Abstract
Background
Conventional catheter-based and wireless pH monitoring continue to be widely used for the evaluation of GERD symptoms despite the emergence of pH-impedance monitoring.
Goals
We sought to identify patient- and test-related factors predicting objective GERD parameters on catheter-based and wireless pH monitoring.
Study
Ambulatory pH studies in 2,067 subjects (50.4 ± 0.3 years, 66.3% female) were assessed for presenting symptoms, antireflux therapy status, test characteristics, distal esophageal total acid exposure time (AET), symptom index (SI), and symptom-reflux association (Ghillebert probability estimate, GPE). Univariate and multivariate logistic regression analyses were performed to identify predictors of GERD evidence, both off and on antireflux therapy.
Results
Catheter-based pH monitoring was performed in 77.6%, and 90.1% of the studies were performed off anti-reflux therapy. The likelihood of finding GERD evidence was significantly higher off therapy (65.8 vs. 21.4% on therapy, p <0.0001); this held true for both strong GERD evidence (elevated AET and positive GPE) and elevated AET alone. The SI did not complement AET and GPE. Extended pH recording with the wireless technique consistently increased diagnostic yield. On multivariate logistic regression, the status of antireflux therapy and frequency of symptoms dictated finding of GERD evidence, and yields were highest for perceptive symptoms (heartburn, chest pain, and cough).
Conclusions
Ambulatory pH testing off antireflux therapy improves detection of all degrees of GERD evidence. pH testing provides highest yields with frequent perceptive symptoms and least with isolated regurgitation and ENT symptoms. Extending pH-recording with wireless monitoring uniformly augments yield.
Keywords: pH monitoring, Catheter-based monitoring, GERD symptoms, Antireflux therapy
Introduction
Gastroesophageal reflux disease (GERD) is one of the most common gastrointestinal problems encountered in outpatient practice, affecting as much as 15–25% of the US population [1, 2]. Medical management of GERD has been revolutionized by the introduction of proton pump inhibitors (PPIs). These agents are so effective in resolving GERD symptoms and healing esophagitis that empiric PPI therapy is now regarded as the initial diagnostic approach in symptomatic uncomplicated GERD, with a sensitivity of 78–80% in the diagnosis of GERD when compared to ambulatory pH monitoring [3–5]. Consequently, invasive diagnostic testing is typically reserved for situations where empiric therapy fails, the diagnosis of GERD is in question, the patient manifests atypical symptoms, complications are suspected, or where quantification of acid exposure and/or symptom-reflux association is desired prior to antireflux procedures.
Invasive diagnostic testing for GERD symptoms typically starts with an upper endoscopic examination in clinical practice. However, up to 70% of patients who fail PPI therapy can have normal esophageal mucosa on visual endoscopic examination [6, 7]. For this reason in particular, ambulatory pH testing serves an important role in the evaluation of persisting symptoms, both when the relationship of presenting symptoms to GERD is under question, and when symptoms do not resolve with seemingly adequate PPI therapy [1, 8]. The catheter-based ambulatory 24-h pH study has been the standard for many years, but is limited by poor tolerance of the pH probe, variability in probe location throughout the study, and suboptimal diagnostic yields when testing is performed on PPI therapy [4, 6]. The wireless pH probe resolves some of these limitations, and has been shown to increase the detection of abnormal GERD parameters [9–12]. Drawbacks of wireless pH monitoring include the additional expense of an endoscopy for probe placement in some instances, and the single site of pH recording [9, 11]. Combined pH-impedance monitoring is now available, which can detect not just acid reflux episodes but also weakly acidic and non-acid events. This has been shown in early studies to improve diagnostic yield over pH recording alone, especially in patients tested on PPI therapy and those with symptoms related to volume regurgitation [3, 6]. However, combined pH-impedance testing is not universally available, interpretation parameters remain incompletely standardized, and data on outcome of therapy directed by pH-impedance testing is limited [3]. Further, pH-impedance testing is performed using a catheter-based technique and suffers from the same drawbacks as traditional catheter-based pH monitoring. Therefore, catheter-based and wireless pH testing continue to be used widely in the evaluation of GERD symptoms.
The objectives of the current study were twofold: (1) to determine the role of antisecretory therapy and study duration on the yield of ambulatory pH monitoring, and (2) to determine the optimal patient population to be tested on and off PPI therapy.
Methods
Adult patients (>18 years) referred for ambulatory pH testing for all indications at our tertiary care medical center over a 7-year period between 2001 and 2008 were eligible for inclusion. Subjects who had undergone prior antireflux procedures or esophageal surgery, incomplete studies with <14 h of interpretable data, and studies contaminated by artifact were excluded from analysis. Demographics, presenting symptom, indication for pH monitoring, and anti-reflux regimen at time of pH study were extracted from the medical record. Importantly, testing at our center is performed on a referral basis, and treating physicians determined if pH studies were performed on or off medications and with conventional catheter-based or wireless pH monitoring. The review of clinical data for the purpose of this study was approved by the Institutional Review Board at Washington University School of Medicine.
