Abstract
Beta-cell dysfunction and impaired insulin production are hallmarks of diabetes1, but despite the growing diabetes epidemic the molecular mechanisms involved have remained unclear. We identified thioredoxin-interacting protein (TXNIP), a cellular redox regulator, as a critical factor involved in beta-cell biology and showed that beta-cell TXNIP was upregulated in diabetes, whereas TXNIP deficiency protected against diabetes by preventing beta-cell apoptosis2–3. Here we show that TXNIP and diabetes induce beta-cell expression of a specific microRNA, miR-204, which in turn blocks insulin production by directly targeting and downregulating MafA, a known insulin transcription factor. After discovering miR-204 to be induced by TXNIP in a microRNA microarray, we confirmed the findings using INS-1 beta-cells, islets of TXNIP-deficient mice, diabetic mouse models and primary human islets. We further discovered that TXNIP induces miR-204 by controlling the activity of STAT3, a transcription factor involved in miR-204 regulation4–5 and identified MafA as a novel target downregulated by miR-204. Taken together, our results demonstrate for the first time that TXNIP controls microRNA expression and insulin production, and that miR-204 is involved in beta-cell function. The identified novel TXNIP/miR-204/MafA/insulin pathway may contribute to diabetes progression and provides new insight into TXNIP function and microRNA biology in health and disease.
Production of adequate amounts of insulin in pancreatic beta-cells is a prerequisite for maintaining normal glucose homeostasis. Indeed, beta-cell dysfunction and impaired insulin production are key factors in the pathogenesis of diabetes1, but despite the growing diabetes epidemic worldwide the molecular mechanisms involved have only started to be unravelled. Recently, we identified thioredoxin-interacting protein (TXNIP), a cellular redox regulator6, as a critical factor involved in beta-cell biology and showed that beta-cell TXNIP was upregulated in diabetes, whereas TXNIP deficiency protected against type 1 and type 2 diabetes by preventing beta-cell apoptosis and increasing whole pancreas beta-cell mass2–3,7–11. Furthermore, we revealed the pathways by which TXNIP induces apoptosis2,10 and discovered that TXNIP shuttles within the beta-cell and translocates from the nucleus into the mitochondria where it initiates the mitochondrial apoptotic cascade10. In fact, the discovery that, under normal conditions, TXNIP is primarily localized in the nucleus combined with our previous gene expression profiling studies demonstrating that ~95% of all altered genes are downregulated by TXNIP9, raised the possibility that TXNIP might be involved in the control (and especially inhibition) of beta-cell gene expression, which prompted us to study the potential effects of TXNIP on microRNA expression.
microRNAs (small, 20–24 nucleotide, non-coding RNAs) recognize and bind to target mRNAs through imperfect base pairing leading to mRNA degradation or translational inhibition of the target mRNA and downregulation of target gene expression12–14. Now, microRNAs are rapidly emerging as important regulators of gene expression in health and disease and recently have also been discovered to play various roles in diabetes and beta-cell biology15–21.
Comparison of our TXNIP overexpressing INS-1 beta-cell line (INS-TXNIP) and INS-LacZ control cell line using miRCURY LNA microRNA Arrays (Exiqon) and a threshold of 0.7 absolute difference in LogMedianRatio (1.6-fold change) revealed five microRNAs that were upregulated in response to TXNIP (i.e. miR-139-5p; miR-193; miR-204; miR-200c; miR-141) (Supplementary Table 1). After confirming these findings by quantitative real-time PCR, we started to investigate the role of these microRNAs by systematically knocking them down using specific inhibitor oligonucleotides and assessing the effects on insulin production, a key aspect of beta-cell function. However, only knockdown of microRNA-204 (miR-204), led to any significant effect and to an increase in insulin expression. Moreover, only overexpression of miR-204, but not of any of the other microRNAs resulted in a marked decrease in insulin mRNA (Supplemental Fig. S1). Notably, miR-204 (which is fully conserved between human, rat and mouse) (Supplementary Fig. 1b) has not been implicated in beta-cell biology, but was found to be highly expressed in insulinomas22. Consistent with this observation, miR-204 was readily detectable in INS-1 cells, but in alignment with other microRNAs its expression was even higher in primary human islets, whereas expression in mouse islets was lower than in the INS-1 cells (Supplementary Fig. 1c). Of note, human pancreatic islets were also one of the major sites of miR-204 expression according to the microRNA.org website, but its function and target genes remained unknown. Taken together, these findings suggested that miR-204 might play an important role in beta-cell biology and we therefore decided to focus on this microRNA.
