Abstract
Lipin-1 regulates lipid metabolism via its function as an enzyme in the triglyceride synthesis pathway and as a transcriptional co-regulatory protein and is highly up-regulated in alcoholic fatty liver disease. In the present study, using a liver specific lipin-1-deficient (lipin-1LKO) mouse model, we aimed to investigate the functional role of lipin-1 in the development of alcoholic steatohepatitis and explore the underlying mechanisms. Alcoholic liver injury was achieved by pair feeding wild-type (WT) and lipin-1LKO mice with modified Lieber-DeCarli ethanol-containing low fat diets for 4-wks. Surprisingly, chronically ethanol-fed lipin-1LKO mice showed markedly greater hepatic triglyceride and cholesterol accumulation, and augmented elevation of serum liver enzymes accompanied by increased hepatic pro-inflammatory cytokine expression. Our studies further revealed that hepatic removal of lipin-1 in mice augmented ethanol-induced impairment of hepatic fatty acid oxidation and lipoprotein production likely via deactivation of PGC-1α, a prominent transcriptional regulator of lipid metabolism. Our findings demonstrate that liver-specific lipin-1 deficiency in mice exacerbates the development and progression of experimental alcohol-induced steatohepatitis. Pharmacological or nutritional modulation of hepatic lipin-1 may be beneficial for the prevention or treatment of human alcoholic fatty liver disease.
Keywords: Alcoholic liver steatosis, Lipid metabolism, Inflammmation, Lipin-1, Signal transduction
Introduction
Alcoholic fatty liver disease (AFLD) is a major health problem in the United States.1 The preponderance of studies have shown that excessive lipid accumulation in hepatocytes can lead to more harmful forms of liver injury such as steatohepatitis, fibrosis, cirrhosis and end stage liver injury in humans.1 The cellular and molecular mechanisms by which ethanol consumption causes steatohepatitis are complex and elusive.
There is little doubt that intrahepatic lipid accumulation is a key constituent of the pathogenesis of AFLD.1 The primary storage form of lipid, triglyceride (TG), is primarily synthesized from the acylation of glycerol-3-phosphate in liver. A critical step in this process is the dephosphorylation of phosphatidic acid to form diacylglycerol. Studies from several decades ago clearly showed that hepatic phosphatidic acid phosphatase (PAP) activity is markedly increased in liver by chronic ethanol exposure but the genes encoding the PAP enzymes remained elusive for many years.2–4 We now know that lipin family proteins are the mammalian Mg2+-dependent PAP enzymes.5,6 The first member of the lipin family, lipin-1, has now emerged as a vital regulator of lipid metabolism in several organs including liver, adipose tissue, skeletal muscle and heart.6,7 Not surprisingly, we have shown that lipin-1 expression is strongly induced by chronic ethanol exposure in mice, suggesting that increased lipin-1-mediated PAP activity contributes to AFLD via it role in driving glyceride synthesis.8,9
Interestingly, lipin-1 displays a second distinct function in regulating lipid metabolism that is dependent on its subcellular localization. Lipin-1 enters the nucleus and directly interacts with several transcription factors to function as a transcriptional co-regulatory protein.6,7 Nuclear lipin-1 co-activates the peroxisome proliferator-activated receptor α (PPARα) and PPARγ coactivator 1α (PGC-1α) and inhibits the functions of sterol regulatory element binding protein-1 (SREBP-1), leading to enhanced levels of enzymes involved in fatty acid oxidation and reduced levels of genes encoding lipogenic enzymes.10–13 Lipin-1-mediated PAP activity is dispensable for its ability to co-activate PPARα and PGC-1α, but is required for the ability to repress SREBP-1.10
Several lines of evidence have suggested that ethanol-mediated dysregulation of lipin-1 function may contribute to the abnormalities in hepatic lipid metabolism associated with alcoholic liver steatosis.8,9 As discussed above, the development of AFLD in rodents and humans is associated with increased hepatic PAP activity.2–4 Our group recently discovered that chronic ethanol feeding to mice significantly increased hepatic lipin-1 gene expression and elevated cytosolic lipin-1 protein levels, suggesting that this accentuates the cytoplasmic pro-lipogenic activity of lipin-1.8,9 In contrast, ethanol administration blocked lipin-1 nuclear entry in mouse livers, demonstrating that the nuclear functions of lipin-1, which would promote fat oxidation and suppress de novo lipogenesis, are attenuated.9,14 The net consequence of these ethanol-mediated effects on lipin-1 localization is predicted to exacerbate fat accumulation in the liver. However, the causal role of lipin-1 in the development of alcoholic fatty liver remains unknown.
To determine the metabolic effects of loss of lipin-1 on the development and pathogenesis of AFLD, we developed and characterized mice with liver-specific lipin-1 deficiency.15 Unexpectedly, these mice actually exhibited increased hepatic TG accumulation at baseline and with ethanol feeding. Loss of lipin-1 also exacerbated liver injury caused by ethanol. For the first time, we provide evidence demonstrating that lipin-1 plays a crucial role the hepatic response to chronic ethanol exposure and the present data suggest that lipin-1 plays a protective role likely via transcriptional regulation of genes involved in fatty acid catabolism.
Materials and Methods
Animal Experiments
Hepatocyte-specific lipin-1-deficient (lipin-1LKO) mice were generated by crossing lipin-1flox/flox mice with albumin-promoter Cre (AlbCre) transgenic mice to generate lipin-1flox/floxAlbCre+ mice.15,16 Lipin-1flox/flox mice not expressing Cre were used as littermate LOX (WT) controls (genetic background: C57BL/6J and SV129).
Statistical Analysis
Data are presented as means±SEM. All data were analyzed by two-way ANOVA followed by Tukey's multiple comparison procedure with a statistically significant difference defined as a p value < 0.05.
Additional Materials and Methods are described in the Supporting information.