Placement of the pH probes was performed at the out-patient endoscopy and motility facility at our medical center. A dominant symptom was identified for each subject for the purposes of symptom-association analysis within the following categories: heartburn, chest pain, cough, regurgitation, and ear–nose–throat (ENT) symptoms. The dominant symptom was designated “regurgitation” only when perceptive symptoms were absent, and the patient complained of a liquid or taste at the back of the throat; for instance, when a patient presented with both heartburn and regurgitation, the dominant symptom was designated heartburn. When subjects underwent studies for evaluation of non-episodic symptoms (globus, nausea, dyspepsia), the studies were designated “no episodic symptom.” Subjects evaluated off PPI were asked to discontinue proton pump inhibitors at least 7 days prior and histamine-2 receptor antagonists (H2RAs), prokinetic agents, and antacids at least 3 days prior to the pH study. All patients studied on antireflux medications were on once or twice daily PPIs and not H2RAs alone. Following discharge from the endoscopy facility, all subjects were instructed to resume normal activity and diet, maintain a daily diary that included symptoms, activities, and meal periods, and to activate the symptom indicator button of the pH recorder every time they experienced their GERD symptom.
Analyses of pH data included quantification of acid exposure time (AET), determination of reflux-symptom association through probability testing, and calculation of a symptom index (SI). AET was defined as the percentage of time the esophageal pH remained below 4 in the distal esophagus. When the wireless pH probe was used, averaged 24-h data were generated to conform to that obtained from catheter-based pH studies [10, 11]. An AET value ≥4% was used to define elevated acid exposure for the complete study. The symptom index (SI) was calculated as the proportion of reflux symptoms while pH <4 within the total number of symptom episodes recorded, expressed as a percentage [13]. A value of ≥50% was considered indicative of a high proportion of reflux-associated symptoms [13]. The Ghillebert probability estimate (GPE) was employed for symptom -association probability testing, and a p value<0.05 was required for significance [10, 11, 14]. A detailed description of this method of symptom association has been reported elsewhere [10]. Like the SAP calculated by the Weusten method [15], this symptom-association test determines the likelihood that a symptom and acid reflux event co-occur solely by chance. The overall likelihood is calculated as a sum of partial probabilities for exact numbers of reflux-associated symptoms within the context of the total number of symptoms, the proportion of time “at risk” (2 min following a reflux event) for linking a symptom to a low pH value, and the total recording time [10, 11, 14].
In assessing the yield of ambulatory pH monitoring, the finding of either elevated AET or positive symptom association (positive GPE) was considered “GERD evidence,” indicating that either AET was abnormally elevated, or acid reflux events in some fashion contributed to the symptomatic state (positive symptom association). GERD evidence was further categorized as follows: (a) strong GERD evidence: elevated AET with positive symptom association (GPE); (b) elevated acid exposure alone: AET ≥ 4.0% with negative symptom association (GPE); (c) acid sensitivity: normal AET with positive symptom association (GPE); (d) no GERD evidence: normal AET with negative symptom association. These categories were also calculated for each of the symptom groups (heartburn, regurgitation, chest pain, cough, ENT symptoms) and the proportions compared.
Data are reported as mean ± standard error of the mean, unless otherwise indicated. Grouped continuous variable data were compared using two-tailed Student’s t test; a p value of <0.05 was required for statistical significance. Intergroup comparisons were made using the Chi-squared test and Fisher’s exact test where appropriate. Univariate analyses were performed to determine the role of demographic variables (age, gender), presenting symptoms, status of antireflux medications, and pH monitoring technique on the diagnostic yield of ambulatory pH monitoring as measured by the detection of GERD parameters (AET, GPE). Multivariate logistic regression analyses were utilized to confirm independent significance of demographic and physiologic parameters based on the presenting symptom(s). In order to further understand the role of patient and test characteristics in predicting GERD evidence, Nagelkerke R2 values were calculated as estimates of the variance explained by each of the independent variables in the models. All analyses were performed using PASW 17.0 (SPSS, Inc., Chicago, IL).
