Abstract
OBJECTIVES
The value of gastroesophageal reflux disease (GERD) indicators (acid exposure time (AET), symptom association probability (SAP), and symptom index (SI)) in predicting therapeutic success in noncardiac chest pain (NCCP) has not been systematically evaluated in outcome studies.
METHODS
We retrospectively identified 98 subjects with NCCP (age 51.8±1.2 years, 75 women, mean duration of symptoms 7.3±0.4 years) who underwent pH monitoring off antireflux therapy. Distal esophageal AET (abnormal if ≥4.0%), SAP (measured as Ghillibert probability estimate, abnormal if P < 0.05), and SI (abnormal if ≥50%) were calculated; symptom severity and change after therapy were assessed on a 10-point Likert scale. Subjects were interviewed 2.8±0.9 years after the pH study to determine the degree of symptom change (high-degree response (HDR), with definite, sustained symptom improvement) after antireflux therapy. Regression analysis was used to determine the independent predictors of HDR.
RESULTS
GERD indicators were present in 61 subjects (62.2%); 52 subjects (53.1%) had abnormal AET, 26 (26.5%) had positive SAP, and 25 (25.5%) had positive SI. With therapy, mean symptom scores improved from 6.3±0.3 at the time of the pH study to 2.9±0.3 at the time of interview (P < 0.001). A total of 58 subjects (59.2%) achieved HDR, and another 29.6% had moderate symptom improvement. On univariate analysis, HDR was associated with positive SAP (P = 0.003) and elevated AET (P = 0.015) but not with demographics, SI, or esophageal motor pattern. In regression analysis containing demographics, GERD indicators, psychiatric comorbidity, and esophageal motor pattern, positive SAP was retained as a significant predictor of HDR (P = 0.003); elevated AET trended toward significance (P = 0.055). Frequency of HDR was highest when subjects had all three GERD parameters abnormal (93.3% HDR) or both elevated AET and positive SAP (88.2% HDR, P < 0.001 compared with only one or no GERD parameter abnormal).
CONCLUSIONS
Positive statistical tests of symptom association predict the therapeutic success of GERD management in NCCP. When used hierarchically, response to antireflux therapy is best predicted when GERD parameters are all abnormal and poorest when parameters are normal. These results support the importance of GERD, the relevance of symptom association testing during ambulatory pH monitoring, and the value of intensive antireflux therapy in NCCP.
INTRODUCTION
Noncardiac chest pain (NCCP) is the most common atypical clinical manifestation of gastroesophageal reflux disease (GERD). Reflux events account for symptoms in as many as 50% with recurrent NCCP (1–3). The underlying mechanisms are incompletely understood, but may involve hypersensitivity to intraesophageal stimuli and altered cerebral perception of esophageal sensory input (1). Identification of a GERD-mediated etiology for NCCP is thought to reduce repeated testing for alternate etiologies, hence ameliorating patient concerns, improving global well-being, and decreasing functional disability (3, 4).
As the prevalence of endoscopically visible esophagitis is low (< 20%) even in treatment-naive patients with NCCP, ambulatory pH monitoring is often the test of choice in these patients, particularly when no alarm features are identified (2, 5, 6). Ambulatory pH monitoring may implicate GERD as the etiology for NCCP in as many as 60% of subjects with normal upper endoscopy (7). Pathological acid exposure times (AETs) suggest the presence of GERD, but temporal correlation between reflux events and chest pain is required to attribute GERD as the etiology of chest pain (5, 6). Therefore, differential levels of GERD evidence exist, the combination of an abnormal AET and symptom association testing intuitively offering the best evidence for a GERD association. We previously proposed a hierarchical approach for the evaluation of GERD evidence, and suggested that the combination of abnormal AET and positive symptom association probability (SAP) offers a higher value to ascribing a GERD etiology to NCCP than either parameter alone (8). Furthermore, within this patient cohort, a positive symptom index (SI) may identify patients with the highest likelihood for symptomatic improvement with antireflux therapy, as suggested by our findings in a cohort of patients with chronic cough in the setting of GERD (9). However, despite general enthusiasm with regard to the use of ambulatory pH monitoring in this setting, the value of esophageal physiological parameters in ascribing GERD as the etiology of esophageal symptoms has been called into question (5, 10). Moreover, no study has evaluated the effectiveness of NCCP therapy guided by pretreatment ambulatory pH testing in a real-world setting.