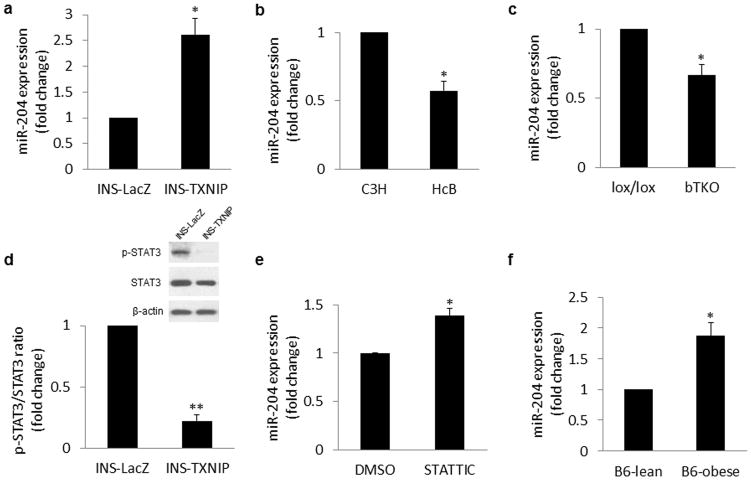
Using quantitative real-time RT-PCR (qRT-PCR), we found that miR-204 expression was >2-fold higher in INS-TXNIP cells as opposed to control INS-LacZ cells (Fig. 1a) confirming our microarray findings. In contrast, primary islets from TXNIP-deficient HcB-19 mice (harbouring a natural nonsense mutation in the Txnip gene) showed a significant reduction in miR-204 expression (Fig. 1b). Similarly, miR-204 was significantly reduced in islets from our bTKO beta-cell-specific Txnip knockout mice (Fig. 1c) further indicating that TXNIP regulates beta-cell miR-204 expression in vivo.
Figure 1. Effects of TXNIP and diabetes on beta-cell miR-204 expression.
Expression of miR-204 was assessed by qRT-PCR in (a) INS-1 cells overexpressing TXNIP (INS-TXNIP) and control (INS-LacZ) cells, (b) primary islets of TXNIP-deficient HcB-19 and C3H control mice, and (c) primary islets of beta-cell-specific Txnip knockout bTKO and lox/lox control mice. (d) TXNIP effects on STAT3 activation were determined by immunoblotting for phospho-STAT3 (p-STAT3) and total STAT3 in INS-TXNIP and control INS-LacZ cells. (e) To determine the role of STAT3 in miR-204 expression, INS-1 cells were incubated with the STAT3 inhibitor STATTIC (2μM for 48h) or vehicle (DMSO) and the expression of miR-204 was detected by qRT-PCR. (f) To assess the effect of diabetes on miR-204 expression, primary islets of 10-week old, male, diabetic ob/ob or lean control mice were analyzed by qRT-PCR. Bars represent means ±SEM; *P<0.05, **P<0.01; n=3–5 independent experiments.
miR-204 is encoded within intron 6 of the TRPM3 gene (transient receptor potential melastatin 3, a cation-selective channel) and is transcribed in the same direction as TRPM323. Since miR-204 and TRPM3 are therefore sharing the same promoter, we hypothesized that, if TXNIP were to regulate miR-204 expression at the transcriptional level, TRPM3 would be co-regulated in parallel. Indeed, we found that TRPM3 expression was >3-fold higher in TXNIP overexpressing INS-TXNIP cells as opposed to control INS-LacZ cells (Supplementary Fig. 1d), whereas primary islets from TXNIP-deficient HcB-19 mice showed a significant reduction in TRPM3 expression (Supplementary Fig. 1e). These findings are very similar to the results obtained for miR-204 and suggest that in fact, TXNIP upregulates miR-204 by inducing its transcription. Since TXNIP is not known to act as a transcription factor, we hypothesized that it acts through regulating another factor. Of note, the signal transducer and activator of transcription 3 (STAT3) has recently been implicated in the downregulation of miR-204 expression by multiple reports4–5 and, given the observed upregulation of miR-204 in response to TXNIP, we investigated whether TXNIP might inhibit STAT3. Indeed, while TXNIP had no effect on STAT3 mRNA expression (Supplementary Fig. 1g) or total protein levels, STAT3 phosphorylation/activation (which is critical for STAT3-mediated transcription) was dramatically reduced in response to TXNIP (Fig. 1d). Using STATTIC, a small molecule that selectively inhibits the activation of the STAT3 transcription factor by blocking its phosphorylation and dimerization (sc-202818, Santa Cruz Biotechnology, Santa Cruz, CA), we investigated whether this could mimic the effects of TXNIP. In fact, similarly to TXNIP, STATTIC significantly induced miR-204 expression (Fig. 1e) as well as its host gene TRPM3 (Supplementary Fig. 1f), suggesting that TXNIP confers its effects on miR-204 at least in part through inhibition of STAT3.
Given the involvement of TXNIP in diabetes3,8,24, we investigated whether beta-cell miR-204 expression might also be altered in diabetes. To this end we used the well-established leptin-deficient, obese and diabetic B6-obese mice as a model of type 2 diabetes (Supplementary Fig. 2a,b). Islets of obese and diabetic mice showed dramatically elevated Txnip levels (Supplementary Fig. 2c). Most notably though, miR-204 expression was also significantly higher in diabetic B6-obese mice as opposed to lean control mice (Fig. 1f) suggesting that this microRNA might play a role in the beta-cell dysfunction of diabetes. Of note, we also found that miR-204 expression was significantly elevated in two additional models of diabetes, i.e. BTBRob/ob and A-ZIP/F-1 mice (Supplementary Fig. 3a–f). Unlike B6-obese mice, which typically have rather mild diabetes, BTBRob/ob mice are not able to compensate for their leptin-deficiency-induced obesity and insulin resistance and develop severe type 2 diabetes consistent with a more pronounced islet phenotype that includes disrupted islet architecture and reduced whole pancreas insulin content25. In contrast, A-ZIP/F-1 mice are not obese and lack white adipose tissue (due to adipose-specific transgenic expression of a dominant-negative protein (A-ZIP/F) that blocks C/EBP and Jun-mediated transcription), but are severely diabetic26. Despite these differences, both models showed again higher Txnip expression, consistent with our previous findings3,8 as well as higher miR-204 levels as compared to their non-diabetic controls.
