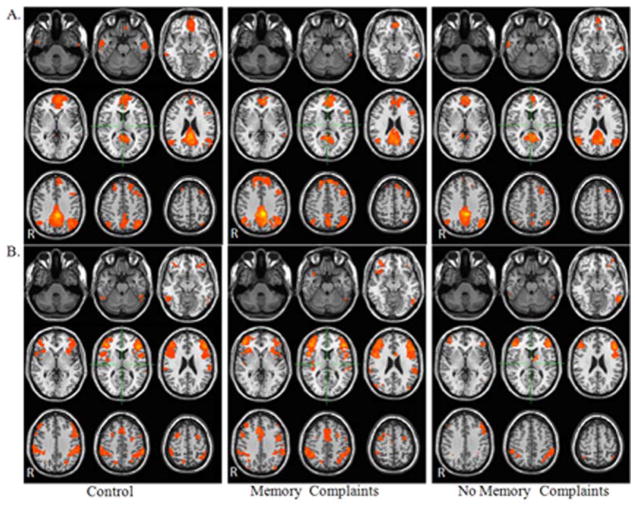
Visual inspection of RSNs for both the DMN (Fig. 2a) and TPN (Fig. 2b) demonstrated that the connectivity maps were consistent with prior reports in the literature of control populations (Fox et al., 2005; Greicius et al., 2003; Raichle et al., 2010; van den Heuvel and Hullshoff Pol., 2010).
Figure 2.
Comparison of resting state networks for mTBI patients with and without memory complaints and control group (a) DMN (b) TPN. Maps were thresholded at voxel wise p-value of 0.001 (uncorrected) and cluster extent threshold of p=.001 using a family wise error correction for multiple comparisons for the regions positively correlated with the seed ROI within each network.
DMN
Within the DMN functional connectivity map, control participants demonstrated positive functional connectivity with the anterior prefrontal cortex, PCC/precuneus, bilateral angular gyrus, bilateral middle temporal gyrus, left premotor and right dorsal frontal cortex. Patients without memory complaints demonstrated positive functional connectivity with the anterior prefrontal cortex, PCC/precuneus, bilateral angular gyrus, and left middle temporal gyrus. Similar regions of connectivity were observed in mTBI group with memory complaints but also included the right middle temporal gyrus and left dorsal frontal cortex (Fig. 2a).
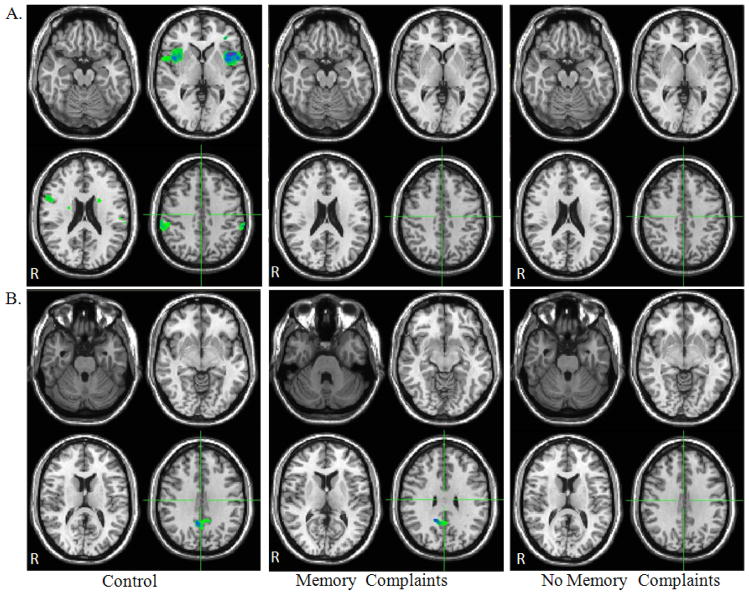
In control participants, DMN anti-correlated functional connectivity maps demonstrate negative functional connectivity with the bilateral insular cortex, left premotor, and bilateral supramarginal gyrus. None of these regions demonstrated significantly anti-correlated functional connectivity with the DMN in either mTBI group with or without memory complaints (Fig. 3a)
Figure 3.
Comparison of anti-correlated functional connectivity maps for mTBI patients with and without memory complaints and control group (a) DMN (b) TPN. Maps were thresholded at voxel wise p-value of 0.005 (uncorrected) and cluster extent threshold of p=.05 using a family wise error correction for multiple comparisons for the regions negatively correlated with the seed ROI within each network.
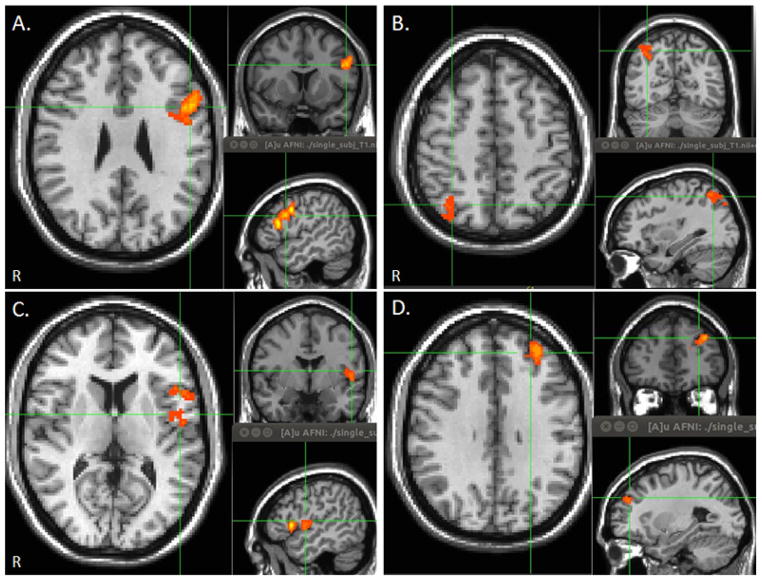
Between group functional connectivity maps noted significantly increased functional connectivity with the left inferior frontal gyrus (L IFG) and the right superior parietal lobule (R SPL) in the mTBI group reporting memory complaints compared to the group without memory complaints (Fig. 4). There were no regions of increased functional connectivity in the mTBI group without memory complaints. In addition, significantly increased functional connectivity with the left superior temporal gyrus/insular cortex (L In) and the left dorsolateral prefrontal cortex (L DLPFC) was noted in the mTBI group reporting memory complaints compared to the control group, while significantly reduced functional connectivity with the right angular gyrus was noted in the mTBI group without memory complaints compared to the control group.