Results
A total of 2,067 subjects (50.4 ± 0.3 years, 66.3% female) fulfilled study inclusion criteria. Catheter-based studies were performed in 77.6%, and 90.1% of the studies were performed off antireflux therapy. Baseline demographics and clinical characteristics were similar between subjects studied using catheter-based and wireless techniques, and between on and off PPI therapy (Table 1). Average recording time was 21.1 ± 2.3 h with catheter-based studies and 42.3 ± 4.5 h with wireless studies. Prolonged pH recording with the wireless system resulted in more reported symptoms (mean 26.5 ± 2.0 vs. 9.8 ± 0.7 with catheter-based pH monitoring, p <0.0001). While symptom reporting was similar off or on therapy (mean 14.8 ± 2.2 vs. 13.3 ± 0.54, p = ns), reflux-associated symptoms were more frequently seen off therapy (4.9 ± 0.2 vs. 1.5 ± 0.5 on therapy, p <0.001); this finding held true even when catheter-based and wireless studies were evaluated separately. On assessment of symptom association, the SI was concordant with the GPE in 86.1%, and discordant in 13.9%. When discordant, the GPE was positive with a negative SI in 10.0%, but the GPE was negative with a positive SI in 3.9%. Over 99% of subjects with a positive SI had either elevated AET or a positive GPE. Only 14 subjects (0.7% of the study population) had positive SI as the only GERD indicator. In all of these cases, the SI was only “borderline positive,” each subject reporting a total of two symptoms of which one occurred within 2 min of a reflux event, yielding a symptom index of exactly 50.0%. Therefore, the symptom index did not significantly alter the diagnostic yield for GERD evidence, and was redundant in the setting of abnormal AET or positive GPE; it thus was not used further as a GERD indicator in this study.
Table 1.
Clinical and demographic data
| All subjects (n = 2,067)
|
Catheter based (n = 1,605)
|
Wireless (n = 462)
|
||||
|---|---|---|---|---|---|---|
| Off PPI | On PPI | Off PPI | On PPI | Off PPI | On PPI | |
| Number of subjects | 1,862 | 205 | 1,493 | 112 | 369 | 93 |
| Mean age (± SEM) | 50.2 ± 0.3 | 52.7 ± 1.1 | 50.4 ± 0.4 | 54.7 ± 1.3 | 49.3 ± 0.7 | 50.2 ± 1.8 |
| Gender (F/M) | 1,246/616 | 132/73 | 1,014/479 | 78/34 | 232/137 | 54/39 |
| Duration of study (h) | 25.3 ± 0.2 | 30.6 ± 0.8 | 21.1 ± 0.6 | 21.2 ± 0.2 | 42.1 ± 0.3 | 42.3 ± 0.5 |
| Mean number of symptoms | 13.3 ± 0.6 | 14.9 ± 2.2 | 9.9 ± 0.4 | 8.3 ± 1.7 | 27.3 ± 2.3 | 23.0 ± 4.2 |
| Dominant symptoms, n (%) | ||||||
| Heartburn | 1,180 (63.4) | 100 (48.8) | 937 (62.8) | 45 (40.2) | 243 (65.9) | 55 (59.1) |
| Chest pain | 395 (21.2) | 54 (26.3) | 324 (21.7) | 29 (25.9) | 71 (19.2) | 25 (26.9) |
| Cough | 205 (11.0) | 36 (17.6) | 170 (11.4) | 26 (23.2) | 35 (9.5) | 10 (10.8) |
| Regurgitation | 48 (2.6) | 5 (2.4) | 33 (2.2) | 3 (2.7) | 15 (4.1) | 2 (2.2) |
| ENT symptoms | 34 (1.8) | 3 (1.5) | 29 (1.9) | 2 (1.8) | 5 (1.4) | 1 (1.1) |
| No episodic symptoms | 0 | 7 (3.4) | 0 | 7 (6.3) | 0 | 0 |
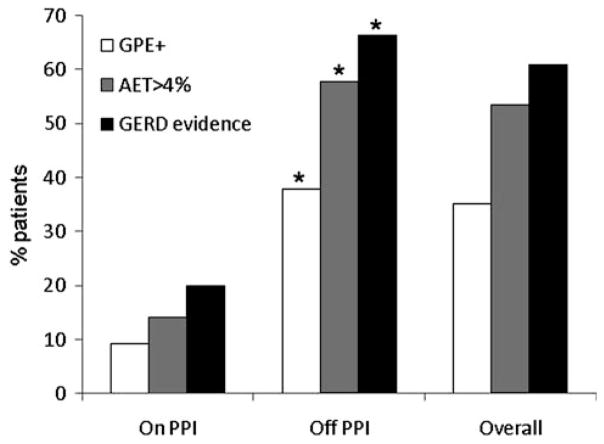
Evidence for GERD measured as either elevated AET or a positive GPE was significantly more frequent when testing was performed off antireflux therapy (66.3% compared to 20.0% on therapy, p <0.0001, Fig. 1). Further, strong GERD evidence was noted most often off PPI therapy compared to on therapy (30.4 vs. 3.4%, respectively, p <0.001; Fig. 2). An elevated AET alone also followed the same trend (27.3 vs. 10.7%, respectively, p <0.001). Acid sensitivity was found numerically more often when testing was performed off therapy (7.5% off therapy vs. 5.9% on therapy, p = 0.39). Finally, the proportion of subjects with no GERD evidence, as expected, was significantly higher when testing was performed on therapy (80.0% off therapy vs. 34.7% on therapy, p <0.001).