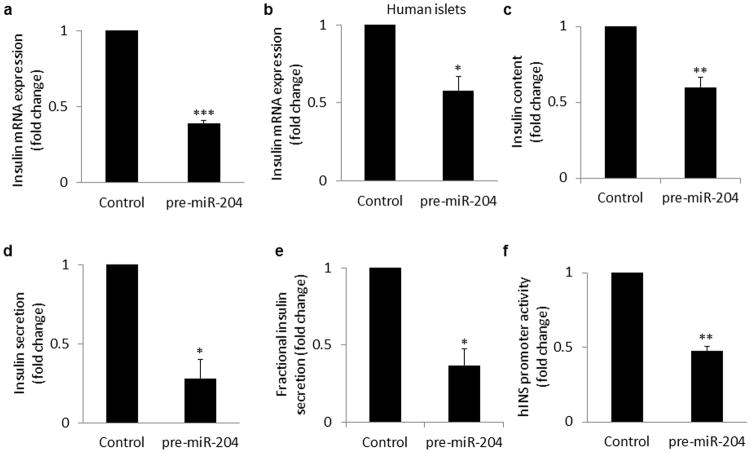
Given the pro-apoptotic effects of TXNIP, we also tested the possibility that miR-204 might induce beta-cell apoptosis. However, compared to scrambled control, miR-204 overexpression did lead to no significant increase in the Bax/Bcl2 ratio (p=0.313), no increase in cleaved caspase-3 and no increase in TUNEL positive beta-cells (data not shown), indicating that unlike TXNIP this microRNA does not induce beta-cell apoptosis. We therefore followed up on our initial observation and tested how miR-204 might affect beta-cell function, i.e. insulin production. In fact, miR-204 overexpression in INS-1 cells led to a >200% reduction in insulin mRNA expression (Fig. 2a). (Unlike humans, rodents have two insulin genes and while data shown was obtained with primers detecting expression from both rat insulin genes, primers specific for Ins1 or Ins2 showed the same effect.) Of note, in human islets miR-204 overexpression also decreased insulin mRNA similar to INS-1 cells (Fig. 2b) demonstrating that this effect was also relevant to human islet biology. In addition, the decrease in insulin mRNA expression translated at the protein level into significantly reduced insulin content in miR-204 overexpressing cells (Fig. 2c) as well as decreased insulin secretion (Fig. 2d) and fractional insulin secretion (Fig. 2e). (The latter suggests that miR-204 might have additional direct effects on insulin secretion.) Moreover, the higher miR-204 levels observed in diabetic B6-obese mice were also associated with significantly lower insulin gene expression (Supplementary Fig. 2d,e). Similarly, elevated miR-204 levels were also associated with reduced insulin gene expression in the two additional diabetes models tested (Supplementary Fig. 3a–f). In contrast, transfection of miR-204 inhibitor oligonucleotides not only resulted in effective inhibition of miR-204 (Supplementary Fig. 3g), but also in a significant increase in insulin mRNA (Supplementary Fig. 3h) suggesting that miR-204 regulates insulin gene expression. However, we observed that miR-204 inhibited insulin promoter activity (Fig. 2f), rather than having classical post-transcriptional microRNA effects on mRNA stability or translation. This suggested that the effect was indirect and likely mediated by miR-204-induced downregulation of factor(s) involved in insulin transcription.
Figure 2. miR-204 effects on insulin production.
(a) INS-1 cells and (b) human islets were transfected with miR-204 precursor (pre-miR204) or scrambled control. 72h after transfection RNA was extracted and analysed for insulin mRNA by qRT-PCR. (c) Cellular insulin protein content and (d) insulin secretion in INS-1 cells overexpressing miR-204 or scrambled control was assessed by ELISA and normalized for cellular DNA content. (Control insulin content: 7.5 and secretion 1.7ng ml−1 per μgDNA) (e) Fractional insulin secretion as calculated by normalizing insulin secretion for insulin content. (f) INS-1 cells were cotransfected with the human insulin promoter reporter construct Ins-Luc, and pre-miR204 or control plasmid. Cells were harvested 72h after transfection, and insulin promoter activity was assessed by firefly luciferase (and corrected for transfection efficiency with pRL-TK renilla luciferase). Bars represent means ±SEM of 3 independent experiments; *P<0.05, **P<0.01, ***P<0.001.