Figure 4.
Between group correlation maps of the DMN demonstrating clusters with increased functional connectivity with DMN in mTBI patients with memory complaints compared to mTBI patients without memory co mplaints (a–b) and control group (c–d). Maps were thresholded at voxel wise p-value of 0.005 (uncorrected) and cluster extent threshold of p=.05 using a family wise error correction for multiple comparisons for regions positively correlated with the seed ROI. (a) Left inferior frontal gyrus (L IFG). (b) Right superior parietal lobule (R SLP). (c) Left insula (L In). (d) Left dorsolateral prefrontal cortex (L DLPFC).
TPN
Within the TPN functional connectivity maps, control participants demonstrated positive functional connectivity with the bilateral DLPFC, bilateral supramarginal gyrus, bilateral inferior temporal gyrus, bilateral premotor, dorsal anterior cingulated cortex, and right somatosensory association area. Within the TPN functional connectivity map, patients without memory complaints demonstrated positive functional connectivity with the bilateral DLPFC, bilateral supramarginal gyrus, bilateral inferior temporal gyrus, and L orbitofrontal cortex. Similar regions of connectivity were observed in mTBI group with memory complaints with the addition of the bilateral premotor, right insular cortex, left associative visual cortex, and dorsal anterior cingulate cortex (Fig. 2b).
In control participants as well as mTBI patients without memory complaints, TPN anti-correlated functional connectivity maps demonstrate negative functional connectivity with the dorsal posterior cingulate cortex (dPCC). However, in the mTBI patients with memory complaints there were no regions that were significantly anti-correlated with the TPN (Fig. 3b)
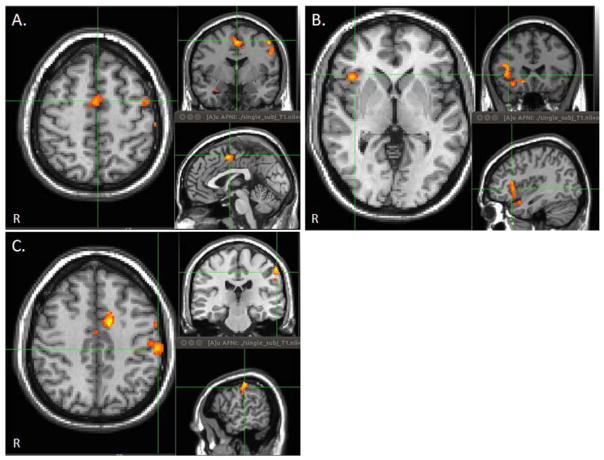
Between group functional connectivity maps noted significantly increased functional connectivity with the right anterior insula (R AIn), left supramarginal gyrus (L SMG), and supplemental motor area (SMA) in the mTBI group reporting memory complaints compared to the group without memory complaints (Fig. 5). There were no regions of increased functional connectivity in the mTBI group without memory complaints. In addition, there were no regions of increased or decreased functional connectivity between either mTBI group and the control group for the TPN functional connectivity maps.
Figure 5.
Between group correlation maps of the TPN demonstrating clusters with increased functional connectivity with EN between mTBI patients with and without memory complaints. Maps were thresholded at voxel wise p-value of 0.005 (uncorrected) and cluster extent threshold of p=.05 using a family wise error correction for multiple comparisons for regions positively correlated with the seed ROI. (a) Supplemental motor area (SMA). (b) Right anterior insula (R AnI). (c) Left supramarginal gyrus (L SMG).
Salience Network (SN)
It has been suggested that the Salience Network (SN) is involved in coordinating the switching between the DMN and the TPN (Menon and Uddin, 2010, Seeley et al., 2007, Sridharan et al., 2008). Therefore, we also investigated functional connectivity within the SN. Within the SN functional connectivity maps, all three groups demonstrated positive functional connectivity with the bilateral Insula, dorsal anterior cingulated cortex, bilateral supramarginal gyrus, and bilateral premotor area (Supplemental Figure 1). Based on voxel wise analysis, no significant differences in functional connectivity were observed between the two groups.
2.4 Cross Network ROI Analysis
DMN
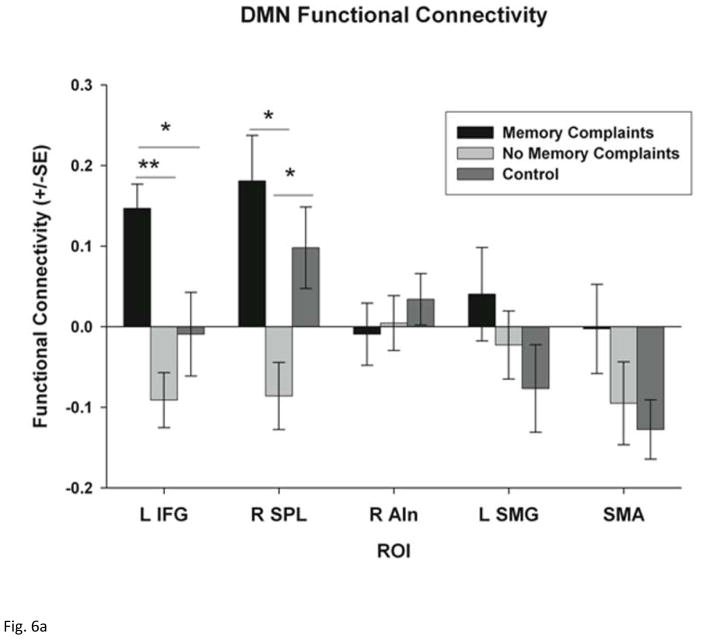
ROI analysis demonstrated a significant difference across the three groups in within DMN functional connectivity for the right dorsal frontal cortex (R DFC) only (F = 3.797, p = 0.032). Post-hoc tests demonstrate that mTBI patients with and without memory complaints have reduced functional connectivity within the R DFC compared to the control group (p = 0.020 and p = 0.032 respectively) (Supplemental Figure 2). In addition, ROI analysis confirmed a significant difference in the strength of functional connectivity between the DMN and regions outside of the DMN across all three groups, including the L IFG (F = 7.969; p = 0.001) and the R SLP (F = 6.262; p = 0.005). No difference in connectivity between the DMN and the R AIn, L SMG, or SMA was observed between the three groups (Fig. 6a). Post-hoc tests demonstrate that mTBI patients with memory complaints have increased functional connectivity with the DMN in the L IFG (p < 0.001) and the R SPL (p = 0.001) compared to mTBI patients without memory complaints. Furthermore, mTBI patients with memory complaints demonstrated a positive functional connectivity (representing a positive correlation) with the L IFG and R SLP indicating that these regions are part of the DMN among these patients. On the other hand, the mTBI patients without memory complaints demonstrated a negative functional connectivity (representing a negative correlation) between the L IFG and R SLP and the DMN indicating that these regions are part of an anti-correlated network.
