Abstract
Background
High-resolution manometry (HRM) can identify obstructive motor features at the esophagogastric junction and abnormalities in esophageal bolus transit. We sought to determine if HRM patterns can differentiate functional from organic mechanical lower esophageal sphincter (LES) obstruction.
Methods
Segmental characteristics of peristalsis were examined using HRM in symptomatic subjects with elevated postdeglutitive residual pressure gradients across the LES (≥5 mmHg). Sixteen consecutive patients with non-achalasic mechanical fixed obstruction were compared with 13 patients with elevated pressure gradients yet no mechanical obstruction and 14 asymptomatic controls. Pressure volumes were determined in mmHg cm s for peristaltic segments defined on HRM Clouse plots using an on-screen pressure volume measurement tool.
Key Results
Residual pressure gradients were similarly elevated in both patient groups. A visually conspicuous and distinctive shift in the proportionate pressure strengths of the second and third peristaltic segments was apparent across groups. Whereas the ratios of peak pressures and pressure volumes between second and third segments approached 1 in controls (0.92, 0.98), pressures shifted to the second segment in mechanical obstruction (peak pressure ratio: 1.2 ± 0.4; pressure volume ratio: 1.8 ± 0.9) and to the third segment in functional obstruction (peak ratio: 0.7 ± 0.2; volume ratio: 0.5 ± 0.2; P < 0.02 for any comparison of either group with controls). A threshold volume ratio of 1.0 correctly segregated 93% of obstruction (P < 0.0001); visual pattern inspection was equally effective.
Conclusions & Inferences
When elevated residual pressure gradients are present in non-achalasic patients, topographic characteristics of peristalsis can differentiate fixed mechanical obstruction from functional obstruction.
Keywords: functional lower esophageal sphincter obstruction, high-resolution manometry, mechanical lower esophageal sphincter obstruction, postdeglutitive residual pressure gradient
INTRODUCTION
Partially obstructing processes at the level of the lower esophageal sphincter (LES; e.g. fundoplication) can be detected in patients with peristalsis by the increase in intra-bolus pressure that precedes the contraction wave in the distal esophagus.1,2 Increased intra-bolus pressure (or ‘ramp pressure’) represents entrapment of the bolus between the propagating peristaltic wave and abnormally high resistance from the obstructing process. One of the most common causes for increased intra-bolus pressure is the functional obstruction produced by incomplete LES relaxation.3,4 In the presence of peristalsis, this manometric abnormality is commonly associated with other features of the spastic motor disorder and likely represents a manifestation of the inhibitory nerve dysfunction responsible for this spectrum of disorders.3,5 Differentiating this type of functional obstruction from other mechanical explanations on manometric grounds would have both diagnostic and therapeutic utility, especially in the postoperative setting. As of yet, no manometric characteristics have been reported to help distinguish partial mechanical from functional LES obstruction.
High-resolution manometric methods with topographic plotting (Clouse plots) have demonstrated that peristalsis is comprised of a chain of coordinated pressure segments.6–8 Two dominant regions of nearly equal size comprise the majority of the esophageal body, presumably representing the transition from cholinergic to non-cholinergic control in the smooth muscle region of the esophagus (Fig. 1A). Preliminary findings in the obstructed opossum model suggest a relative increase in pressure strength of the proximal segment with partial obstruction from fundoplica-tion.9,10 In contrast, spastic disorders are associated with a shift in relative contraction strength to the distal of the two smooth muscle segments.11
Figure 1.