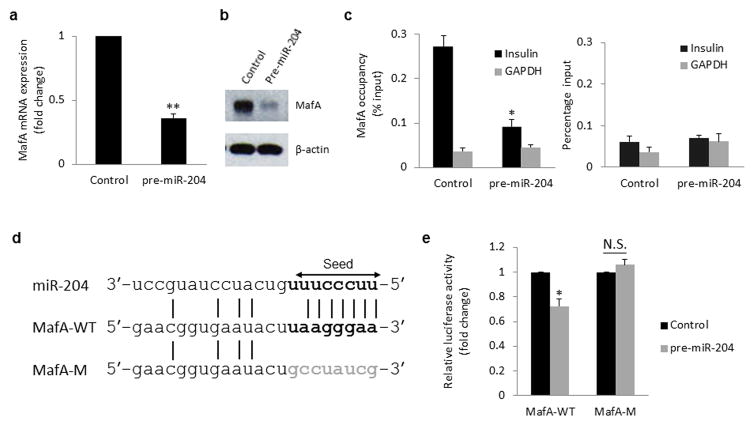
To identify these factors, we next set out to find the putative gene targets of miR-204 and especially those that might play a role in insulin transcription. We therefore tested whether miR-204 could inhibit the expression of any of the key insulin transcription factors, i.e. MafA/B, NeuroD or Pdx-127–29 (which also came up as potential targets using miRWalk algorithms). Indeed, MafA mRNA and protein levels were dramatically reduced in response to miR-204 overexpression (Fig. 3a,b) as well as in vivo in the context of diabetes-induced miR-204 (Supplementary Fig. 2f), whereas miR-204 inhibition led to a >2-fold increase in MafA expression (Supplementary Fig. 3i). In contrast, the other transcription factors were not significantly altered by overexpression or inhibition of miR-204 (Supplementary Fig. 4a–f). While MafA has been show to be capable of activating Pdx-1 promoter driven reporter gene expression30 the expression of MafA and Pdx-1 do not always go hand in hand. Consistent with our findings, MafA expression was found to be lower in diabetic db/db islets and in response to c-Jun, whereas Pdx-1 expression remained unchanged31. In addition, glucose has been shown to induce the expression of MafA, but not that of Pdx-1 in beta-cells32. Furthermore, we found that miR-204-induced reduction in MafA expression also resulted in dramatically reduced MafA binding to the insulin promoter as assessed by chromatin immunoprecipitation (ChIP) studies (Fig. 3c). Together, these findings raised the possibility that MafA was acting as the miR-204 target mediating the effects of this microRNA on insulin gene expression. In fact, comparison of the miR-204 seed sequence and the rat MafA 3′UTR revealed a perfect 7-nucleotide match (Fig. 3d) suggesting that MafA might be a target of miR-204. To address this question, we generated reporter constructs with the wild-type MafA 3′UTR or a mutated MafA 3′UTR (Fig. 3d) cloned downstream of the luciferase gene and assessed miR-204-directed repression of the reporter gene. We found that miR-204 significantly decreased luciferase activity through the wild-type MafA 3′UTR, while no reduction was found with the mutant 3′UTR (Fig. 3e), confirming that MafA is indeed a direct target of miR-204. Of note, MafA is highly conserved across species at the protein as well as at the mRNA level and TargetScan predicted the rat 3′UTR to contain a unique miR-204 binding site, consistent with our findings in rat INS-1 beta-cells. Furthermore, alignment of the rat, human and mouse 3′UTR sequences revealed that 6 of the 7 nucleotides representing the miR-204 seed match were also conserved in human as well as mouse MafA (Supplementary Table 1) and such matching has been reported to be sufficient for the downregulation of target messages33. Nevertheless, we also performed reporter assays using plasmids encoding the wild-type human MafA 3′UTR as well as a human MafA 3′UTR with mutated miR-204 binding seed sequence (Supplementary Fig. 4g). Again miR-204 led to a small, but highly significant and consistent decrease in luciferase activity through the wild-type human MafA 3′UTR and this effect was completely blunted by mutation of the seed sequence (Supplementary Fig. 4h). Moreover, miR-204 also significantly reduced MafA expression in human islets (Supplementary Fig. 4i) and led to an almost 50% reduction in human insulin expression (Fig. 2b). This is very much in line with our findings in rat INS-1 beta-cells, demonstrates the translatability of the results and suggests that this novel miR-204/MafA/insulin pathway is also active in human islets. (Currently, no validated human pancreatic beta-cell line is available, making it necessary to use non-beta-cell lines or primary human islets with lower transfection efficiency, which may explain the slightly smaller effect size found in some of these experiments as compared to the ones using rodent models.) While even small changes in the expression level of a transcription factor such as MafA can lead to much larger effects on target gene expression, e.g. insulin (as observed in our human islets), we can obviously not exclude the possibility that additional factors targeted by miR-204 might be involved in this more complex setting of the human islet.
Figure 3. MafA as a target of miR-204.
miR-204 effects on MafA mRNA (a) or protein expression (b) as assessed by qRT-PCR and immunoblotting. (c) Changes in MafA occupancy of the insulin promoter in response to miR-204 as measured by ChIP and IgG control (right panel). (d) Alignment of miR-204 seed sequence (arrow) and rat MafA 3′UTR target sequence (bold) as well as mutated target sequence (grey). (e) INS-1 cells were cotransfected with the wild-type MafA-WT-3′Luc or mutant MafA-M-3′Luc 3′UTR reporter plasmids and with miR-204 or scrambled control. 24h after transfection, miR-204-directed repression of the luciferase reporter gene bearing the wild-type or mutant MafA 3′UTR segments was assessed. Bars represent means ±SEM of 3 independent experiments and one representative immunoblot is shown; *P<0.05, **P<0.01.