Figure 6.
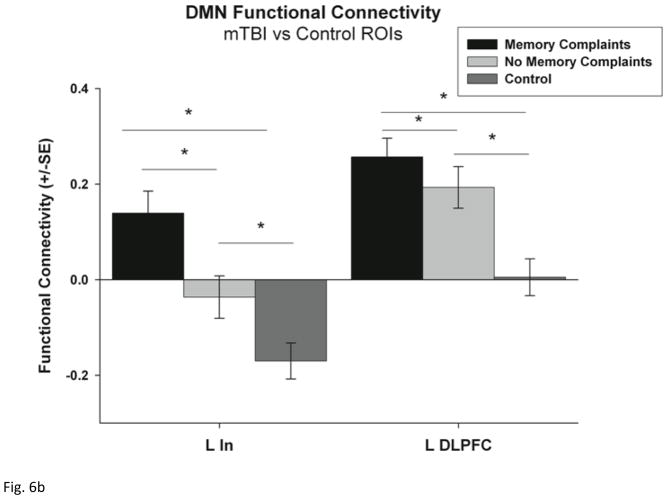
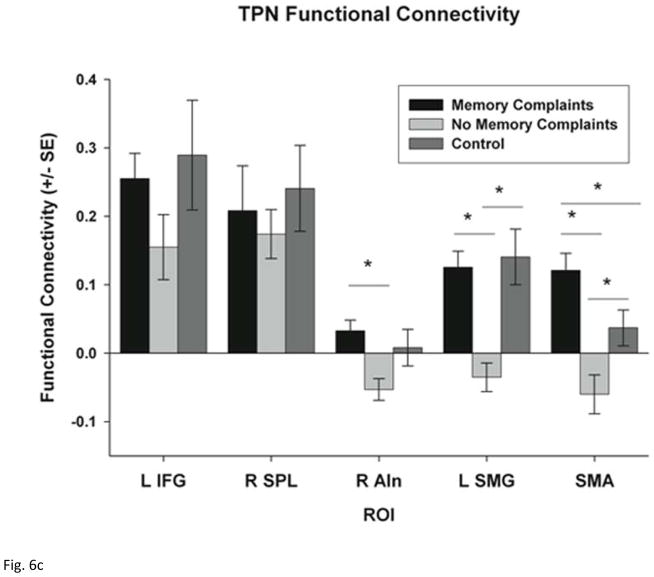
ROI analysis of functional connectivity within each network. (a) functional connectivity between the DMN (PCC seed) and the five selected ROIs including the left inferior frontal gyrus (L IFG), right superior parietal lobe (R SPL), right anterior insula (R AIn), left supramarginal gyrus (L SMG) and the supplementary motor area (SMA). (b) functional connectivity between the DMN (PCC seed) and the left Insula (L In) and the left dorsolateral prefrontal cortex (LDLFPC) (c) functional connectivity between the TPN (bilateral DLPFC seed) and the same five ROIs. Group differences were tested using Student’s t-tests and corrected for multiple comparisons using Bonferroni correction. * p < 0.05 and ** p < 0.001.
In addition, ROI analysis confirmed a difference in strength of functional connectivity between the DMN and the L In and L DLPFC across the three groups (F = 14.34, p < 0.001; F = 11.21, p < 0.001) (Fig. 6b). Post hoc tests indicate that mTBI patients with memory complaints have increased functional connectivity between the DMN and L In compared to controls (p <0.001), but mTBI patients with and without memory complaints demonstrated increased functional connectivity between the DMN and the L DLPFC compared to the control group (p < 0.001, p =0.023 respectively).
TPN
ROI analysis of within TPN functional connectivity failed to demonstrate any significant differences across the three groups. ROI analysis confirmed significant differences in functional connectivity between the regions of TPN and regions outside of the TPN across the three groups including the R AIn (F = 3.907; p = 0.030), L SMG (F = 8.347; p = 0.001), and SMA (F = 10.84; p < 0.001). No difference in connectivity with the L IFG and R SPL between the two groups was observed (Fig. 6c). Post-hoc tests demonstrate that mTBI patients with memory complaints have increased functional connectivity between the TPN and the R AIn (p = 0.009), L SMG (p = 0.002), and SMA (p = 0.001) compared to those without memory complaints. Furthermore, mTBI patients with memory complaints demonstrate a positive functional connectivity with these three regions indicating that these regions are part of the TPN among these patients.