Fig. 1.
GERD evidence by status of antireflux therapy. The likelihood of finding a positive symptom association using the Ghillebert probability estimate (GPE), abnormal AET >4.0%, and overall GERD evidence were significantly higher when testing was performed off antireflux therapy (* p <0.0001). These differences remained statistically significant when analyses were performed for catheter-based and wireless pH monitoring separately
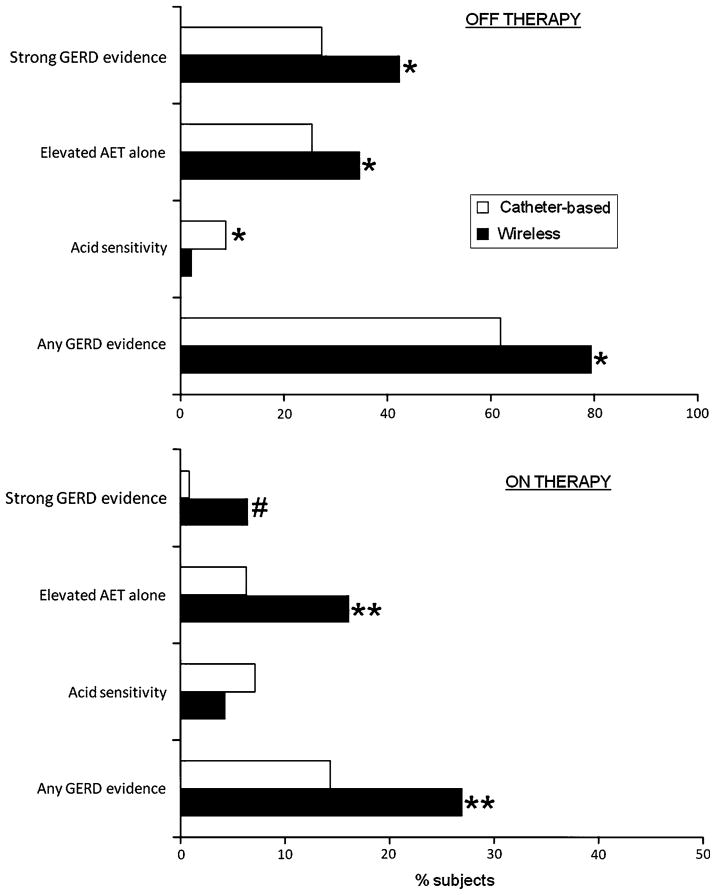
Fig. 2.
Comparison of strength of GERD evidence between catheter-based and wireless pH monitoring, off (top graph) and on (bottom graph) antireflux therapy. The likelihood of finding strong GERD evidence (elevated AET and positive GPE) and elevated AET alone were statistically higher when wireless pH monitoring was utilized, both off and on antireflux therapy. However, acid sensitivity (normal AET with positive GPE) was detected more often with catheter-based pH testing (* p ≤ 0.0001; ** p ≤ 0.04; # p = 0.048)
Wireless Versus Catheter-Based Studies
Off antireflux therapy, there was a 28.5% advantage in the detection of GERD evidence by extending recording duration with a wireless pH study (Fig. 2), with 79.4% having GERD evidence on wireless 48 h studies compared to 61.8% with 24 h monitoring (p <0.001). Looking at individual GERD parameters off therapy, a positive GPE was seen in 36.2% of those evaluated with a catheter-based pH study, and 44.7% of patients undergoing wireless pH studies (p = 0.003). Abnormal AET was seen in 52.9% of catheter-based evaluations and 77.2% of wireless pH studies (p <0.001). Gains were also noted with wireless pH monitoring studies performed on PPI therapy, when compared to catheter-based studies, mainly in the detection of abnormal AET (22.6 vs. 7.1%, p <0.002); symptom-association assessments were similar (Fig. 2). This represented an overall yield of GERD evidence on therapy of 14.3% with catheter-based testing and 26.9% with wireless pH monitoring (p <0.001).
Evaluating the strength of GERD evidence between wireless and catheter-based studies, the proportion of subjects with strong GERD evidence was higher with wireless pH monitoring both off and on PPI therapy (Fig. 2). The proportion of subjects with abnormal AET alone was also higher with wireless monitoring, but higher proportions of subjects had acid sensitivity with catheter-based monitoring, both off and on therapy.