Results
Liver-specific deletion of lipin-1 exacerbates alcoholic fatty liver in mice
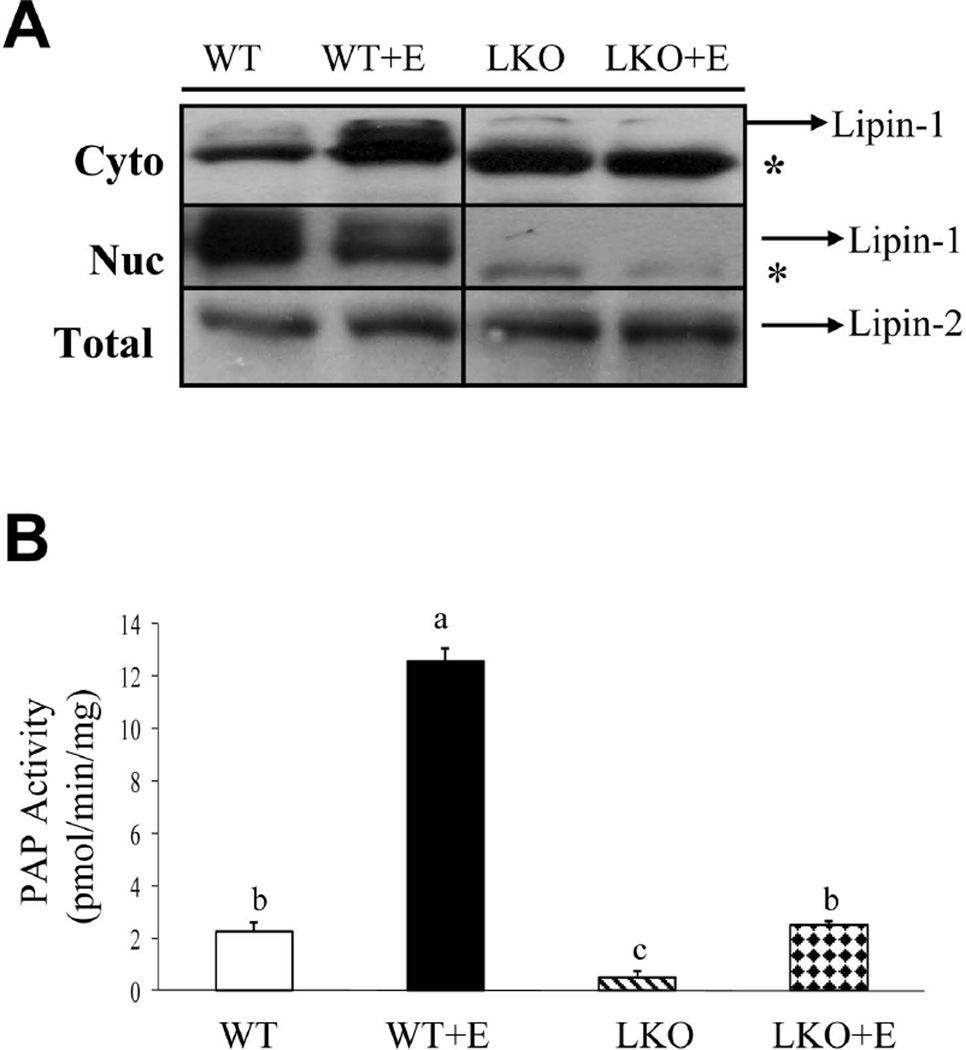
We sought to examine the role of lipin-1 in the development and pathogenesis of AFLD by generating mice with liver-specific knockout of lipin-1 (lipin-1LKO) mice.15 Lipin-1LKO mice were viable and outwardly normal on a chow diet. Western blotting analyses showed the expression of a truncated lipin-1 protein (Fig.1A). The truncated lipin-1 protein results from an alternative translational start site that generates a protein lacking the first 115 amino acids. This protein is completely deficient in PAP activity but seems to retain at least partial transcriptional regulatory function.15 However, in the liver, the nuclear concentration of the truncated lipin-1 protein was nearly depleted either with or without ethanol feeding (Fig.1A). We investigated the effects of hepatic lipin-1 deficiency on ethanol-induced fatty liver by pair-feeding WT and lipin-1LKO mice with a modified Lieber-DeCarli low fat liquid diet with ethanol for 4-wks.17 We tracked the alteration of food intake and body weight for the entire 4-wks period and found that there were no significant differences among the ethanol fed groups in food intake and serum alcohol concentrations were comparable in all ethanol-fed WT or lipin-1LKO mice (Supporting Table 1; data not shown).
Fig. 1.
Liver-specific deletion of lipin-1 exacerbates alcoholic fatty liver in mice. Wild-type (WT) or lipin-1LKO (LKO) mice were pair-fed either a control LF diet or an ethanol-containing diet for 4-wks. (A) Representative Western analysis of lipin-1or lipin-2 protein expression levels. *Truncated lipin-1 protein. (B) Hepatic PAP activity. Results are expressed as means±SEM (n=4–11 mice). Means without a common letter differ, p< 0.05.
Hepatic PAP activity was substantially increased in ethanol-fed WT mice compared to the WT controls (Fig.1B).9 Hepatic PAP activity was severely reduced in lipin-1LKO compared to WT littermate controls. The increase in hepatic PAP activity in response to ethanol feeding was largely abrogated in lipin-1LKO mice (Fig.1B). The expression of lipin-2 was not affected by the loss of lipin-1 nor was it increased by ethanol exposure (Fig.1A). Collectively, these data demonstrate that increased lipin-1 activity accounts for the large increase in hepatic PAP activity after ethanol exposure in WT mice.
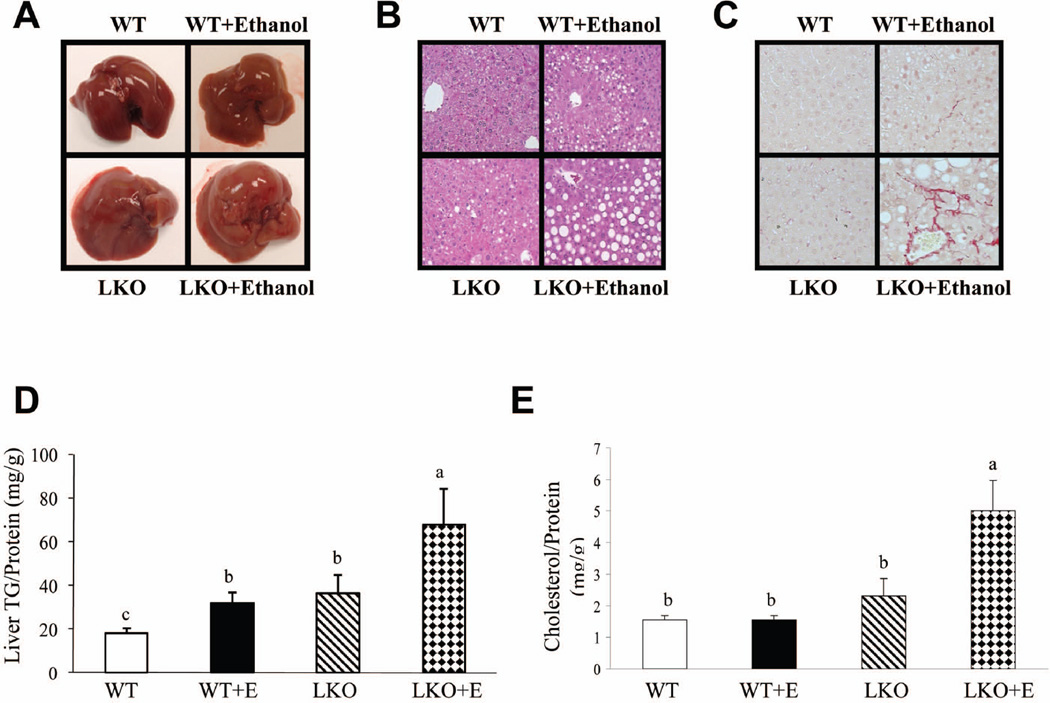
Histopathological analysis revealed that ethanol administration markedly increased microvesicular and macrovesicular steatosis in lipin-1LKO mice compared to all other groups (Fig. 2A,B). Accordingly, significantly higher levels of hepatic triglyceride and cholesterol were detected in ethanol-fed lipin-1LKO mice than in other mice (Fig.2D,E). Ethanol-fed lipin-1LKO mice also displayed significantly higher liver FFA content than did mice in the other groups (Supporting Table 1). Ethanol feeding significantly increased liver weight to body weight ratio and serum levels of ALT and AST in WT mice compared with pair-fed WT controls (Fig.3A,B; Supporting Table 1). The increases in liver weight to body weight ratio and serum ALT and AST levels were more pronounced in ethanol-fed lipin-1LKO mice than respective pair-fed controls (Fig.3A,B; Supporting Table 1).