2.5 Relationship between Cognitive Function and Functional Connectivity
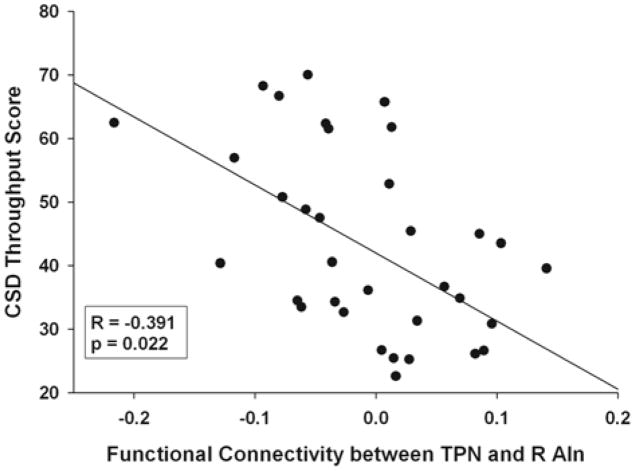
To test the hypothesis that memory deficits in the mTBI population may be associated with the disruption in the balance between the TPN and DMN, we examined correlations between the memory-related cognitive tests (MTS and CSD) and the functional connectivity of each RSN with both the SMA and the R AIn, which are regions associated with the SN due to its proposed role in modulating the interaction between the DMN and TPN. After correcting for the differences in age and educational levels between the three groups, a significant negative correlation remained between functional connectivity with the TPN and the R AIn and the CSD throughput score (R=−0.391, p = 0.022) when all data was used as shown (Fig. 7).
Figure 7.
Partial correlation between the throughput scores of the code substitution delayed (CSD) subtest of the ANAM and functional connectivity between the TPN and the R AIn after correcting for age and level of education.
3. Discussion
Given the association between the DMN, the TPN and memory performance in both healthy controls and patient populations, we predicted that resting state functional connectivity may be a sensitive measure to help distinguish mTBI patients who have memory deficits from those who do not. Based on our findings we arrive at three main conclusions. First, the results of this study do not provide support to previous findings that there is reduced functional connectivity within the DMN or the TPN in our patient population; however, this may be due the small sample size of this pilot study. Second, the results support the idea that there is increased functional connectivity between the nodes of the DMN and the nodes of the TPN among mTBI patients with memory related deficits compared to healthy controls and compared to mTBI patients with no memory related deficits. Patients with memory complaints demonstrated positive correlation between the DMN and several regions of the TPN and the L In of the SN. (Fig. 4). Furthermore, the TPN of the affected group also included the SMA and R AnI which are regions generally included within the SN (Seeley et al., 2007) (Fig. 6). This is further substantiated by the fact that a negative correlation was observed between the functional connectivity with the TPN and R AIn and the throughput scores on the CSD subtest of the ANAM, indicating that individuals with a stronger positive correlation between the TPN and SN performed worse on this test of memory (Fig. 7). Third, the results of this study suggest that in mTBI there is a reduction in anti-correlated networks for both those with and without memory complaints for the DMN, but only a reduction in the anti-correlated network in mTBI patients with memory complaints for the TPN (Fig. 3).
3.1 Implications of altered functional connectivity in the context of the Default Mode Interference Hypothesis
The mTBI patients within this study with memory deficits, similar to previous reports on a general mTBI populations (Mayer et al., 2011) exhibited an increased functional connectivity between the DMN and the nodes of the TPN (L IFG, L DLPFC, and R SPL). The L IFG is located near Broca’s Area (BA 44) and this region is commonly activated during verbal working memory task and has been thought to be involved in sub vocal rehearsal (Smith and Jonides 1998). In addition, it has been noted that as working memory load increases, and an increased functional connectivity between nodes of the DMN and the L IFG (Bluhm et al., 2010). This parametric increase in activation of the L IFG was absent in severe TBI patients during and N-back working memory task (Perlstein et al., 2004). In conjunction with the results presented in our study which demonstrate increased functional connectivity between the L IFG and the DMN, this indicates that TBI patients may have difficulty recruiting additional neural resources as task difficulty increases because they are unable to separate the functions of the DMN and the TPN.
Altered functional connectivity among mTBI patients between the PCC and the R SPL was observed with the affected mTBI group demonstrating an increased functional connectivity than the mTBI group with no memory complaints. While verbal working memory is found to be left hemisphere dominant, spatial working memory is found to be right hemisphere dominant and includes parts of the right posterior parietal cortex. This region has been proposed to be involved in the storage component of working memory (Esposito et al., 2007, Smith and Jonides 1998). Kasahara and colleagues (2011) found that chronic TBI patients had reduced activations of the L inferior parietal gyrus on a verbal N-back working memory task and made more errors during higher working memory loads than controls. Their results indicate a failure of mTBI patients to adequately activate the parietal regions of the TPN during a working memory task. Our observation of the inability of mTBI patients with memory complaints to properly disengage the parietal regions from the DMN are in support of the observations made by Kasahara and colleagues (2011).
In this study, the mTBI population with memory complaints demonstrated increased functional connectivity between the TPN nodes (L IFG, L DLPFC, and R SPL) and the PCC node of the DMN. Functional connectivity between the regions of DMN and TPN have been shown to increase and scale with the difficulty of a cognitive task (Bluhm et al., 2010). The finding that these regions are strongly correlated during the resting state might indicate the inability of mTBI patients with memory complaints to adequately separate the functions of the DMN and TPN. In line with the Default Mode Interference Hypothesis, the mTBI patients with memory deficits may have difficulty in accommodating tasks with increased difficulty because of the inability to adequately deactivate the appropriate regions of the DMN during such a task resulting in interference of DMN activities during goal directed behavior.
Further supporting the Default Mode Interference Hypothesis, the mTBI population with memory deficits demonstrated positive functional connectivity with the TPN (the R AIn, L SMG, and SMA) while the same regions are anti-correlated with the TPN for the mTBI group without memory complaints. This once again argues for the hypothesis that there is increased functional connectivity between the TPN and DMN in mTBI patients with memory complaints.
3.2 Implications of altered functional connectivity and possible role of SN in memory deficits
The mTBI population with memory complaints demonstrated increased functional connectivity of the TPN and DMN with nodes of the salience network (SN). The SN consists of nodes in the dorsal anterior cingulate cortex (dACC), supplementary motor area (SMA), and the anterior insula (AnI) (Seeley et al., 2007). The proposed role of the SN is to detect salient sensory stimuli and coordinate the switching between the TPN and DMN based on this stimuli (Menon and Uddin, 2010). Using Granger causality and a visual attention task, Sridharan and colleagues suggested that the SN modulates the activations and deactivations of the DMN and TPN (Sridharan et al., 2008). Recent work in chronic TBI population found that the failure to adequately deactivate the DMN was associated with damage in white matter tracts connecting the R AIn to other nodes of the SN (Bonnelle et al., 2012) providing further support that the failure of the SN may be partially responsible for the inability to separate the DMN and the TPN. However, at least one resting state study, did not find any associations between TPN functional connectivity with the SN and white matter disruption within tracts of the SN (Bonnelle et al., 2011).