Individual Symptoms
Subjects with heartburn as a dominant symptom formed the largest proportion of study subjects (61.9%) followed by chest pain (21.7%). When strength of GERD evidence was analyzed, strong GERD evidence was highest when tests were performed off therapy, particularly for perceptive GERD symptoms (heartburn, chest pain, cough), while elevated AET alone was seen in up to a third of subjects with heartburn, chest pain, cough, and regurgitation (Table 2). Acid sensitivity was proportionately highest in subjects with cough, both off and on therapy. However, the diagnostic yield of any degree of GERD evidence was substantially lower in subjects studied on therapy for evaluation of any symptom (Table 2).
Table 2.
Strength of GERD evidence by presenting symptom
| Antisecretory therapy | Strong GERD evidencea n (%) | Elevated AET alone n (%) | Acid sensitivityb n (%) | No GERD evidence n (%) | |
|---|---|---|---|---|---|
| Heartburn (n = 1,280) | Off (n = 1,180) | 468 (39.7)* | 301 (25.5)* | 96 (8.1) | 315 (26.7)* |
| On (n = 100) | 6 (6.0) | 11 (11.0) | 4 (4.0) | 79 (79.0) | |
| Chest pain (n = 449) | Off (n = 395) | 52 (13.2)* | 122 (30.9)* | 18 (4.6) | 203 (51.4)* |
| On (n = 54) | 0 (0) | 4 (7.4) | 4 (7.4) | 46 (85.2) | |
| Cough (n = 241) | Off (n = 205) | 30 (14.6)# | 67 (32.7)** | 19 (9.3) | 89 (43.4)* |
| On (n = 36) | 1 (2.8) | 5 (13.9) | 4 (11.1) | 26 (72.2) | |
| Regurgitation (n = 53) | Off (n = 48) | 13 (27.1) | 12 (25.0) | 6 (12.5) | 17 (35.4)* |
| On (n = 5) | 0 (0) | 0 (0) | 0 (0) | 5 (100) | |
| ENT symptoms (n = 37) | Off (n = 34) | 3 (8.8) | 7 (20.6) | 1 (2.9) | 23 (67.6) |
| On (n = 3) | 0 (0) | 2 (66.7) | 0 (0) | 1 (33.3) | |
| No symptoms (n = 7) | Off (n = 0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| On (n = 7) | 0 (0) | 0 (0) | 0 (0) | 7 (100) |
p <0.001,
p = 0.03,
p = 0.057 compared to on therapy in each symptom category
Elevated AET and positive GPE
Normal AET but positive GPE
Predictors of GERD Evidence
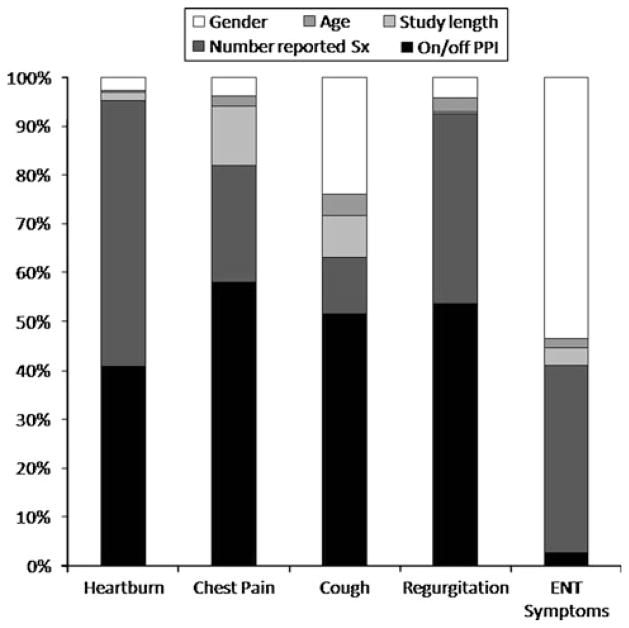
In evaluating the value of pH studies for specific GERD symptoms, logistic regression analyses were performed to determine the contribution of demographics (age, gender), and test characteristics (number of reported symptoms, study duration, and antireflux therapy status) to the finding of GERD evidence for each presenting symptom (heartburn, chest pain, cough, regurgitation, and ENT symptoms). The logistic regression models explained 14–36% of the total variance in GERD evidence findings for individual symptoms. The number of patients with ENT symptoms was small (37 total, and only three patients were on PPI); excluding this symptom, performing pH tests off antireflux therapy accounted for 41–58% of the explained variance in GERD evidence for all other symptoms, while numbers of symptoms reported during the study accounted for 11–54% of the explained variance. None of the variables in the model predicted GERD evidence for isolated regurgitation and ENT symptoms, but the number of subjects included with these symptoms were small and the findings may not be truly representative. Predictors of GERD evidence in subjects with the other perceptive symptoms (heartburn, chest pain, and cough) included study performance off PPI (p ≤ 0.001 for each), number of symptoms reported (p <0.001 for heartburn and chest pain, p = 0.09 for cough) and male gender (p <0.02). Together, testing off therapy and number of symptoms reported accounted for 63–95% of the explained variance in GERD evidence captured by the models for perceptive symptoms; when gender was added to the mix, this number rose to 86–98% (Fig. 3). In addition, study length was a significant predictor for GERD evidence in the evaluation of chest pain (p = 0.004), while older age was a predictor for cough (p = 0.03).