Fig. 2.
Liver-specific deletion of lipin-1 exacerbates alcoholic fatty liver in mice. Mice were fed as described in Fig.1. (A) Pictures of mouse livers. (B) Hematoxylin and eosin (H&E) staining (original magnification×40) of liver sections. (C) Immunohistochemical staining for collagen. (D) Hepatic triglyceride (TG) levels. (E) Hepatic cholesterol levels. Results are expressed as means±SEM (n=4–11 mice). Means without a common letter differ, p< 0.05
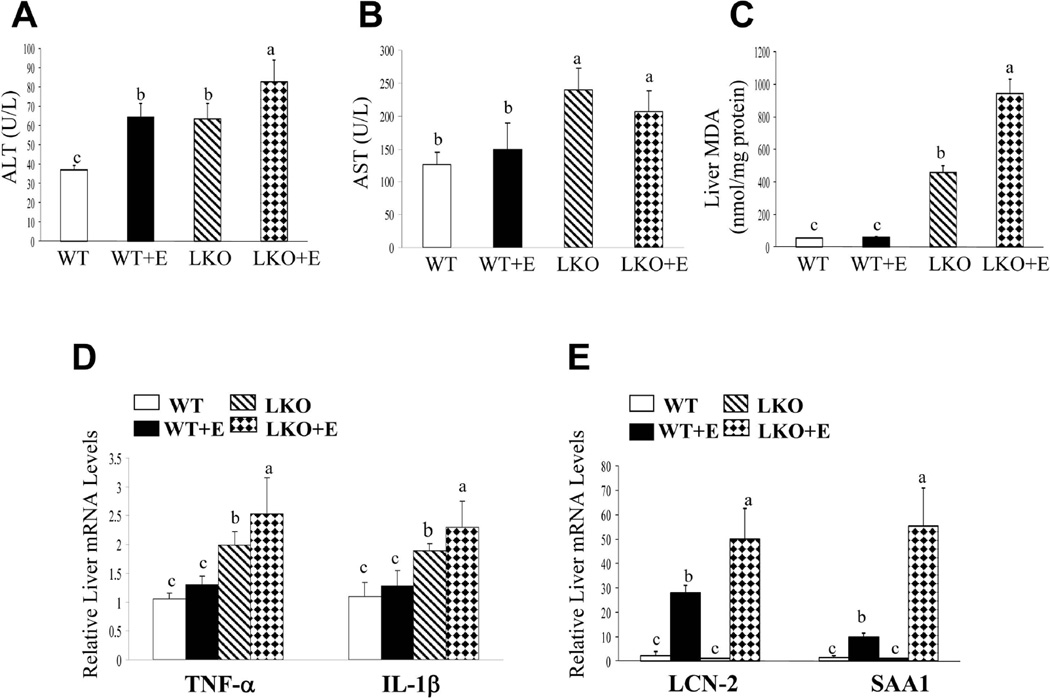
Fig. 3.
Hepatic deletion of lipin-1 leads to hepatic inflammation in mice. Mice were fed as described in Fig.1. (A) Serum ALT levels. (B) Serum AST levels. (C) Hepatic malondialdehyde (MDA) levels. (D) Hepatic relative mRNA levels of TNF-α and IL-1β. (E) Hepatic relative mRNA levels of LCN-2 and SAA1. Results are expressed as means±SEM (n=4–11 mice). Means without a common letter differ, p< 0.05
Immunohistochemical staining for collagen, an indicator of liver fibrosis, revealed modestly higher levels of collagen deposition in the livers of the ethanol-fed lipin-1LKO mice compared with the livers of ethanol-fed WT mice (Fig.2C). The mRNA expression levels of early makers of hepatic fibrosis such as fibronectin and CD68 were highest in the livers of ethanol-fed lipin-1LKO mice compared to all other groups (Supporting Fig.1A). Taken together, our data clearly demonstrate that liver-specific deletion of lipin-1 exacerbates alcoholic steatohepatitis in mice.
Hepatic deletion of lipin-1 leads to hepatic inflammation in mice
Hepatic lipin-1 ablation led to significant increases in mRNA levels of two important cytokines, tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β), up to 2–3 fold in mice fed with a control diet (Fig.3D). Moreover, the magnitude of the increases in mRNA expression levels of TNFα and IL-1β were significantly greater in ethanol-fed lipin-1LKO mice than respective pair-fed controls (Fig.3D). While two pro-inflammatory molecules, lipocalin-2 (LCN-2) and serum amyloid A-1 (SAA-1) were significantly elevated in ethanol-fed WT mice compared to WT controls (Fig.3E)18–21 More strikingly, ethanol feeding to lipin-1LKO mice substantially increased the mRNA levels of LCN-2 and SAA-1 approximately 25-fold and 50-fold, respectively, compared with WT controls, and approximately 5-fold and 2-fold, respectively, compared with the ethanol-fed WT mice (Fig.3E). Accordingly, the circulating levels of LCN-2 and SAA-1 were further increase in the ethanol-fed lipin-1LKO mice (Supporting Fig.2). These observations demonstrate that lipin-1LKO mice fed with or without ethanol are prone to development of hepatic inflammation.
Chronic ethanol administration dramatically exacerbates oxidative stress in the liver of ethanol-fed lipin-1LKO mice
Ethanol administration only slightly induced oxidative stress in WT mice, as demonstrated by the levels of hepatic malondialdehyde (MDA) (Fig.3C).17 Hepatic lipin-1 ablation led to a robust increase in the hepatic MDA levels up to nearly 8-fold in mice fed a control diet compared to WT controls (Fig.3C). Remarkably, ethanol feeding to lipin-1LKO drastically increased MDA levels approximately 16-fold compared with ethanol-fed WT mice, and approximately 2-fold compared with lipin-1LKO fed with control diet (Fig.3C). The data demonstrate that removal of lipin-1 generates oxidative stress in the liver, and the oxidative stress is further augmented in response to ethanol administration in lipin-1LKO mice.