The objective of this retrospective cohort study was to identify the clinical and esophageal physiological parameters that best predict long-term treatment outcomes in patients with NCCP. A secondary goal was to evaluate the value of AET, SAP, and SI, alone and in combination, in predicting response to antireflux therapy.
METHODS
Adult outpatients (≥18 years) referred for ambulatory pH monitoring for the evaluation of unexplained chest pain at Washington University in St. Louis over a 4-year period (2003–2006) were eligible for study inclusion. Study subjects were identified by interrogating the computerized esophageal physiology database at our clinical facility and extracting records of patients who underwent pH testing while off acid suppression for evaluation of NCCP; cardiac causes were excluded in all instances before referral. Subjects were excluded if they had undergone antireflux surgery, if chest pain was not the dominant symptom studied, if pH data were incomplete, or if subjects declined to participate in the follow-up telephone interview. Demographics, presenting symptoms, and clinical characteristics were extracted from electronic medical records. Eligible subjects were then contacted by telephone and interviewed to determine their treatment strategy and outcome by one of the study authors (VK) who was not directly involved in patient testing or management decision-making. The review of clinical data and telephone interviews for the purpose of this study were approved by the institutional review board at the Washington University School of Medicine.
Placement of pH probes was performed at the outpatient endoscopy and motility facility of Barnes-Jewish Hospital, St. Louis, Missouri. Subjects were asked to discontinue proton pump inhibitors (PPIs) at least 7 days before and histamine-2 receptor antagonists, prokinetic agents, and antacids at least 3 days before the pH study. Subjects completed a questionnaire detailing the frequency and severity of symptoms, and rated their overall symptomatic state about their NCCP on a 10 cm visual analog scale (0 = no pain and 10 = disabling pain interfering with activities of daily living). After discharge from the endoscopy facility, all subjects were instructed to resume normal activity and diet; maintain a daily diary that included symptoms, activities, and meal periods; and activate the symptom indicator button of the pH recorder every time they experienced chest pain.
Analyses of pH data included quantification of AET, determination of reflux–symptom association through probability testing, and calculation of SI. AET was defined as the percentage of time that the esophageal pH remained below 4 in the distal esophagus. When the wireless pH probe was used, averaged 1-day data were generated to conform to that obtained from catheter-based pH studies. An AET value ≥4% for the complete study was used to define increased acid exposure. The Ghillibert probability estimate (GPE) was used for symptom association testing, and a P value < 0.05 was required for significance (8, 11, 12). Similar to the SAP calculated by the Weusten method (13), this symptom association test determines the likelihood that symptoms and acid reflux events co-occur solely by chance. The overall likelihood is calculated as a sum of partial probabilities for exact numbers of reflux-associated symptoms within the context of the total number of symptoms, the proportion of time “at risk” for linking a symptom to a low pH value, and the total recording time (8, 11, 12). The SI was calculated as the proportion of chest pain symptoms (pH < 4) divided by the number of chest pain episodes recorded, and was expressed as a percentage (14). A value of ≥50% was considered to be indicative of a high proportion of reflux-associated symptoms (8, 14). Only symptoms occurring during a 2-min window after a reflux event were considered for calculation of symptom association with the GPE and the SI. Symptoms preceding a reflux event were not considered. When available, high-resolution manometry contour plots were evaluated to determine the esophageal motor pattern. Treating gastroenterologists had access to all aspects of the ambulatory pH study including symptom association data, and high-resolution manometry reports when performed.