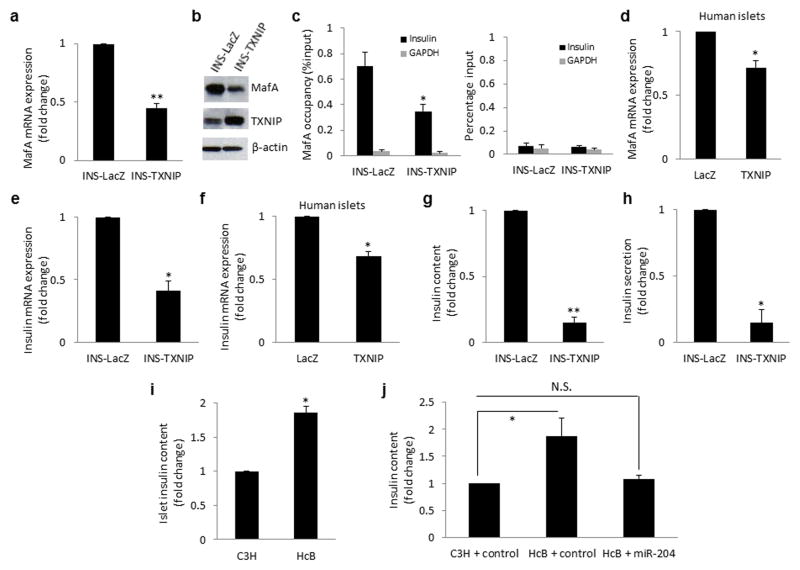
To investigate whether TXNIP (as an upstream regulator of miR-204) could mimic the miR-204 effects on MafA, we analysed our TXNIP overexpressing INS-1 cell line and found a >200% reduction in MafA mRNA expression and MafA protein levels (Fig. 4a,b) similar to the results in response to direct miR-204 overexpression. In contrast, TXNIP had no effect on Pdx-1, MafB or NeuroD expression levels (Supplementary Fig. 5a–c). In addition, ChIP analysis revealed that MafA occupancy of the insulin promoter was reduced to almost half in TXNIP overexpressing cells as compared to INS-LacZ control cells (Fig. 4c). Moreover, TXNIP also reduced MafA expression in human islets (Fig. 4d) confirming the physiological relevance of these findings.
Figure 4. TXNIP effects on MafA and insulin production.
TXNIP overexpressing INS-TXNIP cells were analyzed for (a) MafA mRNA by qRT-PCR, (b) MafA protein by immunoblotting and (c) MafA occupancy of the insulin promoter by ChIP and compared to INS-LacZ control cells; IgG control (right panel). (d) Human islets were transfected with CMV-hTXNIP or LacZ and the effects on MafA mRNA expression were assessed by qRT-PCR. Insulin mRNA as assessed by qRT-PCR in (e) INS-TXNIP cells and (f) human islets transfected with TXNIP. (g) Insulin content and (h) insulin secretion were assessed by ELISA in INS-TXNIP and INS-LacZ control cells. (Control LacZ insulin content: 8.1 and secretion: 1.7ng ml−1 per μgDNA) (i) Islet insulin content was measured by ELISA in primary islets of TXNIP-deficient HcB-19 and control C3H mice. (j) Control C3H and TXNIP-deficient HcB-19 mouse islets were transfected with scrambled control or precursor miR-204 and 2 days later insulin content was measured by ELISA and normalized for DNA content. Bars represent means ±SEM of 3 independent experiments; *P<0.05, **P<0.01.
To further test whether TXNIP would also block insulin production similarly to miR-204, we conducted parallel experiments using our TXNIP overexpressing INS-1 cells as well as human islets and TXNIP deficient HcB-19 mouse islets. Indeed, TXNIP led to a significant decrease in insulin mRNA in INS-1 cells (Fig. 4e) and human islets (Fig. 4f). (This transient TXNIP overexpression in the human islets did not lead to any beta-cell apoptosis as determined by an unchanged Bax/Bcl2 ratio in the same samples, making any confounding effects in this regard extremely unlikely.) At the protein level, TXNIP also caused a dramatic reduction in insulin content (Fig. 4g) and an associated decrease in insulin secretion (Fig. 4h). (This effect seemed to have been caused primarily by the reduced insulin content, as fractional insulin secretion was not significantly affected by TXNIP (Supplementary Fig. 5d).) In contrast, islet of TXNIP deficient mice demonstrated a highly significant, 2-fold higher insulin content (Fig. 4i) strongly supporting the notion that TXNIP inhibits beta-cell insulin production. To further obtain direct evidence for the role of miR-204 in this process, we overexpressed miR-204 in islets of TXNIP-deficient mice, which completely blunted the effect the lack of TXNIP had and reduced the islet insulin content to levels comparable with wild-type control mice (Fig. 4j). This rescue experiment further established the causal relationship between decreased TXNIP and miR-204 and increased insulin production and revealed an important functional link suggesting that TXNIP indeed inhibits insulin production through induction of miR-204 expression.
Based on our discovery that TXNIP inhibits STAT3 activation and that STAT3 inhibition in turn increases miR-204 expression, we also investigated the possibility that STAT3 inhibition could regulate insulin production. Indeed, both insulin and MafA expression were significantly reduced in response to STAT3 inhibition (Supplementary Fig. 5e,f) providing additional evidence for the importance of this newly identified pathway in conferring the observed TXNIP effects.