Liver-specific deletion of lipin-1 augments ethanol-mediated activation of two major inflammatory regulators, nuclear Factor Kappa B (NF-kB) and nuclear factor of activated T cells c4 (NFATc4), in mice
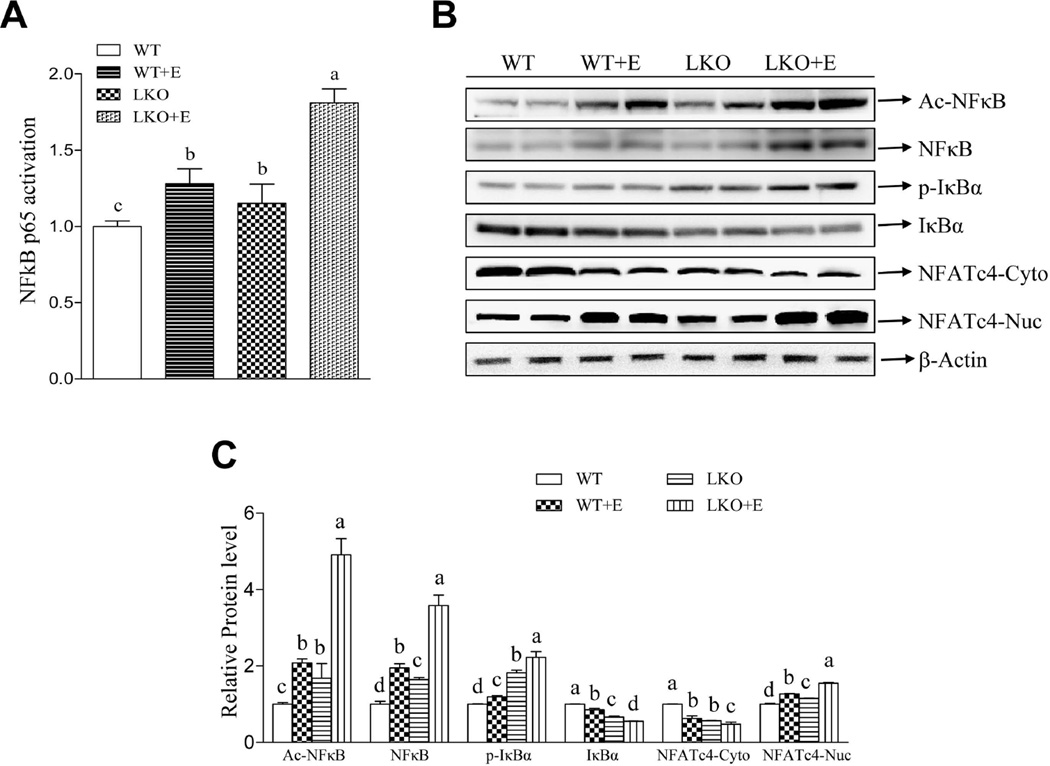
We dissected the mechanism for lipin-1 function in mediating hepatic inflammatory process by investigating two major inflammatory regulators, NF-kB and NFATc4.22,23 Ethanol feeding to WT mice or deletion of hepatic lipin-1 stimulated NFκB activity, demonstrated by increased acetylated NFκB, enhanced phosphorylated IκBα, reduced IκBα protein and elevated NF-κB DNA binding activity compared to WT control mice (Fig.4). The activation of NF-κB was significantly augmented in the livers of ethanol-fed lipin-1LKO mice compared to all other groups (Fig.4).
Fig. 4.
Liver-specific deletion of lipin-1 augments ethanol-mediated activation of two major inflammatory regulators, NF-kB and NFATc4, in mice. Mice were fed as described in Fig.1. (A) Hepatic NFκB binding activity. (B) Representative Western blot analysis of hepatic acetylated NFκB (Ac-NFκB), NFκB, phosphorylated IκBα (p-IκBα), IκBα, and NFATc4. (C) Hepatic relative protein levels. Results are expressed as means±SEM (n=4–11 mice). Means without a common letter differ, p< 0.05
Ethanol feeding significantly increased nuclear accumulation of NFATc4 and decreased the amount of NFATc4 in the cytoplasm in WT mice, and the increased nucleocytoplasmic shuttling of NFATc4 was more pronounced in ethanol-fed lipin-1LKO mice (Fig.4).23,24
Collectively, these results clearly suggesting that deletion of lipin-1 led to activation of both NFκB and NFATc4 and subsequently exacerbated inflammation in ethanol-fed lipin-1LKO mice.
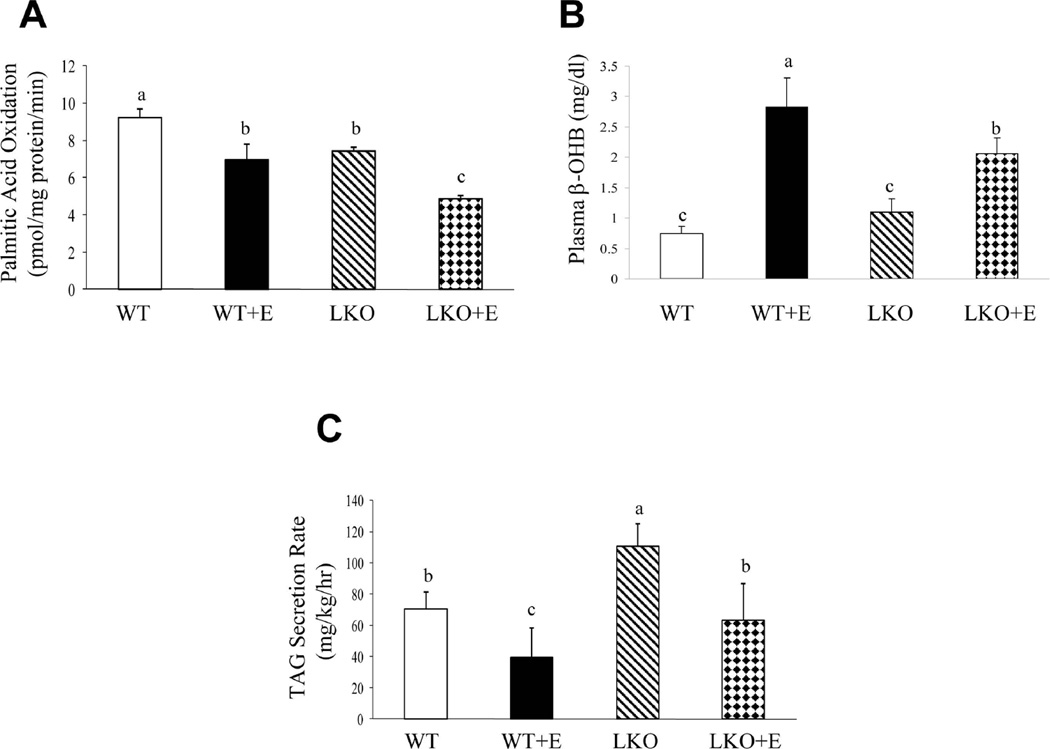
Hepatic removal of lipin-1 potentiates the perturbation of the rate of hepatic fatty acid β-oxidation and VLDL-TAG secretion in mice fed ethanol
We assessed the total hepatic fatty acid β-oxidation capacity and VLDL-TAG secretion in WT and Lipin-1LKO mice fed ethanol. The rate of hepatic fatty acid oxidation was significantly decreased in the lipin-1LKO mice fed with ethanol compared to all other groups (Fig.5A). Accordingly, ethanol feeding modestly but significantly reduced serum β-hydroxybutyrate (β-OHB), a marker for hepatic fatty acid oxidation, in lipin-1LKO mice compared with ethanol-fed WT mice (Fig.5B).
Fig. 5.