Fig. 3.
Independent contribution of demographic and pH study parameters to finding GERD evidence on ambulatory pH monitoring for individual symptom indications. Performing pH studies off antireflux therapy and symptomatic state (higher numbers of reported symptoms) are the most important independent variables. This data emphasizes the importance of performing pH testing off PPI in establishing a diagnosis of GERD, irrespective of the presenting symptom(s)
Discussion
In this study, we sought to determine the factors that would predict the best clinical value of ambulatory pH testing in an era where pH-impedance testing is gaining in acceptance and clinical use; in particular, we analyzed the implications of performing ambulatory pH monitoring off vs. on antisecretory therapy, and evaluated the role of the length of the pH study in predicting GERD evidence. We report that ambulatory pH testing is most likely to yield clinically relevant GERD evidence when the study is performed off antisecretory therapy, especially for perceptive symptoms such as heartburn, chest pain, and cough. This is consistent with reports in the literature using pH- and pH-impedance testing, where studies off therapy have been found to provide the highest yield for GERD evidence, both in the form of elevated AET and positive symptom associations [16]. Further, prolonged recording with the wireless pH probe provided overall higher diagnostic yields, even in subjects studied on antisecretory medications. This increased yield was partly from increased detection of abnormal AET, but also from prolonged duration of the study, thereby allowing higher symptom reporting for symptom-reflux association, as has been demonstrated in prior reports [11, 12]. Finally, pH monitoring provided the best yield for perceptive symptoms (heartburn, chest pain, and cough) but none of the evaluated variables predicted the finding of GERD evidence for isolated regurgitation and ENT symptoms, suggesting that these symptoms are better evaluated by alternate techniques.
In assessing the yield of ambulatory pH testing in settings where an empiric PPI trial has failed to resolved symptoms, the primary intent of the study to either “rule in” or “rule out” abnormal acid exposure and/or a relationship of symptoms to reflux events need to be considered [6, 10]. In evaluating this, we subcategorized findings on ambulatory pH testing to strong GERD evidence (consisting of both abnormal AET and symptom-reflux correlation using GPE), elevated AET alone, acid sensitivity (symptom-reflux correlation alone), and lack of GERD evidence. Categorization in this fashion may have diagnostic and therapeutic utility in that antireflux therapy can be escalated when evidence for GERD is strong, neuro-modulators added when acid sensitivity is encountered, and alternate etiologies sought for symptoms when evidence for GERD is lacking. From our findings and other reports, it is apparent that “rule in” or “rule out” of GERD is best accomplished by testing off therapy [11, 16]. Although our report does not compare catheter-based and wireless pH monitoring head-to-head in the same patient populations, it is also apparent that extending recording time adds to the yield of finding GERD evidence, with higher proportions of abnormal AET with or without positive symptom association [11]. This is despite the fact that we averaged the 2-day AET obtained with wireless pH monitoring to conform to 1-day data from catheter-based pH monitoring. As we have previously reported, this is one of the methods of evaluating AET from wireless pH monitoring [10, 11], but this method may underestimate the yield of abnormal AET when compared to using the “worst-day” AET data [9], and evaluation of “worst-day” data could further augment the diagnostic yield of wireless pH monitoring. Others have reported that increases in study duration to as many as 4 days may further augment the diagnostic yield and reproducibility of pH studies, and may help assess response to PPI therapy if introduced halfway through the study [12, 17].
We used a standard AET cut-off of 4% for both catheter-based and wireless studies for the purpose of this study. Higher AET cut-offs have been suggested for wireless pH monitoring based on the 95th percentile value of 5.3% in normal subjects [9], while systematic assessment of AET in normal subjects determined 4.2% as an acceptable cut-off in another study [18]. However, the number of subjects impacted by this difference in AET cut-off was minimal (3.5%). While it can be argued that extending recording time increases the number of reported symptoms and may allow for an increase in false-positive symptom associations [19], our findings show that the proportion of subjects with only a positive symptom association was actually higher with catheter-based rather than with wireless pH studies. While the “rule-out” value for a GERD diagnosis has been emphasized [19], detecting positive symptom association with reflux events also has clinical value in managing patients. For instance, we have previously shown in other reports that patients with statistically significant symptom associations generally report better symptom improvement and have a higher likelihood of a high degree of symptom response with aggressive GERD therapies [8, 20].