All subjects were subsequently interviewed by an investigator not involved in the interpretation of pH or manometry data (VK). Subjects were asked about all previous GERD therapies and their response to each treatment trial. Each subject’s management was determined by their treating gastroenterologist on the basis of the results of esophageal physiological testing without influence from the study authors. The GERD treatment approaches implemented were assessed and categorized into four groups: (i) minimal medical therapy (over-the-counter antacids, intermittent, or as needed antisecretory medications), (ii) submaximal medical therapy (continuous once daily PPI use), (iii) maximal medical therapy (twice daily PPI use for ≥3 months), and (iv) antireflux surgery. Subjects were asked to quantify the change in their NCCP symptoms in response to antireflux therapy on a 10-point Likert scale (0 = no response and 10 = complete resolution of NCCP symptoms). The use of neuromodulators as primary or adjunctive therapy was also assessed. Subjects then rated their symptoms on a 10-point Likert scale (0 = no pain and 10 = disabling pain, interfering with most activities of daily living). The primary outcome was change in chest pain symptoms from baseline at the time of the pH study to the posttreatment telephone questionnaire. Comparisons were made between the degree of symptom relief between treatment strategies (acid suppression, surgery, neuromodulator therapy). After the interview, subject response to NCCP therapy was quantified into one of three groups: (i) no improvement or transient response, considered ineffective by the subject (range 0–3 on the global Likert scale); (2) partial but sustained response, wherein subjects reported improvement in symptoms but still experienced chest pain requiring additional therapy (range 4–7); and (3) definite sustained response, wherein subjects were asymptomatic or minimally symptomatic on a stable dose of medication or off medication after antireflux surgery (range 8–10). This last category with definite, sustained improvement in symptoms was termed “high-degree response” (HDR).
Data are reported as mean±s.e.m., unless otherwise indicated. Grouped continuous data were compared using two-tailed Student’s t-test; a P value of < 0.05 was required for statistical significance. Fisher’s exact test was used for small group comparisons of binomial data, and analyses of variance were used to establish whether differences across groups were statistically significant. Univariate analyses were performed to determine the association of GERD indicators (SAP, SI, and AET), demographic variables (age, sex), and comorbid conditions (pulmonary disease, coronary heart disease, anxiety, and depression) with HDR. Multivariate linear regression analysis was used to confirm the independent significance of demographic and physiological parameters. All analyses were performed using SPSS version 14.0 (SPSS, Chicago, IL).
RESULTS
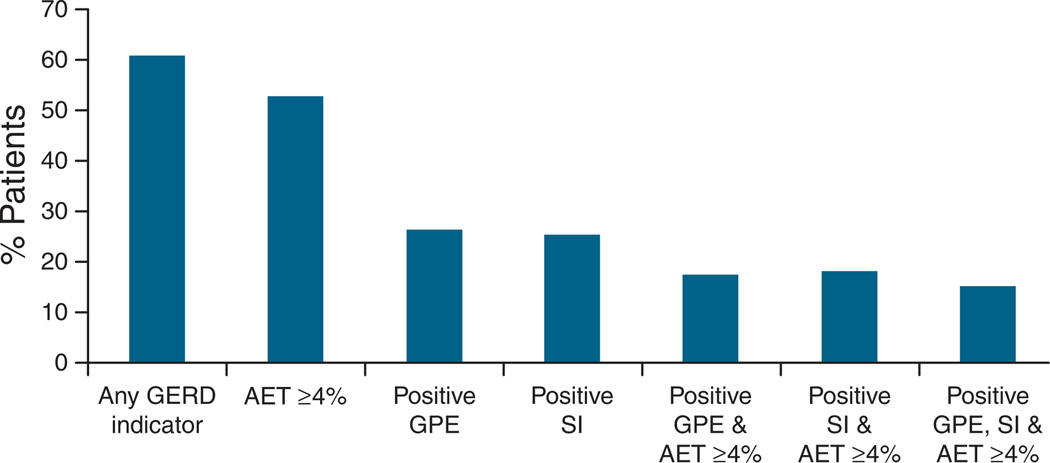
A total of 98 subjects (51.8±1.2 years, 75 women) who underwent ambulatory pH monitoring off antisecretory therapy were available for telephone interview and agreed to participate in the study (Table 1). The mean duration of symptoms was 7.4±4.1 years. Conventional catheter-based pH monitoring was used to evaluate 83 subjects (84.7%), and the remainder were evaluated with the wireless pH system. Chest pain episodes were reported by 79 subjects (80.6%) during the ambulatory pH study, and the mean number of daily symptoms was 8.3±1.3; six subjects reported only one symptom episode during the ambulatory pH study (only two had a positive SI, none had a positive GPE). A total of 61 (62.2%) subjects had at least one GERD indicator on their ambulatory pH study; 53.1% had elevated AET, 26.5% had a positive GPE, and 25.5% had a positive SI (Figure 1). A positive GPE in conjunction with elevated AET was only noted in 17.2% (17 subjects). All subjects received initial pharmacological antireflux therapy with PPIs before and after the initial pH study; 24 subjects later underwent antireflux surgery.