Hepatic removal of lipin-1 potentiates the perturbation of the rate of hepatic fatty acid β-oxidation and VLDL-TAG secretion in mice fed ethanol. Mice were fed as described in Fig.1. (A) Rate of hepatic fatty acid β-oxidation. (B) Serum β-OHB levels. (C) Rate of hepatic VLDL secretion. Results are expressed as means±SEM (n=4–11 mice). Means without a common letter differ, p< 0.05
Ethanol administration significantly decreased the rates of VLDL-TAG secretion in the livers of WT mice compared with WT controls (Fig.5C).25 VLDL-TAG secretion rates were significantly increased in lipin-1LKO mice fed a control diet compared with WT controls.12 Interestingly, ethanol feeding largely abolished the increase in VLDL-TAG secretion in lipin-1LKO mice (Fig.5C).
Taken together, our results demonstrate that genetic removal of hepatic lipin-1 deranges the overall rate of fatty acid oxidation and VLDL-TAG secretion, and these impairments are further aggravated in response to ethanol administration in lipin-1LKO mice.
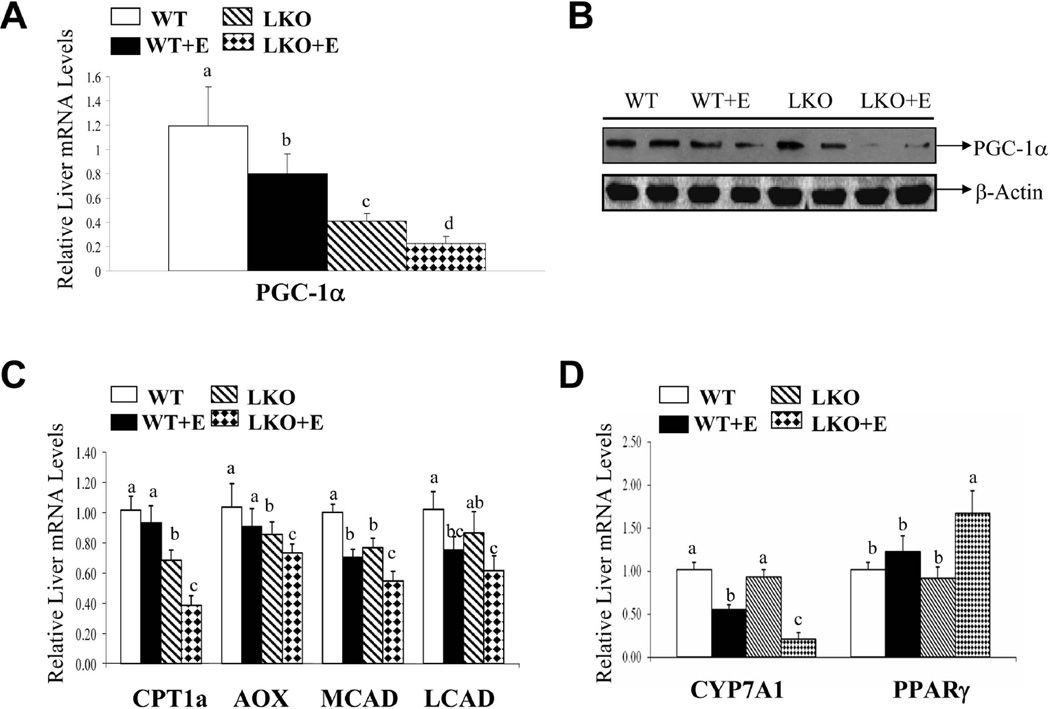
Chronic ethanol ingestion augments the suppression of PGC-1α in livers of lipin-1LKO mice
We investigated the effect of chronic alcohol administration and lipin-1 deficiency on PGC-1α.26,27,29 The mRNA and protein expression levels of PGC-1α were significantly reduced in lipin-1LKO mice on control diet compared to WT littermates (Fig.6A,B). Liver mRNA levels of PGC-1α were also significantly reduced in WT mice after ethanol feeding and depletion of hepatic lipin-1 greatly exacerbated the inhibitory effects of ethanol on PGC-1α (Fig.6A,B; Supporting Fig. 1B).
Fig. 6.
Chronic ethanol ingestion augments the suppression of PGC-1α and its target genes in livers of lipin-1LKO mice. Mice were fed as described in Fig.1. (A) Relative mRNA levels of PGC-1α. (B) Representative Western blot analysis of hepatic PGC-1α protein expression levels. (C) Relative mRNA expression levels of CPT1a, AOX, MCAD, LCAD. (D) Relative mRNA levels of CYP7A1 and PPARγ. Results are expressed as means±SEM (n=4–11 mice). Means without a common letter differ, p< 0.05
Ethanol feeding to lipin-1LKO mice substantially suppressed mRNAs of carnitine palmitoyltransferase 1a (CPT1a), acyl-CoA oxidase (AOX), mitochondrial medium-chain acyl-CoA dehydrogenase (MCAD) and mitochondrial long-chain acyl-CoA dehydrogenase (LCAD) compared with respective controls or ethanol-treated WT mice (Fig.6C). Additionally, ethanol feeding significantly increased hepatic PPARγ mRNA expression in WT mice, and this increase was more pronounced in lipin-1LKO mice after ethanol administration (Fig.6D). The mRNA levels of Cyp7A1, a PGC-1α target gene,28 were markedly decreased by ethanol administration to WT mice and further significantly reduced in ethanol-fed lipin-1LKO mice compared to all other groups (Fig.6D).
Together, these data suggest that liver-specific lipin-1 deficiency disrupts the hepatic lipin-1-PGC-1α complex activity and leads to impaired capacity for fatty acid and cholesterol catabolism.
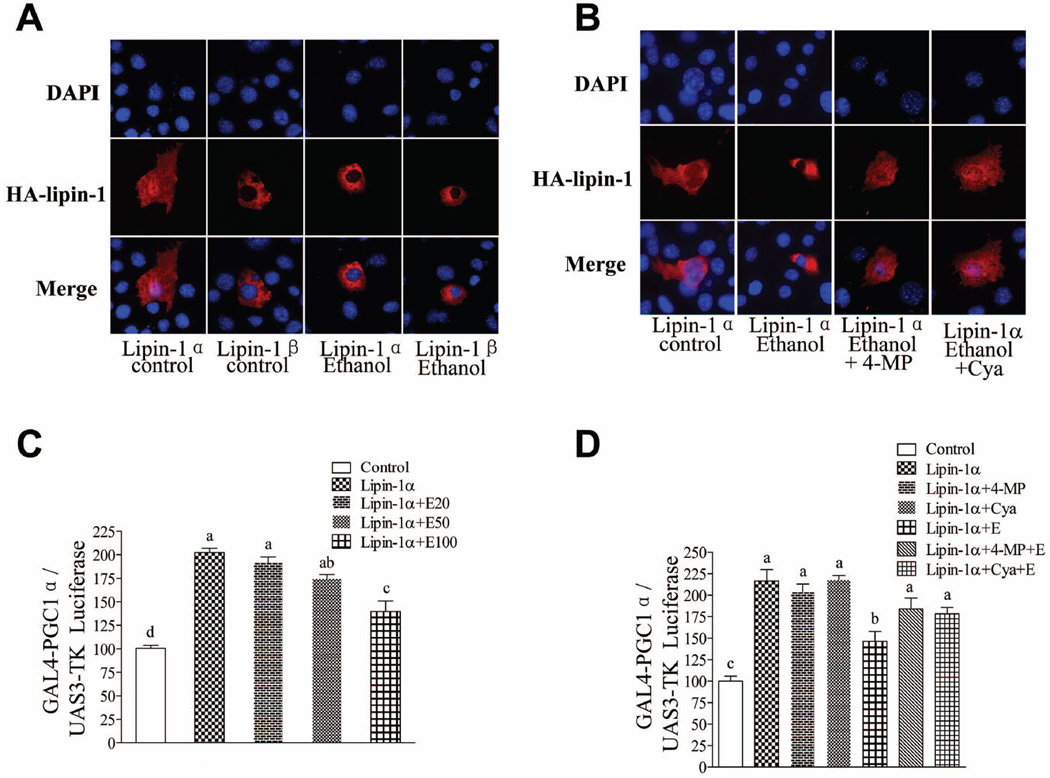
Ethanol perturbs hepatic lipin-1 signaling in mouse AML-12 hepatocytes
We further dissected the mechanisms by which ethanol exposure disrupts nuclear lipin-1 signaling and causes fat accumulation in cultured mouse AML-12 hepatocytes. Immunofluorescent staining of nuclei (blue, DAPI staining) and lipin-1(red) confirmed that lipin-1α was localized in both the cytoplasm and the nucleus. Lipin-1β was also found exclusively in the cytoplasm, and its sub-cellular localization was not affected by ethanol exposure (Fig.7A).14 Ethanol exposure sequestered lipin-1α to the cytosol (Fig.7A)9,14 Treatment with either 4-methylpyrazole (4-MP) (an ADH inhibitor) or cyanamide (Cya) (an ALDH2 inhibitor) essentially blocked the ability of ethanol to interfere with lipin-1α signaling, indicating that ethanol metabolism is required (Fig.7B).