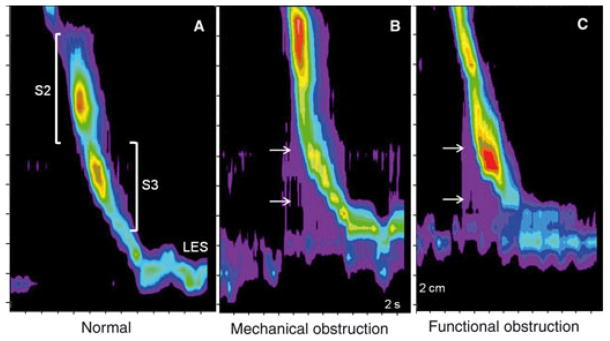
Smooth muscle contraction segments (S2, segment 2; S3, segment 3) in normal controls (A), fixed mechanical obstruction (B) and functional obstruction (C). Note the conspicuous shift in contraction vigor to segment 2 in mechanical obstruction, and to segment 3 in functional obstruction. Both patterns of obstruction are associated with increased intra-bolus pressure in the distal esophagus (arrows), and evidence of increased postdeglutitive residual LES pressure when compared to normals, findings that can be appreciated visually on inspection of Clouse plots.
We presumed from these observations that topographic characteristics of peristalsis might be useful in the manometric diagnosis of partial obstruction at the level of the LES. Cases were identified by their increase in intra-bolus pressure with swallowing, additional clinical data were examined to establish the nature of partial obstruction in each case, and pressure characteristics of the two, distal peristaltic segments were measured using topographic methods.
METHODS
Subjects
The subjects reported in this investigation were identified from review of manometric tracings obtained from dysphagic subjects who had been referred to the motility center of Barnes-Jewish Hospital, St. Louis, MO, for clinical manometric evaluation over a 2-year time span. Included were consecutive subjects demonstrating a postdeglutitive residual pressure gradient across the LES of at least 5 mmHg and intact peristalsis. Subjects were segregated into a mechanical obstruction group and a functional obstruction group based on clinical history and objective findings on endoscopy and radiologic transit studies. The mechanical obstruction group was made up of patients with anatomic obstruction found on endoscopy or barium swallow at the level of the LES. Subjects in the functional obstruction group had a normal endoscopy and/ or barium swallow. Esophageal and gastric pressures to determine the gradient were measured 1–6 s along the swallow at sites within 2 cm above and below the HRM identified region of the LES – the region typically addressed with the use of the ‘esleeve’ with current solid-state HRM techniques.4,12 This method identifies the increased intraesophageal and intra-bolus pressure seen in achalasia, with incomplete LES relaxation associated with spastic disorders, and the partial obstruction produced by fundoplication.4,12 Increased intraesophageal pressure over gastric baseline manifests graphically as stripes of isobaric pressure elevation ending abruptly at the level of the LES (Fig. 1B,C). The threshold value of 5 mmHg was chosen because of its high discriminant value for incomplete LES relaxation present in achalasia.4,5 An asymptomatic cohort of 14 healthy volunteers enrolled for the calculation of institutional normative data for HRM studies formed the control group for this study, and data from this cohort were used to compare findings from the mechanical obstruction and functional obstruction groups. The review of manometric data for the evaluation of topographic techniques was approved by the Human Studies Committee (IRB) of Washington University School of Medicine.
Clinical information was extracted from data accompanying the referral and from the self-report sheets completed by the patients at the time of manometry. All patients had undergone formal clinical evaluation including endoscopy. Final diagnosis was established from this information and augmented by contacting the referring physicians for supplemental test results when required.
Manometric methods
Esophageal HRM had been performed on each patient using a prototype water-perfused HRM system that preceded the development of the current solid-state HRM systems, and therefore without the ‘esleeve’ function for detailed LES interrogation. A water-perfused, silicone catheter with 21 recording sites spaced at 1 cm intervals and a computerized data acquisition system capable of topographic analysis methods was utilized for each HRM study (Medical Measurement Systems, Enchede, Holland). The use of these methods for clinical esophageal manometry has been reported previously.13 In brief, the esophageal motility catheter is passed transnasally in the fasted subject. The catheter is advanced such that the recording ports have an intragastric location, and a pull-through maneuver is performed so that resting LES pressure can be determined as the mean of 10 consecutive recordings sites sampling 360° in circumference. The catheter is then positioned such that ≥2 recording sites remain in an intragastric position while the more proximal sites record from the LES and approximately 80% of the esophageal body. Ten swallows with 4 mL ambient temperature water are spaced at 30 s intervals before the catheter is repositioned such that the proximal recording site rests in the upper esophageal sphincter (UES). In this position, which samples 85% of the esophageal body in addition to UES, an additional ten swallows are taken, and the study is terminated.