Since miR-204 and TRPM3 are co-regulated by TXNIP, we also wanted to address the questions whether any of the observed effects might be mediated by TRPM3. We therefore knocked down TRPM3 using siRNA and assessed whether this could mimic the effects of miR-204 inhibition. However, while we obtained a robust downregulation of TRPM3 (Supplementary Fig. 5g), neither MafA (Supplementary Fig. 5h) nor insulin expression (Supplementary Fig. 5i) were increased and, in contrast to miR-204 inhibition, rather decreased. This indicates that TRPM3 is not conferring the observed TXNIP-mediated inhibition of insulin transcription. Moreover, it suggests that miR-204 is not only able to regulate insulin transcription as demonstrated by miR-204 overexpression and inhibition, but, in the case of TXNIP overexpression, also to outweigh the opposing effects of TRPM3.
Taken together, our findings in INS-1 beta-cells, islets of TXNIP-deficient mice, diabetic mouse models and primary human islets demonstrate that TXNIP inhibits STAT3 and induces beta-cell transcription of a specific microRNA, miR-204, which in turn blocks insulin production by directly targeting and downregulating the critical transcription factor, MafA. This suggests that this novel TXNIP/p-STAT3/miR-204/MafA/insulin pathway may contribute to impaired insulin production, beta-cell dysfunction and the pathogenesis of diabetes (Supplementary Fig. 6).
While our previous work revealed the important role TXNIP plays in diabetic beta-cell death2–3,8,11, the current results demonstrate for the first time that TXNIP also controls in vivo beta-cell function and insulin production. In fact, the higher insulin production associated with TXNIP deficiency might have contributed to the anti-diabetic effects observed3 and may provide an added bonus when targeting TXNIP as a therapeutic approach for diabetes. Our current findings are also consistent with a previous report associating changes in TXNIP with altered insulin secretion34.
We have previously found that TXNIP deficient HcB-19 mice also have significantly lower blood glucose concentrations and are protected against streptozotocin- and obesity-induced diabetes 3. Based on the newly identified signalling pathway, by which TXNIP induces miR-204 expression and thereby downregulates MafA and insulin production, one would anticipate that MafA knockout mice would demonstrate a phenotype that is opposed to the one seen in TXNIP deficient mice. Indeed, MafA knockout mice are characterized by lower insulin transcription, higher blood glucose concentration and at ~1-year of age many progress spontaneously to overt diabetes with blood glucose measurements over 500 mg dL−1 35. These data are not only consistent with our current findings, but also underline the importance this novel TXNIP/miR-204/MafA/insulin signalling cascade seems to play in the physiology and pathophysiology of glucose homeostasis in a whole animal.
Similarly, as we discovered that TXNIP downregulates STAT3 phosphorylation/activation which results in increased miR-204 expression and decreased insulin production, Stat3 deletion would be predicted to impair glucose homeostasis. In fact, pancreas-specific Stat3 knockout mice exhibit glucose intolerance and impaired insulin secretion36, providing additional in vivo support for the novel pathway identified in the present studies.
While multiple microRNAs have been implicated in beta-cell biology15–21 and some even in insulin gene expression18,37–39, miR-204 has not been one of them. Nevertheless, in a study looking at microRNA patterns to distinguish between different pancreatic tumour types, miR-204 was found to be highly expressed in insulinomas and to correlate with immunohistochemical expression of insulin22, which is commonly used as a marker to define the beta-cell origin of these tumours. In this context it is also important to remember that the high systemic levels of insulin found in patients with insulinomas are primarily due to neoplastic growth and abnormal increase in the number of insulin producing beta-cells giving rise to the tumour and not necessarily to increased insulin production per beta-cell. The observed correlation is therefore not able to provide any information about miR-204 regulating insulin expression and just underlines the predominant beta-cell expression pattern of miR-204 and, as stated by the authors, its potential usefulness in distinguishing insulinomas from other pancreatic tumours. As such their study remains very much in line with our findings.
The discovery that miR-204 is upregulated in diabetes and controls insulin transcription reveals a potential novel target for the future development of RNA therapeutics that would address an unmet need for increasing insulin production. In fact, silencing of microRNAs has just begun to be explored for the treatment of cancers and various other diseases including diabetes40. Finally, the surprising observation that TXNIP controls microRNA expression, which in turn can regulate key transcription factors such as MafA and thereby may modulate cell function as well as differentiation, fundamentally changes our understanding of the role TXNIP and microRNA biology play in health and disease.
Online Methods
Tissue culture
INS-1 beta-cells and stably transfected INS-TXNIP overexpressing human TXNIP or control INS-LacZ cells were grown as previously described8. Mouse pancreatic islets were isolated by collagenase digestion as detailed previously2. Human islets were obtained from the UAB Islet Resource Facility and always islets from the same donor were used as control and at least islets from 3 different donors were used per experiment.
Animal studies
All mouse studies were approved by the University of Alabama at Birmingham Animal Care and Use Committee and conform to the NIH Guide for the Care and Use of Laboratory Animals. The C3H congenic TXNIP-deficient HcB-19 (HcB) mice harbouring a naturally occurring nonsense mutation in the Txnip gene and the control C3H/DiSnA (C3H) strain as well as beta-cell-specific Txnip knockout mice (bTKO) and their controls (lox/lox) have been described previously3. Male, 1-year old animals were used for the studies shown.