Table 1.
Demographics and clinical characteristics
| n | 98 |
| Age | 51.8±1.2 years |
| Gender, F/M | 75/23 |
| Duration of symptoms | 7.4± 4.1 years |
| Comorbidities | |
| Coronary disease | 12 (12.2%) |
| Mitral valve prolapse | 14 (14.3%) |
| Anxiety | 13 (13.3%) |
| Depression | 21 (21.4%) |
| Esophageal motor pattern (available in 92 subjects) | |
| Spastic pattern | 47.8% |
| Hypomotility | 16.3% |
| Normal | 35.9% |
F, female; M, male.
Figure 1.
Prevalence of indicators of GERD on ambulatory pH testing off antisecretory therapy in GERD patients. Although any one GERD indicator was noted in over 60% of subjects, a positive GPE in conjunction with abnormal AET was only noted in 17.2%. AET, acid exposure time; GERD, gastroesophageal reflux disease; GPE, symptom association probability measured as the Ghillibert probability estimate; SI, symptom index.
Subjects were contacted for the telephone interview after a mean interval of 2.8±0.9 years from the time of the ambulatory pH study. There was a broad distribution of treatments reported by the subjects, allowing for comparison of outcomes. Overall, 77.5% of subjects received maximal GERD therapy (53.1% maximal dose PPI and 24.5% antireflux surgery), the remaining 22.5% received submaximal antireflux therapy. With therapy, mean symptom scores improved from 6.3±0.3 at the time of the pH study to 2.9± 0.3 at the time of interview (P < 0.001). The subjects’ self-reported mean change in NCCP after therapy was 63.7±3.1%. The average symptom change on the 10-point Likert scale with PPI therapy was rated as 6.0±3.1, and as 8.5±2.5 with antireflux surgery. Neuromodulators were prescribed during the follow-up period to 16 subjects (16.3%) by their treating physicians for persisting symptoms. Of these, 81.3% had GERD evidence and 18.7% did not. Overall, 73.5% of subjects indicated that antireflux therapy was the most effective treatment, whereas 10.2% had the most relief from neuromodulators; 16.3% reported no sustained relief from any form of therapy.
When stratified by degree of response to therapy, 58 subjects (59.2%) achieved HDR and another 29.6% had moderate symptom improvement. In univariate analysis, HDR was associated with positive GPE (P = 0.003) and elevated AET (P = 0.015), but not SI, demographics, comorbid illness, or esophageal motor pattern. Frequency of HDR was highest in subjects who had all three GERD parameters abnormal (93.3% HDR) or both elevated AET and positive GPE (88.2% HDR); frequency was considered to be intermediate when only one parameter was abnormal (elevated AET alone: 71.4% HDR, positive GPE alone: 66.7% HDR), and lowest when neither parameter was abnormal (32% HDR, P < 0.001 across groups by analysis of variance). As demographic characteristics and medical comorbidities could have obscured the effect of GERD parameters in predicting response to antireflux therapy, a multivariate regression model was created. In logistic regression analysis containing demographics, GERD indicators, psychiatric comorbidity, and esophageal motor pattern, positive SAP was retained as a significant predictor of HDR (P = 0.003); elevated AET trended toward significance (P = 0.055) (Table 2). We further evaluated the value of SI in the light of these findings. Among the 15 subjects with elevated AET and positive GPE who achieved HDR, the SI indicated a high proportion of associated symptoms in 14 subjects (93.3%). In the remaining 43 subjects achieving HDR, SI was abnormal in only 5 subjects (11.6%), but SI was also abnormal in 6 subjects not achieving HDR (15.0%). However, of the 25 instances in which SI was abnormal, all other GERD indicators were absent in only one case. Therefore, SI seems to augment evidence for a reflux etiology for chest pain when other GERD parameters are positive.
Table 2.
Results of multivariate regression analysis using HDR as the outcome variable
| Variables used in the regression model | P value |
|---|---|
| Age | 0.59 |
| Gender | 0.61 |
| AET ≥ 4.0% | 0.055 |
| Positive SI | 0.15 |
| Positive GPE | 0.003 |
| Medical and psychiatric comorbidities | 0.17 |
| Esophageal motor pattern on HRM | 0.93 |
AET, acid exposure time; GPE, Ghillibert probability estimate; HDR, high-degree response; HRM, high-resolution manometry; SI, symptom index.