Trans-sphincteric gradient measurements and topographic characteristics of peristalsis were determined for this study when the catheter was in the distal position, as were conventional manometric parameters used to classify the type of motor dysfunction. A combination of four manometric characteristics were used to classify each tracing according to a previously described algorithm: (i) peristaltic swallow performance, extracted from recordings at 3 and 11 cm proximal to the LES, (ii) distal contraction wave characteristics: averaged esophageal body contraction amplitude (3 and 7 cm proximal to the esophagus, normal 36–180 mmHg) and wave duration (evaluated 3 and 7 cm proximal to the LES, normal <5.1 s), (iii) LES resting pressure end-expiratory LES basal pressure (normal 5–25 mmHg), and (iv) LES relaxation as assessed by the trans-LES residual pressure gradient as described above.14–16 Spastic features in the esophageal body were defined as any of the following: double peaked waves >15%, simultaneous swallows >20%, any triple peaked waves, wave duration >5.7 s or averaged wave amplitude >180 mmHg.17 Finally, time to nadir LES pressure was measured from swallow initiation as a further measure of LES relaxation.
Clouse plots from the 10 distal swallows were analyzed to determine the best pressure trough locations separating the peristaltic sequence into individual segments. Based on previous observations regarding shifts in relative pressure activity of the two distal segments, topographic measurements selected for the study focused on these segments.6,8 Locations and amplitudes of the intersegment pressure troughs were made, thereby also allowing extraction of the length of each pressure segment (Fig. 2). Peak segmental amplitude and its location were determined by locating the highest amplitude within each segment. Segmental pressure volume measurements in mmHg cm s were determined for each smooth muscle segment from an on-screen pressure volume measurement tool using methods described previously.8 Briefly, a region of interest is outlined using the onscreen tool; a plane representing 10 mmHg, the lowest pressure of interest is designated; and the cumulative pressure above this plane is recorded in mmHg cm s. A similar measurement has been previously described encompassing both smooth muscle peristaltic segments, when it is termed the distal contraction integral.18
Figure 2.
Segmental pressure amplitudes compared between normal controls, mechanical obstruction and functional obstruction. The most significant difference was seen in segment 3 peak amplitudes, which were highest in functional obstruction and lowest in mechanical obstruction; both values were significantly different from normals.
Statistical methods
Grouped values are reported as mean ± SEM unless indicated otherwise. Categorical and grouped data were compared using Fisher’s exact test, chi-squared test or two-tailed Student’s t-test as appropriate. In each case, P < 0.05 was required for statistical significance.
RESULTS
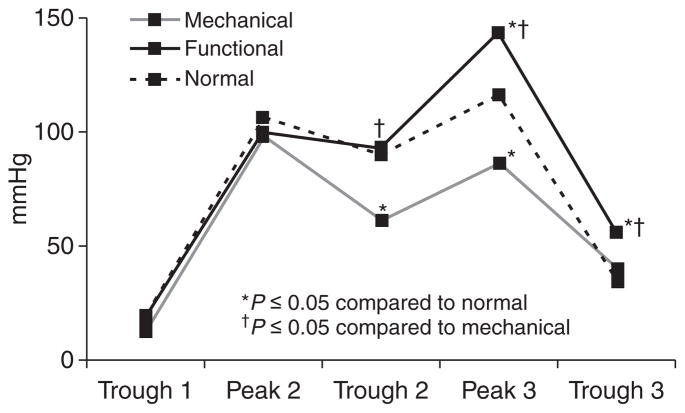
A total of 29 subjects (47.8 ± 2.6 years, 55.2% female) with HRM findings of an elevated trans-sphincteric residual pressure gradient were identified on review of our database over a 2-year period. Of these, 16 (55.2%) had evidence of fixed mechanical LES obstruction; 12 subjects with postfundoplication dysphagia (all with intact fundoplication), two with obstructing para-esophageal hernia, two with tight distal esophageal strictures. The remaining 13 subjects had a persistent elevated gradient across the LES without any fixed mechanical explanation for the obstruction (Table 1); one patient in this group had a 2 cm sliding hiatal hernia which was non-obstructing. All subjects had transit symptoms (dysphagia and/or regurgitation). Manometric findings in the study subjects were compared with 14 asymptomatic controls (38.7 ± 3.7 years, 62.3% female). The two groups with obstruction resembled each other from the standpoint of basal end-expiratory LES pressure (P = 0.38) and elevated trans-sphincteric pressure gradient (P = 0.53). The gradient in each group was significantly elevated compared with controls (Table 1). The time from swallow initiation to nadir LES pressure was longer in the mechanical obstruction group compared with the functional obstruction group (P = 0.005); both patient groups were significantly different from normals (P < 0.0001 compared with either patient group). Spastic findings in the esophageal body were noted more frequently in functional obstruction (46%) compared with the other two groups (P < 0.05).