Plasmid construction, transfection and luciferase assays
The TXNIP expression plasmid has been described previously8. The human insulin promoter region was amplified from genomic DNA with the listed primers (Supplementary Table 2) and subcloned into the MluI and HindIII restriction sites of the pGL3 enhancer vector (Promega, Madison, WI) providing the Ins-Luc reporter plasmid. The wild-type rat MafA 3′UTR region containing the miR-204 binding site was amplified from rat genomic DNA using primers designed based on the sequence found in the UCSC genome browser (http://genome.ucsc.edu) between the stop codon and poly A site of rat MafA (Supplementary Table 2). The human MafA 3′UTR was cloned based on the sequence available in NCBI (NM_201589). To generate the MafA mutant reporter plasmids, mutations were introduced by PCR and the primers listed in Supplementary Table 2. PCR products were subcloned into the SpeI and PmeI sites of the pMIR-REPORT Luciferase vector (Applied Biosystems, Foster City, CA) yielding the rat MafA-WT-3′Luc and MafA-M-3′Luc 3′UTR reporter plasmids as well as the human plasmids hMAFA-WT-3′Luc and hMAFA-M-3′Luc. All plasmids were confirmed by sequencing. The presence of predicted rat and mouse MafA 3′UTR sequences was also confirmed using RNA from rat INS-1 beta cells and mouse islets and the 3′RACE System for Rapid Amplification of cDNA End (Invitrogen, Grand Island, NY). For transfection experiments, INS-1 cells were plated in 6-well plates and grown overnight to ~60% confluence. Human islets (500 per tube) or mouse islets (100 per tube) were gently dispersed by incubation for 5 min in 200 μl of 0.05% Trypsin-EDTA (Invitrogen) at 37°C washed and resuspended in culture medium. Cells were transfected with hsa-miR-204 precursor or pre-miR negative control 2 (Applied Biosystem) at a final concentration of 25 nM using DharmaFECT1 transfection reagent (Dharmacon/Thermo Scientific, Chicago, IL). For luciferase assays, INS-1 cells (or human HEK293 cells) were grown overnight in 12-well plates and cotransfected with Ins-Luc, MafA-WT-3′Luc or MafA-M-3′Luc (or hMAFA-WT-3′Luc or hMAFA-M-3′Luc) and hsa-miR-204 or negative control using DharmaFECTDuo transfection reagent (Dharmacon/Thermo Scientific). To control for transfection efficiency, cells were cotransfected with pRL-TK (Promega) control plasmid expressing renilla luciferase and firefly as well as renilla luciferase activity were determined using the Dual Luciferase Assay Kit (Promega).
Quantitative real-time RT-PCR
qRT-PCR was performed as described previously3 using a lightcycler 480 system (Roche, Indianapolis, IN) and the primers listed in Supplementary Table 2. miR-204 expression was quantified using a TaqMan microRNA Assay (Applied Biosystems). Gene and microRNA expression results were corrected for 18S and U6 (or snoRNA135 for comparisons involving diabetic mouse models) run as internal standards, respectively. Internal standards were stable throughout all experiments and experiments were run in duplicates.
Immunoblotting
Protein extracts were prepared and analysed as described previously2. MafA was detected by Rabbit antibody to MafA (sc-66958X, Santa Cruz Biotechnology, Santa Cruz, CA). Total and phospho-STAT3 were detected by rabbit antibody to STAT3 (4904, Cell Signaling Technology, Danvers, MA) and rabbit phospho-STAT3-specific antibody (9145, Cell Signaling Technology).
Insulin content and secretion
Insulin content and secretion of isolated islets and INS-1 cells was assessed by ELISA and normalized for DNA content as described previously2. In brief, INS-1 cells were plated in 24-well plates and after overnight incubation at 5mM glucose the media was removed and cells were incubated in KRB buffer with 2.5 mM glucose (135 mM NaCl, 3.6 mM KCl, 10 mM Hepes [pH7.4], 5 mM NaHCO3, 0.5 mM NaH2PO4, 0.5 mM MgCl2, 1.5 mM CaCl2) for 1h. After stimulation with KRB buffer containing 16.7 mM of glucose for 1h, media was harvested for later insulin assay. Cells were lysed with 300 μl lysis buffer (100 mM Tris-HCl [pH8.0], 300 mM NaCl, 10 mM NaF, 2 mM NaOrthovanadate, 2%NP-40, 2 Protease cocktail tablets [Roche]) and lysates stored overnight at −20°C. After centrifugation at 5000 rpm for 5 min, the supernatants were harvested for insulin assay with the Ultra Sensitive Rat Insulin ELISA Kit (Crystal Chem Inc., Downers Groves, IL) and transfected mouse islets were analyzed with the Mouse Insulin Assay kit (ALPCO Diagnostics, Salem, NH). Results were normalized for DNA content as determined by Quant-iTPicoGreendsDNA Assay kit (Invitrogen).
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed as described previously41. 5 μg of rabbit antibody to MafA (A300-611A, Bethyl Laboratories, Montgomery, TX) or normal rabbit IgG (sc-2027, Santa Cruz) were used for immunoprecipitation and purified DNA fragments were quantified by qPCR with primers described in Supplementary Table 2.
Statistical analysis
Student’s t-tests or ONE-WAY-ANOVA were used to calculate the significance of a difference between two or more groups, respectively.
Supplementary Material
Acknowledgments
This work was supported by grants to A.S. from the US National Institutes of Health (R01DK-078752), the American Diabetes Association (7-12-BS-167) and the Juvenile Diabetes Research Foundation and JNJSI (40-2011-1). A-ZIP/F mice were a generous gift of C. Vinson, US National Institutes of Health.