Fig. 7.
Ethanol perturbs hepatic lipin-1 signaling in mouse AML-12 hepatocytes. (A)&(B) AML-12 cells were transfected with expression vector driving expression of HA-lipin-1α, HA-lipin-1β or control vector in the presence or absence of 4-methylpyrazole (4-MP), cyanamide (Cya) or ethanol (E) (100 mM) for 18 h. The cells were subjected to immunostaining with anti-HA (lipin-1) antibody (red), and nuclei were stained with DAPI (blue). Representative photomicrographs of HA-lipin-1 (red) or DAPI (blue) immunofluorescence in AML-12 cells. Original magnification×200. (C)&(D) AML-12 cells were transfected with plasmids for (UAS)3-TK Luciferase and expression vector driving expression of a Gal4-PGC-1α fusion protein along with control vector or HA-lipin-1α in the presence or absence of ethanol (E) or inhibitors for 24 h. Cells then were harvested for assay of luciferase activity. All data are means±SEM from at least 3 experiments. Means without a common letter differ, p< 0.05.
Ethanol significantly abolished the increase in PGC-1α co-transcriptional activity mediated by lipin-1α in a dose-dependent manner in AML-12 cells (Fig.7C). Again, Treatment with either 4-MP or Cya largely abolished the ability of ethanol to interfere with lipin-1α signaling (Fig.7C).
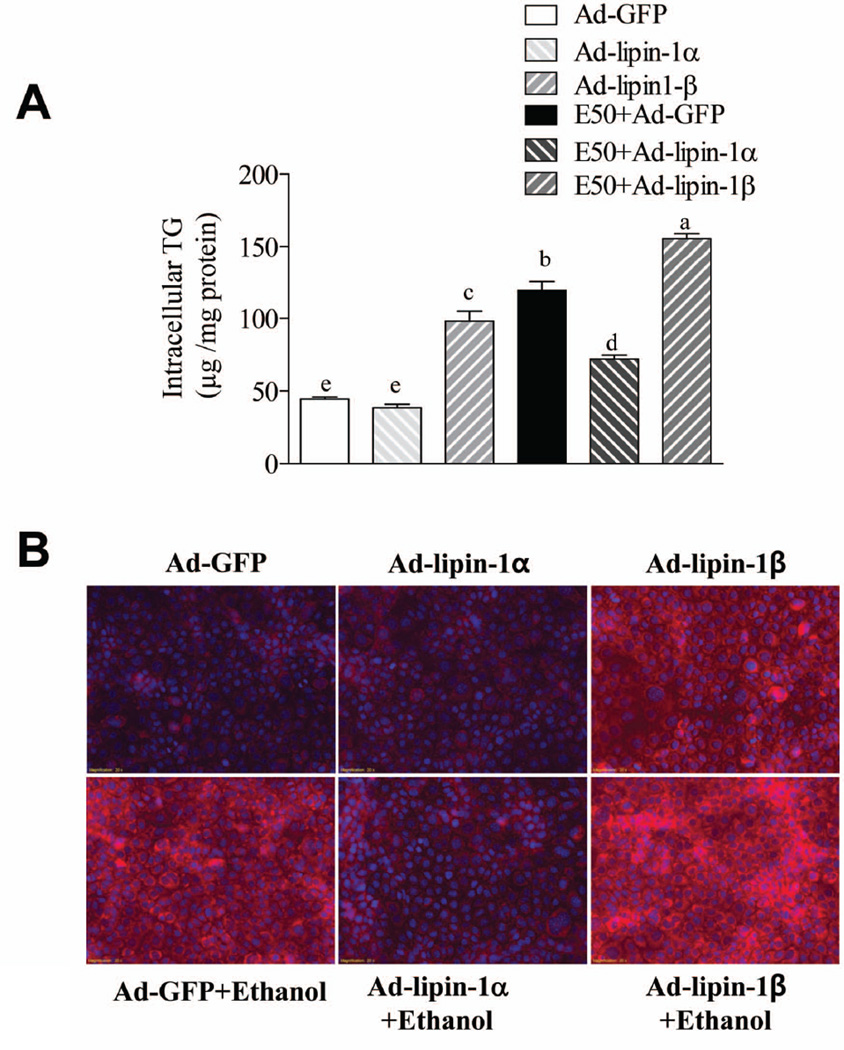
Ethanol or overexpression of lipin-1β significantly increased the TG accumulation in AML-12 cells compared with controls and lipin-1β overexpression also mildly enhanced ethanol-mediated TG accumulation (Fig.8).13,14 Importantly, the ethanol-mediated fat accumulation was largely prevented in Ad-lipin-1α-overexpressing AML-12 cells compared to Ad-GFP controls. Collectively, these data, taken with the results of lipin-1LKO mouse studies, suggest that while lipin 1 is not required for alcohol-induced steatosis in mice, lipin-1β may enhance ethanol-induced fat accumulation. On the other hand, lipin 1α, which is more nuclear, may suppress lipid accretion possibly by enhancing fatty acid catabolism and loss of this activity may enhance the development of steatosis in the lipin-1LKO mice.
Fig. 8.
Overexpression of lipin-1α alleviates ethanol-mediated TG accumulation in AML-12 cell. AML-12 cells were infected with AD-GFP, Ad-lipin-1α, Ad-lipin-1β. 48 h after infection, ethanol (50 mM) was added. After incubation for 48 h, cellular TG content was measured by a cellular enzymatic kit. (B) cellular TG content was measured by Nile Red Staining. All data are means±SEM from at least 3 experiments. Means without a common letter differ, p< 0.05.