Table 1.
Clinical and manometric characteristics of the study groups
| Mechanical obstruction (n = 16) | Functional obstruction (n = 13) | Normal controls (n = 14) | |
|---|---|---|---|
| Age (years)* | 51.1 ± 3.2 | 43.9 ± 3.9 | 38.7 ± 3.7 |
| Gender (M : F) | 8 : 8 | 5 : 8 | 5 : 9 |
| Peristaltic sequences | 95.0 ± 1.8% | 93.7 ± 2.4% | 96.4 ± 2.4% |
| Segment 1 (% eso length) | 6.4 ± 0.2 cm (27.9%) | 6.0 ± 0.7 cm (25.7%) | 5.5 ± 0.3 cm (26.3%) |
| Segment 2 (% eso length) | 10.2 ± 0.6 cm (44.8%) | 9.1 ± 0.8 cm (39.0%) | 8.6 ± 0.6 cm (41.2%) |
| Segment 3 (% eso length)< | 5.5 ± 0.4 cm (24.3%) | 7.6 ± 0.3 cm (33.1%) | 6.5 ± 0.4 cm (31.1%) |
| Spastic motor pattern3 | 3 (19%) | 6 (46%) | 1 (7%) |
| LES basal pressure (mmHg) | 14.6 ± 0.4 | 15.1 ± 0.3 | 15.9 ± 0.3 |
| Trans-LES gradient (mmHg)§ | 13.5 ± 1.0 | 12.5 ± 1.2 | 3.1 ± 0.4 |
| Time to nadir LES pressure (s)§ | 5.8 ± 0.3– | 4.5 ± 0.2 | 1.9 ± 0.1 |
Normals significantly younger than subjects with mechanical obstruction, P = 0.02.
Mechanical obstruction group significantly different from other two groups, P ≤ 0.004.
Includes any of the following: >15% double peaked waves, any triple peaked waves, mean distal esophageal amplitude ≥180 mmHg, mean wave duration ≥5.7 s; P = 0.049 across groups.
Normals significantly lower than other two groups, P < 0.0001 for each comparison.
P = 0.005 compared to functional obstruction.
Mechanical obstruction produced an increase in length of the second segment relative to the third segment (98.6% increase). This change was significantly different from both controls (41.5%) and subjects with functional obstruction (22.2%, P ≤ 0.04 for each comparison with mechanical obstruction). In the group with mechanical obstruction, the second segment represented nearly 65% of the combined lengths of the smooth muscle segments; in comparison, the two segments were of approximately equal proportionate length in the control and functional obstruction groups. No difference was noted in the length of the skeletal muscle segment across the three groups (Table 1).