The role of performing pH monitoring on antireflux therapy has been an area of controversy, and the utility has been called into question due to the lack of validated normative values [5, 21]. Our overall yield of pathologic acid exposure on antireflux therapy of 14.1% is slightly higher than that typically reported in the literature [21]; when this is broken down by degree of GERD evidence, strong GERD evidence was encountered in only 3.9%, and AET alone in an additional 10.7%. We endorse the additional yield of pH-impedance monitoring in this setting [3, 5]. However, yields of GERD evidence were higher with wireless monitoring compared to catheter-based techniques (strong GERD evidence, 6.5 vs. 0.9%, and elevated AET alone, 16.5 vs. 6.3% respectively, p <0.001 in both instances, Fig. 2). Our data shows that ENT symptoms and isolated regurgitation do not provide predictable GERD evidence with ambulatory pH monitoring, another setting wherein pH-impedance monitoring may uncover additional useful information [3, 5]. However, our method of designating regurgitation as the dominant symptom only when it was an isolated symptom likely impacted the low value of pH testing in this instance; others have reported that “acid” regurgitation is a symptom that is most often perceived with significant pH drops on ambulatory pH monitoring [22]. Our findings also suggest that if pH-impedance testing is unavailable, extended recording with the wireless probe on therapy has a higher clinical value than catheter-based recording. Nevertheless, current trends suggest that pH-impedance monitoring will likely replace pH monitoring alone on therapy [5], as pH testing alone is unlikely to “rule out” GERD in this setting.
We propose that the clinical value of ambulatory pH monitoring can be augmented if patient symptoms and treatment histories are reviewed by trained personnel, either a trained nurse or a physician, and the most appropriate study recommended. For instance, evaluation of conventional esophageal symptoms (heartburn, chest pain) with standard catheter-based or wireless pH monitoring may be appropriate, but the overall yield for a GERD diagnosis with standard pH monitoring is much lower with regurgitation and ENT symptoms. If refractory symptoms drive the need for objective data where pre-procedure GERD evidence is strong, pH-impedance monitoring may have the edge over pH monitoring alone for testing on therapy in situations [3, 5], but pH testing off therapy, especially with the wireless technique may be adequate when a “rule in” or “rule out” of GERD is required. These decisions may be best made by personnel familiar with the yield and performance characteristics of the various pH and pH-impedance monitoring options available at the present day.
Our study has a few limitations. First, since this is a retrospective study, there was no standard protocol for which the mode of pH monitoring was used (catheter-based vs. wireless) or whether the test was performed on or off therapy; these decisions were made by the referring physicians. Second, subjects evaluated with wireless pH monitoring and those tested on therapy were proportionately small, and we could not assess the duration or quantity of PPIs taken by the subjects. Despite this, we were still able to show robust differences in the outcome variables between groups. Third, our patient population represents tertiary referrals; while our findings may not directly apply to the evaluation-naive or treatment-naive patient, we feel the concepts emerging from our data are pathophysiologically sensible, and may help direct investigation even in these groups. Our study indirectly assumes that testing was performed for ruling in GERD rather than ruling out the presence of abnormal AET or statistically significant reflux symptom associations. These assumptions may not necessarily be true in some instances, especially since our motility center operates on an open-access basis. However, we feel that our observations reflect a real-world clinical situation that provides representative and valuable insights into the optimal use of these tests for the practicing physician. Finally, we did not compare pH monitoring to combined pH-impedance monitoring, since this technique was not widely used during the period of the current study.
In conclusion, we have shown that ambulatory pH testing will most likely yield clinically relevant data when performed off antisecretory therapy in patients with perceptive symptoms, specifically heartburn, chest pain, and cough. Additionally, extending the study duration with a wireless pH monitoring system increases the diagnostic yield for GERD evidence when evaluating patients both on and off PPI. Finally, if pH monitoring is to be performed on PPI therapy, prolonged monitoring with a wireless device has better yield than traditional catheter-based methods. Further study is needed to determine pre-procedure predictors of GERD evidence to optimize the utilization of pH and pH-impedance testing.
Acknowledgments
This study was funded in part by the Washington University Mentors in Medicine program, whereby the Department of Internal Medicine provides grants to residents in training (Vladimir Kushnir). No writing assistance was obtained.
Footnotes
Conflict of interest No conflicts of interest exist.
This work was presented in preliminary form at the Annual Meeting of the American Gastroenterological Association, Chicago, May 2009.