Test performance characteristics were calculated for elevated AET, positive GPE, positive SI, and the combination of the three parameters (Table 3). As expected, an abnormal AET had the highest sensitivity for predicting HDR. Individual GERD parameters in general had lower specificity compared with combinations of tests. In this analysis, GPE and SI were comparable. The best performance characteristics were the specificity and negative predictive value of combinations of tests, especially when all three GERD parameters were abnormal.
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive value for HDR to antireflux therapy
| Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |
|---|---|---|---|---|
| AET ≥ 4.0 | 69 | 70 | 53 | 47 |
| Positive GPE | 36 | 88 | 27 | 74 |
| Positive SI | 33 | 85 | 26 | 74 |
| AET ≥ 4.0 and positive GPE | 26 | 95 | 17 | 83 |
| AET ≥ 4.0 and positive SI | 28 | 95 | 18 | 82 |
| AET ≥ 4.0, positive SI, and positive GPE | 24 | 98 | 15 | 85 |
AET, acid exposure time; GPE, Ghillibert probability estimate; HDR, high-degree response; SI, symptom index.
DISCUSSION
In this retrospective cohort study, we show that a positive statistical test of symptom association (GPE in this report) is an independent predictor of a high symptomatic response to therapy of NCCP, whereas elevated AET trends strongly toward being an independent predictor. The combination of a positive GPE and elevated AET on ambulatory pH testing performed off therapy identified almost 90% of subjects who were most likely to attain an HDR to antireflux therapies including antireflux surgery, with further marginal increment when all three GERD parameters (AET, GPE, and SI) were abnormal. Over 60% of our study cohort had evidence of GERD indicators on the ambulatory pH study. These results emphasize the value of ambulatory pH testing off antisecretory therapy in predicting therapeutic outcome in patients with NCCP.
In recent years, empirical PPI administration has become the initial diagnostic test to establish GERD as the etiology of NCCP, with sensitivity and specificity reported as high as 80 and 74%, respectively (15, 16), and the number needed to treat of 3 (16). In comparison, in nonselected GERD symptoms, the sensitivity of a PPI trial for a GERD diagnosis was similar at 78%, but specificity was lower at 54% (17). A proportion of NCCP patients, especially those with nonerosive reflux disease or acid sensitivity, will fail to respond to short courses of PPI (18). The risk ratio for continued symptoms after a trial of PPI therapy has been reported as 0.54 (95% CI: 0.41–0.71) in meta-analysis (16). In these instances, ambulatory esophageal pH monitoring is frequently used to determine whether GERD is the etiology of NCCP (2, 5, 6). The use of reflux–symptom association testing during ambulatory monitoring is gaining momentum, but has remained less well established compared with the detection of elevated AET during ambulatory pH testing (5, 10).
Both calculation of the simple proportion of reflux-associated symptoms (SI) and SAP testing are used in assessing reflux–symptom associations, but neither test type has been well validated in terms of its accuracy in predicting clinically important reflux–symptom relationships. We used the Ghillibert probability estimate (GPE) for determining significant reflux–symptom associations (8, 11, 12). Unlike the traditional SAP calculation using a two-by-two contingency table (13), the GPE segregates the entire study into periods of “at risk” or “not at risk” for a reflux–symptom linkage, and evaluates the proportionate distribution of symptoms within these domains. The GPE can be calculated post hoc from data routinely collected during a pH study, such as number of symptoms, number of reflux-associated symptoms, number of reflux events, amount of time pH < 4, and total recording time (12). Preliminary comparisons of symptom correlation using the traditional SAP calculation and the GPE showed a good correlation of the two tests, but the GPE tended to underestimate statistically significant associations (19). The use of GPE, therefore, does not enhance GERD diagnoses, and may have a lower sensitivity for detecting reflux–symptom associations. For these reasons, we believe that our results can be extrapolated to the use of the traditional SAP calculation by the Weusten method. We chose to use GPE for SAP calculation to maintain uniformity in symptom correlation, as the symptom–reflux information for each 2-min period was unavailable in some instances.