The increased functional connectivity between the TPN and the SMA and R AIn observed in this study could represent a compensatory mechanism among mTBI patients with memory deficits due to an increased need for the TPN to have top down control in suppressing the DMN. We postulate this may be necessary in these patients to prevent interference of internally directed thoughts in goal-oriented behavior. In addition to altered functional connectivity between the SN and TPN, we note altered functional connectivity between the SN and DMN, suggesting a disruption in the balance between all three networks. While this cannot be directly ascertained from the present data, further research in this area including simultaneous acquisition of both resting state fMRI and working memory task related fMRI may elucidate the mechanisms for this increased functional connectivity that leads to memory impairments following mTBI.
Finally, results demonstrate a reduction in the anti-correlated network of the DMN in mTBI patients regardless of memory deficits, but only a reduction in the anti-correlated network of the TPN in the mTBI patients with memory deficits. These findings not only support the Default Mode Interference Hypothesis in all mTBI populations, but also points to a specific deficit in the anti-correlated network of the TPN in mTBI patients with memory problems that in the future may help to predict who will develop these impairments in the months following a mTBI.
3.3 Limitations
The findings of increased interaction between the TPN and DMN among mTBI patients with memory deficits should be taken in the context of the limitations of the study. This is a pilot study with limited sample size to probe the network differences associated with memory deficits among mild TBI populations in the subacute stage. More importantly, the results from this study indicate the need to study such network changes in a larger population in conjunction with working memory related functional MRI studies and other techniques that provide physiological information from these regions such as arterial spin labeling (ASL). In addition, possibly due to our limited sample size, we only found minor differences in cognitive performance between mTBI patients with memory complaints and the control group (while there were greater differences in cognitive performance between mTBI patients with and without memory complaints). However, the cognitive deficits experienced following mTBI are very subtle and our measures of measures of cognitive assessment may not be sensitive enough to pick up these differences. Therefore, additional research using neuropsychological assessments with more sensitive measures of cognitive performance is warranted.
In addition, we recruited mTBI patients from a top tier shock trauma center so our patient population may be biased towards those patients who sought treatment following an injury and may present with a greater extent of injuries. This may explain why our patient population had a larger portion of patients with positive CTs than would be generally expected.
While the age difference was not statistically significant between the two mTBI groups, there was a trend in the mTBI patients with memory complaints being older than those without memory complaints. We do not think that this played a major role in our results as it is generally found that as an individual ages, the strength of functional connectivity of regions positively and negative correlated with the DMN decreases (Wu et al., 2011). In our results, however, we observed the opposite effect with the regions exhibiting increased anti-correlations in our mTBI group with memory complaints who are in fact older. This implies that the effect of the memory problems outweighs the possible effects of age in this sample of mTBI patients.
It is important to note that while the difference in gender was not significant, there is an increased proportion of females reporting memory complaints compared to males (6 females reporting memory problems with only 1 female not reporting memory problems). Although previous literature suggests that women are more likely to develop post concussive symptoms (Bazarian et al., 2010) this gender difference in behavior may result in variation of self-report of memory. While a study on a larger cohort of patients would help substantiate the findings of this study, it should also be focused on understanding the interactions between the various networks of DMN, TPN and SN. Of interest would be to see the evolution of these networks over time and verify whether the alterations noted in this study persist during the chronic stage following injury and how they may relate to the final outcome of the patient
In this pilot study, we used seed based analysis to understand the interaction of the various networks involved to test a specific hypothesis. Future studies may benefit from data driven analysis methods such as Independent Component Analysis (ICA) (Calhoun et al., 2008), which may provide further insights into the interactions between the various networks (Stevens et al., 2012). In addition, while future studies addressing alterations in these three networks during working memory task would provide additional information on how mTBI affects these networks, this was not the objective of this study.
4. Materials and Methods
4.1 Participants
Twenty-three mTBI patients (39.5+/−16.4yrs, 11M:12F) were prospectively recruited from the Adam Cowley Shock Trauma Center at the University of Maryland Medical Center as part of a larger imaging protocol using a combination of advanced MR imaging, and neuropsychological assessments including the Automated Neuropsychological Assessment Metrics (ANAM) (Kane et al., 2006). Fourteen neurologically intact subjects (37.1+/−14.3yrs, 8M:6F) served as a control population. All participants were over the age of 18. Patients were screened and excluded for history of neurological and psychiatric illness, history of stroke, history of brain tumors or seizures, and contraindications to MR. In addition, patients were excluded for history of previous brain injury or concussion. Our inclusion criteria consisted of an admission GCS of 13–15 and mechanism of injury consistent with trauma, positive head CT, or altered mental status and/or loss of consciousness. Due to our inclusion criteria, the patient population presented in this study consists of mTBI patient with positive CT results (complicated mild) and negative CT results (uncomplicated mild). Mechanisms of injury included 8 motor vehicle accidents, 3 bicycle accidents, 4 assaults, 6 falls, and 2 recreational sports related accidents. The average GCS of the participants was 14.9.
All 23 mTBI patients received resting state fMRI in the sub-acute stage of approximately 1 month post injury (average 36 days, range 25–58 days) and the 14 control participants received resting state fMRI at one time point. Both groups received MR structural scans as part of the imaging protocol. These structural MR scans included FLAIR, DWI, SWI, and T1 and T2 imaging. Both CT results and structural MR images were read by a board certified trauma radiologist (K.S.). Based on clinical CT and MR scans, 5 out of the 23 mTBI patients had evidence of injury. None of the mTBI patients classified as negative CT demonstrated any trauma related injury on structural MR. Individual patient results are shown in Table 2.
Table 2.