References
- 1.Kahrilas PJ. Gastroesophageal reflux disease. N Engl J Med. 2008;359:1700–1707. doi: 10.1056/NEJMcp0804684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Hansen RA, et al. The burden of gastrointestinal and liver diseases. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirano I, Richter JE. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol. 2007;102:668–685. doi: 10.1111/j.1572-0241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 4.Kahrilas PJ, Shaheen NJ, Vaezi MF, et al. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392–1413. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Pandolfino JE, Vela MF. Esophageal-reflux monitoring. Gastrointest Endosc. 2009;69:917–930. doi: 10.1016/j.gie.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut. 2009;58:295–309. doi: 10.1136/gut.2007.145581. [DOI] [PubMed] [Google Scholar]
- 7.Richter JE. How to manage refractory GERD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:658–664. doi: 10.1038/ncpgasthep0979. [DOI] [PubMed] [Google Scholar]
- 8.Kushnir VM, Sayuk GS, Gyawali CP. Abnormal GERD parameters on ambulatory pH monitoring predict therapeutic success in noncardiac chest pain. Am J Gastroenterol. 2010;105:1032–1038. doi: 10.1038/ajg.2009.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandolfino JE, Richter JE, Ours T, et al. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98:740–749. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]
- 10.Prakash C, Clouse RE. Wireless pH monitoring in patients with non-cardiac chest pain. Am J Gastroenterol. 2006;101:446–452. doi: 10.1111/j.1572-0241.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 11.Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2005;3:329–334. doi: 10.1016/s1542-3565(05)00021-2. [DOI] [PubMed] [Google Scholar]
- 12.Garrean CP, Zhang Q, Gonsalves N, Hirano I. Acid reflux detection and symptom-reflux association using 4-day wireless pH recording combining 48-h periods off and on PPI therapy. Am J Gastroenterol. 2008;103:1631–1637. doi: 10.1111/j.1572-0241.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 13.Wiener GJ, Richter JE, Copper JB, et al. The symptom index: a clinically important parameter of ambulatory 24-h esophageal pH monitoring. Am J Gastroenterol. 1988;83:358–361. [PubMed] [Google Scholar]
- 14.Ghillebert G, Janssens J, Vantrappen G, Nevens F, Piessens J. Ambulatory 24 h intraoesophageal pH and pressure recordings v provocation tests in the diagnosis of chest pain of oesophageal origin. Gut. 1990;31:738–744. doi: 10.1136/gut.31.7.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weusten BL, Roelofs JM, Akkermans LM, Van Berge-Henegouwen GP, Smout AJ. The symptom-association probability: an improved method for symptom analysis of 24-h esophageal pH data. Gastroenterology. 1994;107:1741–1745. doi: 10.1016/0016-5085(94)90815-x. [DOI] [PubMed] [Google Scholar]
- 16.Hemmink GJ, Bredenoord AJ, Weusten BL, Monkelbaan JF, Timmer R, Smout AJ. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: ‘on’ or ‘off’ proton pump inhibitor? Am J Gastroenterol. 2008;103:2446–2453. doi: 10.1111/j.1572-0241.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 17.Scarpulla G, Camilleri S, Galante P, Manganaro M, Fox M. The impact of prolonged pH measurements on the diagnosis of gastroesophageal reflux disease: 4-day wireless pH studies. Am J Gastroenterol. 2007;102:2642–2647. doi: 10.1111/j.1572-0241.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 18.Ayazi S, Lipham JC, Portale G, et al. Bravo catheter free pH monitoring: normal values, concordance, optimal diagnostic thresholds, and accuracy. Clin Gastroenterol Hepatol. 2009;7:60–67. doi: 10.1016/j.cgh.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Conner J, Richter J. Increasing yield also increases false positives and best serves to exclude GERD. Am J Gastroenterol. 2006;101:446–452. doi: 10.1111/j.1572-0241.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- 20.Hersh M, Sayuk GS, Gyawali CP. Long-term outcome of patients undergoing ambulatory pH monitoring for chronic unexplained cough. J Clin Gastroenterol. 2010;44:234–236. doi: 10.1097/MCG.0b013e3181b8e97b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charbel S, Khandwala F, Vaezi MF. The role of esophageal pH monitoring in symptomatic patients on PPI therapy. Am J Gastroenterol. 2005;100:283–289. doi: 10.1111/j.1572-0241.2005.41210.x. [DOI] [PubMed] [Google Scholar]
- 22.Bredenoord AJ, Weusten BL, Curvers WL, Timmer R, Smout AJ. Determinants of perception of heartburn and regurgitation. Gut. 2006;55:313–318. doi: 10.1136/gut.2005.074690. [DOI] [PMC free article] [PubMed] [Google Scholar]