Recent preliminary data indicate that SAP may have significant independent ability over other clinical and physiological data to predict outcome from laparoscopic antireflux surgery in patients with a mixture of typical and atypical GERD symptoms (20). The SI was not predictive in this multivariate model, possibly because some patients with relatively low SI have more reflux-related symptoms than would be expected by chance. Others have also evaluated the use of SI in GERD-related NCCP; in a prospective study, Fass and co-workers found the prevalence of a positive SI in only 19% of patients with chest pain and documented reflux. They concluded that SI did not aid the diagnosis of GERD as the etiology of NCCP when combined with a positive AET (21). We noted a discordance in the SI and GPE in 23.3% of patients with either test positive, which likely explains why the GPE retained an independent predictability of HDR on multivariate analysis, whereas the SI did not—this discordance probably arises from low symptom reporting in which the SI can be ‘falsely’ high. However, the SI continues to have value in symptom association, especially when used hierarchically with the other two GERD parameters. In pH-impedance monitoring evaluation of atypical symptoms such as cough, the SI (calculated using a 5 min symptom window after reflux events) has been shown to be predictive of symptomatic response after antireflux surgery in a limited number of patients in one study (22).
Our results suggest that there is value in ambulatory pH testing off antisecretory therapy in subjects with NCCP. The best sensitivity and specificity for an HDR were observed with an elevated AET; whereas GPE and SI had robust specificity and a negative predictive value. There was an advantage to evaluating AET, GPE, and SI in a hierarchical manner, and this combination of abnormal tests indicated a likelihood of HDR of over 90% with antireflux therapy. These results are consistent with previous reports of the value of pathological esophageal acid exposure and SAP in predicting successful outcome after antireflux surgery in GERD patients; the subset of NCCP patients with the combination of elevated AET and positive SAP likely represents the population with the greatest likelihood of reflux-triggered chest pain (20, 23). Patients with esophageal hypersensitivity to noxious stimuli may also present with chest pain correlating with acid reflux events with a positive symptom association (typically in the absence of abnormal elevations of AET). This would explain the lower sensitivity of a positive GPE alone in predicting HDR. Despite this, a positive GPE emerged as a strong independent predictor of long-term symptom response on multivariate analysis, establishing an important role for this parameter in ambulatory pH analysis.
The implications of our study results are severalfold. First, in the evaluation of NCCP with an inadequate response to an empirical PPI trial, testing off antisecretory identifies a GERD relationship in over 60%. Although we were unable to determine the intensity or length of the PPI trial in our subjects, these results indirectly suggest a need for longer, and perhaps more aggressive, PPI trials in NCCP patients. Second, SAP testing seems to have a predictive value in determining outcome from antireflux therapy. As our group has previously suggested, SI could be used to allocate the proportionality of reflux-associated symptoms in subjects with a positive SAP; those with a higher proportion of associated symptoms having a higher likelihood of symptom response (8). Third, esophageal motor pattern did not have a direct role in predicting therapeutic outcome in subjects referred for ambulatory pH testing. Finally, we speculate that subjects with incomplete symptom relief could have a component of visceral hypersensitivity. We previously showed the value of tricyclic antidepressants as sensory neuromodulators in patients with NCCP (24). Therefore, the use of neuromodulators may have an important adjunctive role in this setting (24, 25), and indeed 10.2% of our study population rated these agents as providing the most symptomatic benefit.
Our study has some notable limitations. We used visual analog scales for initial symptom assessment, and generated Likert scales with similar parameters for the telephone interview to assess symptomatic response, which is subject to bias. The role of comorbidities in symptom production was limited to patient recall and electronic chart notes, determined on a retrospective basis. Nevertheless, all efforts were made to determine adequate cardiac workup in all subjects, particularly when coronary artery disease was reported by the subjects. We had a small number of subjects studied with wireless pH monitoring, which may have a higher yield in detecting symptom–reflux associations compared with conventional catheter-based testing in NCCP patients. In addition, we averaged AET from wireless pH studies to conform to data from catheter-based pH studies. We reported this as a valid method of assessing GERD evidence, especially in the assessment of reflux-symptom association (8, 11), but others have used ‘worst day’ data instead (26). However, on direct comparison of results between the two cohorts, no significant differences in frequency of HDR were determined (wireless pH testing, 66.6%; catheter-based testing, 57.8% P = NS). Furthermore, as this is a retrospective report, therapies offered to subjects with NCCP were not standardized. However, we were stringent in designating any deviation from long-term double-dose PPI therapy as submaximal therapy. Finally, we did not use pH-impedance testing, which could potentially uncover additional subjects with abnormal weak acid reflux events potentially correlating with chest pain episodes.