Footnotes
The authors declare no competing interest.
Author Contributions
G. X. designed, performed and analysed the experiments and helped prepare the manuscript. J. C. was responsible for the mouse studies and islet isolations. G. J. performed most of the cloning and helped with some of the experiments. A. S. conceived the project, supervised the work and wrote the manuscript.
References
- 1.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-Interacting Protein: A Critical Link between Glucose Toxicity and Beta Cell Apoptosis. Diabetes. 2008;57:938–944. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, et al. Thioredoxin-Interacting Protein Deficiency Induces Akt/Bcl-xL Signaling and Pancreatic Beta Cell Mass and Protects Against Diabetes. FASEB J. 2008;22:3581–3594. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courboulin A, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulin R, et al. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2011;301:H1798–1809. doi: 10.1152/ajpheart.00654.2011. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life. 2001;52:29–33. doi: 10.1080/15216540252774739. [DOI] [PubMed] [Google Scholar]
- 7.Shalev A, et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology. 2002;143:3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- 8.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 9.Minn AH, et al. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2005;336:770–778. doi: 10.1016/j.bbrc.2005.08.161. [DOI] [PubMed] [Google Scholar]
- 10.Saxena G, Chen J, Shalev A. Intracellular Shuttling and Mitochondrial Function of Thioredoxin-interacting Protein. J Biol Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Fontes G, Saxena G, Poitout V, Shalev A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated beta-cell death. Diabetes. 2010;59:440–447. doi: 10.2337/db09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, et al. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32:e188. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Valverde SL, Taft RJ, Mattick JS. MicroRNAs in beta-cell biology, insulin resistance, diabetes and its complications. Diabetes. 2011;60:1825–1831. doi: 10.2337/db11-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes. 2011;60:1832–1837. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynn FC, et al. MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes. 2007;56:2938–2945. doi: 10.2337/db07-0175. [DOI] [PubMed] [Google Scholar]
- 18.Melkman-Zehavi T, et al. miRNAs control insulin content in pancreatic beta-cells via downregulation of transcriptional repressors. Embo J. 2011;30:835–845. doi: 10.1038/emboj.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poy MN, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 20.Poy MN, et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A. 2009;106:5813–5818. doi: 10.1073/pnas.0810550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tattikota SG, Poy MN. Re-dicing the pancreatic beta-cell: do microRNAs define cellular identity? Embo J. 2011;30:797–799. doi: 10.1038/emboj.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roldo C, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 23.Krol J, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 24.Xu G, Chen J, Jing G, Shalev A. Preventing beta-Cell Loss and Diabetes With Calcium Channel Blockers. Diabetes. 2012;61:848–856. doi: 10.2337/db11-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clee SM, Nadler ST, Attie AD. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am J Ther. 2005;12:491–498. doi: 10.1097/01.mjt.0000178781.89789.25. [DOI] [PubMed] [Google Scholar]
- 26.Moitra J, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artner I, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Lay J, Stein R. Involvement of PDX-1 in activation of human insulin gene transcription. J Endocrinol. 2006;188:287–294. doi: 10.1677/joe.1.06510. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, et al. The NeuroD1/BETA2 sequences essential for insulin gene transcription colocalize with those necessary for neurogenesis and p300/CREB binding protein binding. Mol Cell Biol. 1999;19:704–713. doi: 10.1128/mcb.19.1.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanhoose AM, et al. MafA and MafB regulate Pdx1 transcription through the Area II control region in pancreatic beta cells. J Biol Chem. 2008;283:22612–22619. doi: 10.1074/jbc.M802902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuoka TA, et al. Regulation of MafA expression in pancreatic beta-cells in db/db mice with diabetes. Diabetes. 2010;59:1709–1720. doi: 10.2337/db08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanderford NL, Andrali SS, Ozcan S. Glucose induces MafA expression in pancreatic beta cell lines via the hexosamine biosynthetic pathway. J Biol Chem. 2007;282:1577–1584. doi: 10.1074/jbc.M605064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Rani S, et al. Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell Physiol Biochem. 2010;25:667–674. doi: 10.1159/000315086. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostromina E, et al. Glucose intolerance and impaired insulin secretion in pancreas-specific signal transducer and activator of transcription-3 knockout mice are associated with microvascular alterations in the pancreas. Endocrinology. 2010;151:2050–2059. doi: 10.1210/en.2009-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolmeson C, et al. Differences in islet-enriched miRNAs in healthy and glucose intolerant human subjects. Biochem Biophys Res Commun. 2011;404:16–22. doi: 10.1016/j.bbrc.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 38.El Ouaamari A, et al. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes. 2008;57:2708–2717. doi: 10.2337/db07-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao X, Mohan R, Ozcan S, Tang X. MicroRNA-30d induces insulin transcription factor MafA and insulin production by targeting mitogen-activated protein 4 kinase 4 (MAP4K4) in pancreatic beta-cells. J Biol Chem. 2012;287:31155–31164. doi: 10.1074/jbc.M112.362632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krutzfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 41.Cha-Molstad H, Saxena G, Chen J, Shalev A. Glucose-stimulated Expression of Txnip Is Mediated by Carbohydrate Response Element-binding Protein, p300, and Histone H4 Acetylation in Pancreatic Beta Cells. J Biol Chem. 2009;284:16898–16905. doi: 10.1074/jbc.M109.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.