Mechanism of injury and conventional imaging findings on patients with and without memory complaints.
| Patient | CT Results | MR Results | Mechanism | Group |
|---|---|---|---|---|
| MRTBI010 | Atraumatic | Atraumatic | MVC | Memory Complaint |
| MRTBI016 | Atraumatic | Atraumatic | MVC | Memory Complaint |
| MRTBI019 | Atraumatic | Atraumatic | Fall | Memory Complaint |
| MRTBI077 | Atraumatic | Atraumatic | MVC | Memory Complaint |
| MRTBI078 | Atraumatic | Atraumatic | Fall | Memory Complaint |
| MRTBI091 | Atraumatic | Atraumatic | bicycle | Memory Complaint |
| MRTBI093 | Sub-centimeter hemorrhage right thalamus | Sub-centimeter hemorrhage right thalamus, left dorsal brainstem contusion | MVC | Memory Complaint |
| MRTBI098 | Atraumatic | Atraumatic | Assault | Memory Complaint |
| MRTBI105 | Sub-centimeter right frontal hemorrhage | Sub-centimeter right frontal hemorrhage | Fall | Memory Complaint |
| MRTBI111 | Atraumatic | Atraumatic | MVC | Memory Complaint |
| MRTBI134 | Epidural hematoma and hemorrhagic contusion in left occipital lobe | Epidural hematoma and hemorrhagic contusion in left occipital lobe | Assault | Memory Complaint |
| MRTBI151 | Atraumatic | Atraumatic | Fall | Memory Complaint |
| MRTBI152 | Subdural hemorrhage along falx | Subdural hemorrhage along falx | Fall | Memory Complaint |
| MRTBI047 | Atraumatic | Atraumatic | MVC | No Memory Complaint |
| MRTBI068 | Atraumatic | Atraumatic | Fall | No Memory Complaint |
| MRTBI074 | Left frontal lobe cortical contusion | Left frontal lobe cortical contusion | Bicycle | No Memory Complaint |
| MRTBI079 | Atraumatic | Atraumatic | Assault | No Memory Complaint |
| MRTBI088 | Atraumatic | Atraumatic | Sports | No Memory Complaint |
| MRTBI089 | Atraumatic | Atraumatic | MVC | No Memory Complaint |
| MRTBI095 | Atraumatic | Atraumatic | MVC | No Memory Complaint |
| MRTBI103 | Atraumatic | Atraumatic | Sports | No Memory Complaint |
| MRTBI135 | Atraumatic | Atraumatic | Bicycle | No Memory Complaint |
| MRTBI146 | Atraumatic | Atraumatic | Assault | No Memory Complaint |
Based on self-reported symptoms from the Modified Rivermead Post-Concussion Symptoms Questionnaire (RPQ) (Savola and Hillbom 2003), the group of mTBI was subdivided into those who self reported memory problems (N=13) and those who did not self-report memory problems (N=10). The RPQ asks participants to rate a series of common symptoms following TBI on a scale of 0–4. Patients were included in the memory complaints group if they self-reported memory problems to any degree on the RPQ. Four patients with memory complaints had positive CT scans while one patient without memory complaints had a positive CT scan. Specific injuries are shown in Table 1. Patient demographic information for all subjects is shown in Table 2.
4.2 Neuropsychological Assessment
All patients underwent neuropsychological assessment. Level of cognitive functioning was assessed by the administration of the Mini Mental State Exam (MMSE) and Military Acute Concussion Evaluation (MACE) (McCrea et al., 2000), and patient outcome was determined by the Glasgow Outcome Scale Extended (GOSE) (Teasdale et al., 1998). In addition to the total score on the MACE mental status section, we also assessed individual scores for each of the four subtests of the MACE (Orientation, Immediate Memory, Concentration, and Delayed Recall). Along with the self-reported symptoms using the RPQ (Savola and Hillbom 2003),39 the computerized cognitive assessment, the ANAM, was administered to the participants (Kane et al., 2006).35 The ANAM consists of seven subtests assessing processing speed, memory, and attention. Given our focus on memory deficits in the mTBI population, we constrained our analysis to the two subtests of the ANAM assessing memory functioning, the match to sample (MTS) and code substitution delayed (CSD) subtests. The MTS assesses spatial working memory and the CSD assesses short-term memory. The throughput score for each of the two subtests which is a single measure encompassing both accuracy and reaction time was used for further analysis. Specifically, the throughput score is the number of correct responses per total amount of time a participant took to respond for each trial, expressed as the number of correct responses per minute (Ivins et al., 2009).40 A lower throughput score indicates a poorer cognitive performance.
4.3 MR Data Acquisition
All imaging was performed on a Siemens Tim-Trio 3T MRI scanner using a 12-channel receive only head coil. A high resolution T1-weighted-MPRAGE (TE = 3.44 ms, TR = 2250ms, TI = 900ms, flip angle = 9°, resolution = 256 × 256 × 96, FOV = 22 cm, sl. Thick. = 1.5 mm) was acquired for anatomic reference. For the resting state fMRI scan, T2*-weighted images were acquired using a single-shot EPI sequence (TE = 30 ms, TR =2000 ms, FOV = 220 mm, resolution = 64 × 64) with 36 axial slices (sl. thick. = 4 mm) over 5 min 42 s that yielded 171 volumes. During all resting state scans extraneous auditory and visual stimuli were removed, and the participants were instructed to rest peacefully with eyes closed.
4.4 Resting State Data Pre-processing
Preprocessing of the imaging data was performed using SPM 8 (http://www.fil.ion.ucl.ac.uk/spm) and included motion correction of the time series, slice timing correction, band pass filtering (.009Hz < f < .08Hz), and registration of all the 171 volumes to the first volume of the time series. The resting state series were then registered to the individual’s T1-MPRAGE images and spatially normalized to standard space using the Montreal Neurological Institute (MNI) template available in SPM 8. Spatial blurring was then applied to the resting state data using a 6mm Gaussian kernel. Individual T1-MPRAGE images in MNI space were segmented into white matter (WM), gray matter (GM) and cerebral spinal fluid (CSF) using SPM8 default settings. The average BOLD time series for the individual T1-MPRAGE segmented masks for CSF and WM were used in later analysis to account for time series variance related to non-neuronal contributions.