In conclusion, we show the important role of GERD in symptom production in subjects with NCCP, with a vast majority of NCCP subjects reporting lasting symptomatic benefit from GERD therapy over long-term follow-up. We establish the value of symptom association testing during ambulatory pH monitoring in predicting long-term symptomatic improvement, and identify SAP as an important factor in considering therapeutic options, given its high specificity and independent predictive value. Further prospective outcome studies are needed to address the true value of symptom association testing in NCCP, perhaps using pH-impedance testing rather than pH testing alone.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Gastroesophageal reflux disease is an important cause of noncardiac chest pain.
-
✓
The value of ambulatory pH test parameters in predicting symptomatic response is not well characterized.
WHAT IS NEW HERE
-
✓
Ambulatory pH testing off antireflux therapy is useful in noncardiac chest pain.
-
✓
A positive symptom association probability is independently predictive of a symptomatic response to antireflux therapy.
-
✓
Abnormal acid exposure time may also be predictive, and has best value when combined with positive symptom association.
ACKNOWLEDGMENTS
We acknowledge the contributions of the late Ray E. Clouse to the field of esophagology in general and to this study in particular. Dr Clouse was involved in the initial conception and design of this study, but passed away before the study was completed. We dedicate this paper to his memory.
Financial support: This study was supported in part by a grant from Mentors in Medicine, Washington University School of Medicine, St. Louis, awarded to Vladimir M. Kushnir. Mentors in Medicine provides competitive grants to internal medicine residents at the Department of Internal Medicine, Washington University School of Medicine, who wish to engage in clinical or basic research during their residency. This organization had no role in planning and/or conducting the study, collecting and/or interpreting data, or drafting the paper. All authors have reviewed and approved the final paper.
Footnotes
This research was presented in preliminary form at the Annual Meeting of the American College of Gastroenterology, Orlando, Florida, in October 2008.
CONFLICT OF INTEREST
Guarantor of the article: C. Prakash Gyawali, MD, MRCP.
Specific author contributions: Vladimir M. Kushnir: study design, data collection, analysis, and paper preparation; Gregory S. Sayuk: study design, data analysis, and paper preparation; and C. Prakash Gyawali: study concept and design, data analysis, and paper preparation.
Potential competing interests: None.
REFERENCES
- 1.Faybush EM, Fass R. Gastroesophageal reflux disease in noncardiac chest pain. Gastroenterol Clin North Am. 2004;33:41–54. doi: 10.1016/S0889-8553(03)00131-6. [DOI] [PubMed] [Google Scholar]
- 2.Vaezi MF. Review article: the role of pH monitoring in extraoesophageal gastro-oesophageal reflux diseases. Aliment Pharmacol Ther. 2006;23:40–49. doi: 10.1111/j.1365-2036.2006.02797.x. [DOI] [PubMed] [Google Scholar]
- 3.Eslick GD, Jones MP, Talley NJ. Non-cardiac chest pain: prevalence, risk factors, impact and consulting—a population-based study. Aliment Pharmacol Ther. 2003;17:1115–1124. doi: 10.1046/j.1365-2036.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 4.Ward BW, Wu WC, Richter JE, et al. Long-term follow-up of symptomatic status of patients with noncardiac chest pain: is diagnosis of esophageal etiology helpful? Am J Gastroenterol. 1987;82:215–218. [PubMed] [Google Scholar]
- 5.Pandolfino JE, Vela MF. Esophageal-reflux monitoring. Gastrointest Endosc. 2009;69:917–930. doi: 10.1016/j.gie.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Hirano I, Richter JE. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol. 2007;102:668–685. doi: 10.1111/j.1572-0241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 7.Richter JE. Chest pain and gastroesophageal reflux disease. J Clin Gastroenterol. 2000;30(Suppl):S39–S41. [PubMed] [Google Scholar]