Discussion
Intrahepatic lipid accumulation plays a pathogenic role in liver injury in response to chronic ethanol exposure.1 Lipin-1 plays an important role in regulating lipid metabolism via its cytoplasmic and nuclear effects.5–7 In the present study, we provide evidence demonstrating that hepatic lipin-1 deficiency led to dramatically pronounced changes in terms of steatosis, inflammation, and fibrosis in response to chronic ethanol administration compared to WT mice. This suggests that the induction in lipin-1 in alcoholic fatty liver disease may play a protective role by limiting inflammation, promoting efficient lipid storage, and/or controlling the transcriptional regulation of fatty acid catabolism. Correlating closely with the rapid onset and progression of steatosis and inflammation, hepatic PGC-1α abundance was found to be severely diminished in ethanol-fed lipin-1LKO mice leading to reduced expression of several PGC-1α target genes encoding fatty acid oxidation enzymes, decreased rates of hepatic fatty acid oxidation, reduced generation of ketone bodies, and impaired VLDL secretion. These findings can be interpreted to suggest that the loss nuclear lipin-1 leads to these deleterious effects since lipin-1 is known to regulate these processes at the transcriptional level.5–7,10 Indeed, lipin 1α overexpression suppressed alcohol-induced TG accumulation potentially by transcriptionally activating fatty acid catabolism. However, it is possible that loss of lipin-1 enzymatic activity may somehow be affecting signaling pathways that lead to PGC-1α deactivation. For example, adipocyte-specific lipin 1 deletion led to impaired cAMP signaling in that cell type,15 and this pathway seems to be very important for regulation of PGC-1α in liver. Altogether, our results demonstrate that genetic ablation of hepatic lipin-1 aggravates experimental alcohol-induced steatohepatitis in mice.
A marked increase in hepatic PAP activity has long been known to occur in alcoholic fatty liver in animals and humans.2–4,9 We have previously shown that lipin-1 is strongly induced in ethanol-induced fatty liver in mice and we sought to determine whether loss of lipin-1 would attenuate the increased PAP activity and intrahepatic triglyceride accumulation in response to ethanol feeding. Interestingly, our present study showed that removal of lipin-1 from the liver effectively abolished the increase in hepatic PAP activity caused by ethanol, but dramatically exacerbated ethanol-induced fatty liver in mice. Studies have demonstrated that fld mice display liver steatosis partly due to increased hepatic lipin-2-mediated PAP activity.6 Our data, however, suggest that it is unlikely that lipin-2-mediated PAP activity contributes to aggravated fatty liver in ethanol-fed lipin-1LKO mice because the hepatic total PAP activity was largely abolished. It is possible that the residual activity is sufficient to maintain high rates of TG synthesis and that the accumulation of TG is a function of reduced hepatic mobilization of fatty acids and catabolism of these metabolites in the mitochondrial β-oxidation pathway. Indeed, we have recently shown that lipolytic rates are reduced in adipose tissue of mice lacking lipin-1 in adipocytes.15
Nucleo-cytoplasmic localization of lipin-1 is a vital factor in the regulation of lipin-1 function as either a PAP enzyme or a transcriptional co-activator.6,7 Among the enzymes involved in a number of lipid metabolism pathways we have examined (Supporting Fig.2), the major altered signaling molecule that most closely associated with fatty liver in ethanol-fed lipin-1LKO mice was PGC-1α. PGC-1α is a pivotal regulator of lipid metabolism through interacting with various transcriptional factors.26,27 Genetic ablation of lipin-1 not only caused loss of PAP activity but also diminished the nuclear levels of lipin-1 in mouse livers and the drastic liver responsiveness to ethanol administration in lipin-1LKO mice may be due to loss of nuclear lipin-1 function and impaired capacity for fatty acid catabolism. Indeed, we found that removal of lipin-1 led to exacerbated inhibition of a panel of enzymes involved in fatty acid oxidation and augmented impairment of fatty acid oxidizing capacity in the livers of ethanol-fed mice. The alterations in the rate of fatty acid oxidation in ethanol-fed lipin-1LKO mice may suffice to elicit profound hepatic fat accumulation.
Induction of PGC-1α in cultured hepatic cells or in mice inhibits TG synthesis and stimulates VLDL-TG secretion, which, in turn, attenuates high fat diet-induced hepatic steatosis in mice.29 Interesting, while the precise role of lipin-1 in promoting or attenuating hepatic VLDL secretion is still controversial, the effects of lipin-1 on VLDL-TG secretion is dependent on its nuclear signaling and independent of its PAP activity.12 Therefore, it is logical to speculate that impairment of the lipin-1-PGC-1α axis may induce TG synthesis and suppress VLDL-TG secretion, ultimately leading to excess fat accumulation in the livers of ethanol-fed lipin-1LKO mice.
Another intriguing discovery was that hepatic lipin-1 ablation markedly increased the expression of pro-inflammatory cytokines and caused hepatic oxidative stress in mice fed with or without ethanol, suggesting that endogenous lipin-1 has potent anti-inflammatory properties. Recent accumulating evidence indicates an essential role of lipin-1 in the regulation of the inflammatory process.23,30 For instance, in adipocytes, lipin-1 interacts with NFATc4 to repress NFATc4 transcriptional activity, which in turn suppresses pro-inflammatory cytokines such as TNF-α. Interestingly, we found that loss of hepatic lipin-1 resulted in the activation of both NF-κB and NFATc4 in mice, suggesting a possible cross-talk between the NFATc4 and NF-κB pathways regulated by lipin-1. On the other hand, the exacerbated inflammatory response could just be secondary to increased hepatic lipid accumulation in ethanol-fed lipin-1LKO mice. Up-regulation of hepatic pro-inflammatory cytokines and excess production of reactive oxygen species (ROS) in lipin-1LKO mice are likely contribute to markedly elevated serum makers of liver injury. Additional studies evaluating the ability of lipin-1 to repress the activity of transcriptional factors such as NFATc4 or NF-κB and to attenuate the production of cytokines or ROS in response to LPS or ethanol in Kupffer cells are currently under investigation in our laboratory.
The Lieber-DeCarli liquid diets enriched in polyunsaturated fat promotes ethanol-induced liver injury in rodents.1 Our present study has utilized a modified Lieber-DeCarli low fat ethanol containing liquid diet.17 It is possible that hepatic lipin-1 may be influenced by dietary fat and composition. We are current investigating the effects of dietary fat and composition on ethanol-mediated impairments of lipin-1.
The present study demonstrates that ethanol metabolism via ADH and ALDH2 induces nucleocytoplasmic shuttling of lipin-1α, inhibiting PGC-1α activity and causing fat accumulation in cultured hepatocytes. These in vitro findings further support the notion that depletion of hepatic nuclear lipin-1 in lipin-1LKO mice may largely contribute to the drastic liver responsiveness to ethanol challenge. The role of hepatic ethanol metabolism-induced production of metabolites, redox state shift or reactive oxygen species in regulation of lipin-1α nucleocytoplasmic shuttling merits investigation.