4.5 Resting State Analysis
The CONN-fMRI Functional Connectivity toolbox v13.h (http://www.nitric.org/projects/conn) was used to process the resting state data and create RSNs. For the DMN, the reference time series was selected from a 5mm spherical ROI in the posterior cingulate cortex (PCC) centered at (−5,−53, 41) on the MNI template. For the TPN, we selected two reference time series from bilateral 5mm spheres in the dorsolateral prefrontal cortex (DLPFC) centered at (−42,34,20) and (44,36,20) on the MNI template. Coordinates for the seed ROIs were chosen as described Fox et al., (2005).23 The mean BOLD time series for the above seed ROI’s were extracted and correlated with the time series of each voxel of the entire brain. For the TPN, the time series from the right and left DLPFC were averaged before correlating with each voxel’s time series from the entire brain. Reference time series from the right anterior insula (R AIn) was obtained to create RSN of the salience network (SN) and was based on the coordinates from Seeley et al., 2007. In addition, the mean BOLD time series from the WM mask, CSF mask, and the 6 motion correction parameters were included in the model as regressors to remove the variance related to non-neuronal contributions and motion.
Within group functional connectivity maps of the DMN and TPN were created using SPM8. Positive functional connectivity maps were thresholded at voxel-wise p-value of 0.001 (uncorrected) and cluster extent threshold of p-value of 0.001 using a family wise error correction for multiple comparisons. Separate anti-correlated functional connectivity maps were created using SPM for regions negatively correlated with the seed ROIs for each network. The anti-correlated functional connectivity maps were thresholded at voxel wise p-value of 0.005 (uncorrected) and cluster extent threshold p-value of 0.05 using a family wise error correction for multiple comparisons. Between group functional connectivity maps included both positive and negative functional connectivity and were thresholded at voxel-wise p-value of 0.005 (uncorrected) and cluster extent threshold of p-value of 0.05 using a family wise error correction for multiple comparisons.
In addition to the voxel based analysis, ROI analysis was also performed between the three groups. To assess within network resting state functional connectivity differences between the three groups, 10mm spherical ROIs were created around the peak correlated voxels from the control group for the DMN (Supplemental Table 1) and TPN (Supplemental Table 2). Additional ROIs were chosen based on the between group contrast maps obtained between mTBI patients with and without self reported memory complaints as described above for both the DMN and TPN. For each significant cluster, a 5mm spherical ROI was created centered on the peak voxel, and the mean BOLD time series was extracted. For each participant, the individual mean BOLD time series for each ROI was correlated with the mean BOLD time series of the PCC node of the DMN and the average of the two DLPFC nodes of the TPN. Group differences were tested using One Way ANOVAs and post hoc tests were corrected for multiple comparisons using Fisher’s Least Significant Difference (LSD) test.
5. Conclusions
In conclusion, our data suggests a disruption of the segregation of the DMN and the TPN at rest through two possible pathways, (a) through increased FC between various nodes of these two networks and (b) through an indirect pathway that links the TPN and DMN through nodes of the SN. This study supports the idea that disruption of the balance between the DMN and TPN is detrimental to memory performance. In particular, an increased functional connectivity between the TPN and the R AIn node of the SN network appears to be associated with poorer performance on cognitive tests that assess short-term memory. Further investigation is needed to see if such a disruption will have an impact on the long-term outcome of patients that exhibit memory deficits in the sub-acute stage.
Supplementary Material
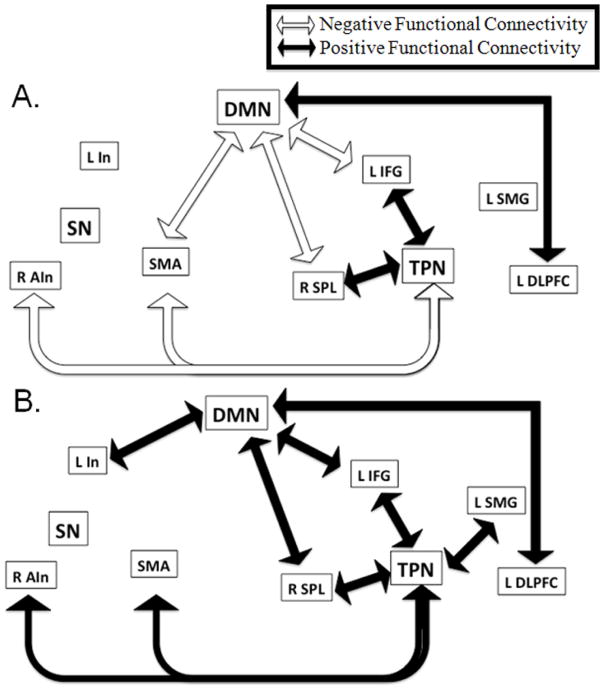
Figure 8.
Summary figure representing significant positive and negative functional connectivity between nodes of the DMN, TPN, and SN. Arrows included represent group correlations that were significantly different than zero (p<0.05, uncorrected for multiple comparisons). Positive functional connectivity represented by black arrows. Negative functional connectivity represented by white arrows (a) Networks functional connectivity in mTBI patients without memory complaints. (b) Network functional connectivity in mTBI patients with memory complaints.
Highlights.
RS-fMRI was performed at the sub-acute stage on mTBI patients with self-reported memory complaints
We examined the interactions between the Default Mode (DMN) & Task Positive Networks (TPN)
Increased connectivity at rest between TPN, DMN, and SN observed in patients with memory issues
Connectivity changes correlate with neuropsychological changes.
Changes in sub-acute stage rs-fMRI are consistent with the Default Mode Interference Hypothesis.