- 8.Prakash C, Clouse RE. Wireless pH monitoring in patients with non-cardiac chest pain. Am J Gastroenterol. 2006;101:446–452. doi: 10.1111/j.1572-0241.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 9.Hersh MJ, Sayuk GS, Gyawali CP. Long-term outcome of patients undergoing ambulatory pH monitoring for chronic unexplained cough. J Clin Gastroenterol. doi: 10.1097/MCG.0b013e3181b8e97b. e-pub ahead of print 12 October 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghavi SA, Ghasedi M, Saberi-Firoozi M, et al. Symptom association probability and symptom sensitivity index: preferable but still suboptimal predictors of response to high dose omeprazole. Gut. 2005;54:1067–1071. doi: 10.1136/gut.2004.054981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2005;3:329–334. doi: 10.1016/s1542-3565(05)00021-2. [DOI] [PubMed] [Google Scholar]
- 12.Ghillebert G, Janssens J, Vantrappen G, et al. Ambulatory 24h intraoesophageal pH and pressure recordings v provocation tests in the diagnosis of chest pain of oesophageal origin. Gut. 1990;31:738–744. doi: 10.1136/gut.31.7.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weusten BL, Roelofs JM, Akkermans LM, et al. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology. 1994;107:1741–1745. doi: 10.1016/0016-5085(94)90815-x. [DOI] [PubMed] [Google Scholar]
- 14.Wiener GJ, Richter JE, Copper JB, et al. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83:358–361. [PubMed] [Google Scholar]
- 15.Wang WH, Huang JQ, Zheng GF, et al. Is proton pump inhibitor testing an effective approach to diagnose gastroesophageal reflux disease in patients with noncardiac chest pain? A meta-analysis. Arch Intern Med. 2005;165:1222–1228. doi: 10.1001/archinte.165.11.1222. [DOI] [PubMed] [Google Scholar]
- 16.Cremonini F, Wise J, Moayyedi P, et al. Diagnostic and therapeutic use of proton pump inhibitors in non-cardiac chest pain: a metaanalysis. Am J Gastroenterol. 2005;100:1226–1232. doi: 10.1111/j.1572-0241.2005.41657.x. [DOI] [PubMed] [Google Scholar]
- 17.Numans ME, Lau J, de Wit NJ, et al. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med. 2004;140:518–527. doi: 10.7326/0003-4819-140-7-200404060-00011. [DOI] [PubMed] [Google Scholar]
- 18.Gasiorowska A, Fass R. The proton pump inhibitor (PPI) test in GERD: does it still have a role? J Clin Gastroenterol. 2008;42:867–874. doi: 10.1097/MCG.0b013e31816c47ed. [DOI] [PubMed] [Google Scholar]
- 19.Snedegar CT, Clouse RE. Comparison of reflux-associated symptom probability (RASP) tests from ambulatory pH monitoring. Gastroenterology. 2004;26(Suppl 2):A-323. [Google Scholar]
- 20.Holinga JA, Kumar S, Prakash C, et al. Reflux-associated symptom probability from preoperative ambulatory pH monitoring predicts quality of life (QoL) following laparoscopic antireflux surgery. Gastroenterology. 2007;134(Suppl 2):A-756. [Google Scholar]
- 21.Dekel R, Martinez-Hawthorne SD, Guillen RJ, et al. Evaluation of symptom index in identifying gastroesophageal reflux disease-related noncardiac chest pain. J Clin Gastroenterol. 2004;38:24–29. doi: 10.1097/00004836-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Tutuian R, Mainie I, Agrawal A, et al. Nonacid reflux in patients with chronic cough on acid-suppressive therapy. Chest. 2006;130:386–391. doi: 10.1378/chest.130.2.386. [DOI] [PubMed] [Google Scholar]
- 23.Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg. 1999;3:292–300. doi: 10.1016/s1091-255x(99)80071-7. [DOI] [PubMed] [Google Scholar]
- 24.Prakash C, Clouse RE. Long-term outcome from tricyclic antidepressant treatment of functional chest pain. Dig Dis Sci. 1999;44:2373–2379. doi: 10.1023/a:1026645914933. [DOI] [PubMed] [Google Scholar]
- 25.Achem SR. Noncardiac chest pain—treatment approaches. Gastroenterol Clin North Am. 2008;37:859–878. doi: 10.1016/j.gtc.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfino JE, Richter JE, Ours T, et al. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]