In summary, using liver specific lipin-1-null mice fed an ethanol containing diet, we demonstrated for the first time that liver-specific deletion of lipin-1 leads to the rapid onset and progression of alcoholic steatohepatitis, providing novel insights into the biological function of lipin-1 in alcoholic steatohepatitis. Our present findings suggest that the development of nutritional or pharmacological agents to enhance nuclear lipin-1 activity could be a promising approach toward developing new options for the prevention and treatment of human alcoholic steatohepatitis.
Supplementary Material
Acknowledgments
GRANTS
This study was supported by the National Institute on Alcoholism and Alcohol Abuse (grants AA015951 and AA013623 to M.Y. and AA021228 to B.F.) and the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK078187 to B.F.).
Abbreviations used in the paper
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AFLD
alcoholic fatty liver disease
- AMPK
AMP-activated protein kinase
- ACC
acetyl-CoA carboxylase
- AOX
acyl-CoA oxidase
- β-OHB
beta-hydroxybutyate
- Cya
cyanamide
- CYP7A1
cholesterol 7α-hydroxylase
- CPT1a
carnitine palmitoyltransferase 1a
- DAG
diacylglycerol
- FAS
fatty acid synthase
- Fld
fatty liver dystrophic
- LCAD
mitochondrial long-chain acyl-CoA dehydrogenase
- GPAT1
mitochondrial glycerol-3-phosphate acyltransferase
- LCN-2
lipocalin-2
- MP
methylpyrazole
- MCAD
mitochondrial medium-chain acyl-CoA dehydrogenase
- MDA
malondialdehyde
- NF-kB
nuclear Factor Kappa B
- NFATc4
nuclear factor of activated T cells c4
- PPAR
peroxisome proliferator-activated receptor
- PGC-1α
peroxisome proliferator-activated receptor γ co-activator-1alpha
- PA
phosphatidic acid
- ROS
reactive oxygen species
- SAA-1
serum amyloid A-1
- SIRT1
sirtuin 1
- SREBP-1
sterol regulatory element-binding protein 1
- SCD1
stearoyl-coenzyme A desaturase 1
- TBARS
thiobarbituric reactive substances
- TNFα
tumor necrosis factor-alpha
- IL-1β
interleukin-1beta
- TAG
Triacylglyceride
- VLDL
very low density lipoproteins
Footnotes
No Conflicts of Interest Exist
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brindley DN, Cooling J, Burditt SL, Pritchard PH, Pawson S, Sturton RG. The involvement of glucocorticoids in regulating the activity of phosphatidate phosphohydrolase and the synthesis of triacylglycerols in the liver. Effects of feeding rats with glucose, sorbitol, fructose, glycerol and ethanol. Biochem J. 1979;180:195–199. doi: 10.1042/bj1800195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savolainen MJ, Baraona E, Pikkarainen P, Lieber CS. Hepatic triacylglycerol synthesizing activity during progression of alcoholic liver injury in the baboon. J Lipid Res. 1984;25:813–820. [PubMed] [Google Scholar]
- 4.Simpson KJ, Venkatesan S, Martin A, Brindley DN, Peters TJ. Activity and subcellular distribution of phosphatidate phosphohydrolase (EC 3.1.3.4) in alcoholic liver disease. Alcohol Alcohol. 1995;30:31–36. [PubMed] [Google Scholar]
- 5.Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci. 2006;31:694–699. doi: 10.1016/j.tibs.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 7.Harris TE, Finck BN. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol Metab. 2011;22:226–233. doi: 10.1016/j.tem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Z, Liang X, Rogers CQ, Rideout D, You M. Involvement of adiponectin-SIRT1-AMPK signaling in the protective action of rosiglitazone against alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G364–G374. doi: 10.1152/ajpgi.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu M, Wang F, Li X, Rogers CQ, Liang X, Finck BN, et al. Regulation of hepatic lipin-1 by ethanol: role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology. 2012;55:437–446. doi: 10.1002/hep.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z, Gropler MC, Norris J, Lawrence JC, Jr, Harris TE, Finck BN. Alterations in hepatic metabolism in fld mice reveal a role for lipin 1 in regulating VLDL-triacylglyceride secretion. Arterioscler Thromb Vasc Biol. 2008;28:1738–1744. doi: 10.1161/ATVBAHA.108.171538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bou Khalil M, Sundaram M, Zhang HY, Links PH, Raven JF, Manmontri B, et al. The level and compartmentalization of phosphatidate phosphatase-1 (lipin-1) control the assembly and secretion of hepatic VLDL. J Lipid Res. 2009;50:47–58. doi: 10.1194/jlr.M800204-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Hu M, Zhang R, Shen Z, Flatow L, You M. MiR-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J Biol Chem. 2012;287:9817–9826. doi: 10.1074/jbc.M111.333534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra MS, Chen Z, Ren H, Harris TE, Chambers KT, Hall AM, et al. Mice with an adipocyte-specific lipin 1 separation-of-function allele reveal new roles for phosphatidic acid in metabolic regulation. Proc Natl Acad Sci U S A. 2013;110:642–647. doi: 10.1073/pnas.1213493110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadra K, de Preux Charles AS, Médard JJ, Hendriks WT, Han GS, Grès S, et al. Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 2008;22:1647–1661. doi: 10.1101/gad.1638008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Effect of chronic ethanol consumption on the expression of complement components and acute-phase proteins in liver. Clin Immunol. 2007;124:213–220. doi: 10.1016/j.clim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Chan YR. Lipocalin 2 regulation and its complex role in inflammation and cancer. Cytokine. 2011;56:435–441. doi: 10.1016/j.cyto.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Pruett BS, Pruett SB. An explanation for the paradoxical induction and suppression of an acute phase response by ethanol. Alcohol. 2006;39:105–110. doi: 10.1016/j.alcohol.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000;7:64–69. doi: 10.1097/00062752-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 22.You M, Flick LM, Yu D, Feng GS. Modulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. J Exp Med. 2001;193:101–110. doi: 10.1084/jem.193.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HB, Kumar A, Wang L, Liu GH, Keller SR, Lawrence JC, Jr, Finck BN, Harris TE. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol Cell Biol. 2010;30:3126–3139. doi: 10.1128/MCB.01671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang TT, Yu RY, Agadir A, Gao GJ, Campos-Gonzalez R, Tournier C, Chow CW. Integration of protein kinases mTOR and extracellular signal-regulated kinase 5 in regulating nucleocytoplasmic localization of NFATc4. Mol Cell Biol. 2008;28:3489–3501. doi: 10.1128/MCB.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharbanda KK, Todero SL, Ward BW, Cannella JJ, 3rd, Tuma DJ. Betaine administration corrects ethanol-induced defective VLDL secretion. Mol Cell Biochem. 2009;327:75–78. doi: 10.1007/s11010-009-0044-2. [DOI] [PubMed] [Google Scholar]
- 26.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;29:S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884S–889S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Norris JY, Finck BN. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) stimulates VLDL assembly through activation of cell death-inducing DFFA-like effector B (CideB) J Biol Chem. 2010;285:25996–26004. doi: 10.1074/jbc.M110.141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi N, Yoshizaki T, Hiranaka N, Suzuki T, Yui T, Akanuma M, et al. Suppression of lipin-1 expression increases monocyte chemoattractant protein-1 expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2011;415:200–205. doi: 10.1016/j.bbrc.2011.10.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.