Acknowledgments
The authors would like to thank Joshua Betz for his help with patient recruitment and George Makris and Steve Roys for their help with acquiring and processing the data. This study was supported by DOD award W81XWH-08-1-0725.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazarian JJ, Blyth B, Mookerjee S, He H, McDermott MP. Sex differences in outcome after mild traumatic brain injury. J Neurotrauma. 2010;27(3):527–39. doi: 10.1089/neu.2009.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional Connectivity in the Motor Cortex of Resting. Brain. 1998;(9):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Clark CR, McFarlane AC, Moores KA, Shaw ME, Lanius RA. Default network connectivity during a working memory task. Hum Brain Mapp. 2011;32(7):1029–35. doi: 10.1002/hbm.21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, Greenwood RJ, Sharp DJ. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31(38):13442–51. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta M, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Nat Acad Sci USA. 2012;109(12):4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) National Center for Injury Prevention and Control. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta (GA): Centers for Disease Control and Prevention; 2003. [Google Scholar]
- D’Esposito M. From cognitive to neural models of working memory. Philosophical transactions of the Royal Society of London Series B, Biol Sci. 2007;362(1481):761–72. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control; 2010. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Nat Acad Sci USA. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Nat Acad Sci USA. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Nat Acad Sci USA. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnet Reson Imaging. 2010;28(8):1051–7. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Contreras-Rodríguez O, Soriano-Mas C, López-Solà M, Deus J, Ortiz H, Blanco-Hinojo L, Alonso P, Hernandez-Ribas R, Cardoner N, Menchon JM. Task-induced deactivation from rest extends beyond the default mode brain network. PloS one. 2011;6(7):e22964. doi: 10.1371/journal.pone.0022964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary FG, Genova HM, Medaglia JD, Fitzpatrick NM, Chiou KS, Wardecker BM, Franklin RG, Wang K, DeLuca J. The nature of processing speed deficits in traumatic brain injury: is less brain more? Brain Imaging Behav. 2010;4(2):141–54. doi: 10.1007/s11682-010-9094-z. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J, Good DC, Wylie GR. Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol. 2011;82(1):115–23. doi: 10.1016/j.ijpsycho.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Ivereson GL, Lovell MR, Smith S, Franzen MD. Prevalence of abnormal CT-scans following mild head injury. Brain Injury. 2000;14(12):1057–61. doi: 10.1080/02699050050203559. [DOI] [PubMed] [Google Scholar]
- Johansson B, Berglund P, Rönnbäck L. Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Injury. 2009;23(13–14):1027–40. doi: 10.3109/02699050903421099. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alterations of brain default network in subacute phase of injury in concussed individuals: Resting state fMRI study. Neuroimage. 2012;59(1):511–8. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane RL, Roebuck-Spencer T, Short P, Kabat M, Wilken J. Identifying and monitoring cognitive deficits in clinical populations using Automated Neuropsychological Assessment Metrics (ANAM) tests. Arch Clin Neuropsych. 2007;22(Suppl 1):S115–26. doi: 10.1016/j.acn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Menon DK, Salmond CH, Outtrim JG, Taylor Tavares JV, Carpenter T, Pickard JD, Sahakian BJ, Stamatakis EA. Altered functional connectivity in the motor network after traumatic brain injury. Neurology. 2010;75(2):168–76. doi: 10.1212/WNL.0b013e3181e7ca58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M, Menon DK, Salmond CH, Outtrim JG, Tavares JVT, Carpenter TA, Pickard JD, Sahakian BJ, Stamatakis EA. Traumatic brain injury alters the functional brain network mediating working memory. Brain Injury. 2011;25(12):1170–87. doi: 10.3109/02699052.2011.608210. [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss NE, Wade DT. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. 1995;242(9):587–592. doi: 10.1007/BF00868811. [DOI] [PubMed] [Google Scholar]
- Kwok FY, Lee TMC, Leung CHS, Poon WS. Changes of cognitive functioning following mild traumatic brain injury over a 3-month period. Brain Injury. 2008;22(10):740–51. doi: 10.1080/02699050802336989. [DOI] [PubMed] [Google Scholar]
- Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma. 2008;25(9):1049–56. doi: 10.1089/neu.2008.0566. [DOI] [PubMed] [Google Scholar]
- Marquez de la Plata CD, Garces J, Shokri Kojori E, Grinnan J, Krishnan K, Pidikiti R, Spence J, Devous MD, Moore C, McColl R, Madden C, Diaz-Arrastia R. Deficits in functional connectivity of hippocampal and frontal lobe circuits after traumatic axonal injury. Arch Neurol. 2011;68(1):74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruishi M, Miyatani M, Nakao T, Muranaka H. Compensatory cortical activation during performance of an attention task by patients with diffuse axonal injury: a functional magnetic resonance imaging study. J Neurol Neurosurg Psychiatry. 2007;78(2):168–73. doi: 10.1136/jnnp.2006.097345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo R. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32(11):1825–35. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. NeuroImage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McCrea M, Kelly J, Randolph C. StandardizedAssessment of Concussion (SAC): Manual for Administration, Scoring, and Interpretation. 2. Waukesa, WI: 2000. [Google Scholar]
- McDowell S, Whyte J, D’Esposito M. Working memory impairments in traumatic brain injury: evidence from a dual-task paradigm. Neuropsychologia. 1997;35(10):1341–53. doi: 10.1016/s0028-3932(97)00082-1. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention, and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Cole M, Demery J, Seignourel PJ, Dixit NK, Larson MJ, Briggs RW. Parametric manipulation of working memory load in traumatic brain injury: behavioral and neural correlates. J Int Neurospychol Soc. 2004;10(5):724–41. doi: 10.1017/S1355617704105110. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod M, Snyder Z, Powers WJ, Gusnard D, Shulman GL. A default mode of brain function. Proc Nat Acad Sci USA. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14(4):180–90. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobounov SM, Zhang K, Pennell D, Ray W, Johnson B, Sebastianelli W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: a functional MRI study. Exp Brain Res. 2010;202:341–354. doi: 10.1007/s00221-009-2141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Working memory: a view from neuroimaging. Cogn Psychol. 1997;33(1):5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Nat Acad Sci USA. 1998;95(20):12061–8. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31(7):977–86. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Nat Acad Sci USA. 2008;105(34):12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neusopsychopharmcol. 2010;20(8):519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Xing J, Ma ZL, Yang M, Zhang ZJ, Teng GJ. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer’s Disease. Behav Brain Res. 2009;197(1):103–108. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, Grossman RI, Ge Y. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265(3):882–92. doi: 10.1148/radiol.12120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.