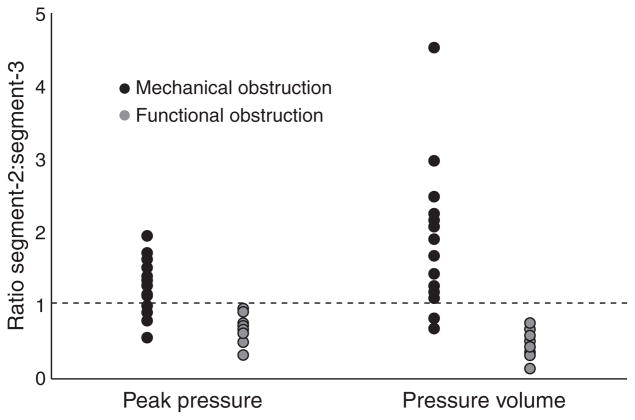
Pronounced alterations in Clouse plot landmarks were seen in the third segment. Compared with the other two groups, mechanical obstruction was associated with a reduction in peak pressure in the third segment, together with a reduction in the trough pressure between the second and third segments (Fig. 2). In contrast, functional obstruction was associated with an increase in peak pressure of the third segment and in the pressure trough separating this segment from LES after-contraction (P ≤ 0.05 compared with mechanical obstruction and controls for both comparisons). Whereas, the ratios of peak pressures between second and third segments approached 1 in controls (0.92), pressures shifted to the second segment in mechanical obstruction (peak pressure ratio: 1.2 ± 0.4) and to the third segment in functional obstruction (peak pressure ratio: 0.7 ± 0.2; P < 0.02 for comparisons of either group with controls). A ratio of 1.0 segregated 11 of 16 subjects (68.6%) of mechanical obstruction from all 13 subjects with functional obstruction, identifying the obstruction pattern in 82.8% of symptomatic subjects (Fig. 3, P = 0.0001). Further, LES relaxation patterns were different between mechanical and functional obstruction (Table 1), but further evaluation is needed with modern HRM systems incorporating electronic interrogation of the LES to better characterize LES relaxation patterns.
Figure 3.
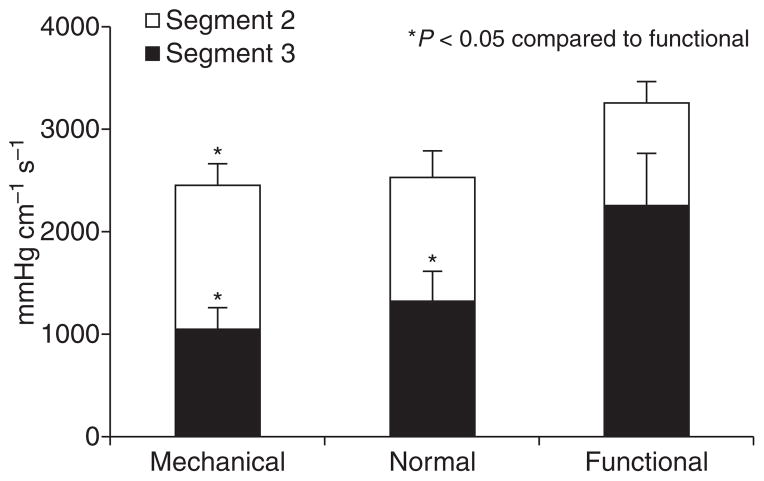
Pressure volume (in mmHg cm s) in the three study groups. Overall contraction vigor was highest in functional obstruction, where pressure volume was proportionately highest in segment 3. The exact opposite was seen in mechanical obstruction, where segment 2 demonstrated more vigorous contraction compared to segment 3.
The most discriminating characteristic, however, was the shift in cumulative pressure volume. Within the obstructed patients, pressure volumes in each segment were significantly different between the mechanical and functional obstruction patients (Fig. 4). Mechanical obstruction was associated with higher pressure volume in the second segment (1410.84 ± 127.8 mmHg cm s) and lower pressure volume in the third segment (1045.76 ± 107.9 mmHg cm s) compared with normals (second segment, 1205.65 ± 171.3 mmHg cm s, third segment, 1325.66 ± 192.5 mmHg cm s). On the other hand, functional obstruction was associated with the reverse direction of change, lower pressure volume in the second segment and higher pressure volume in the third segment compared with controls (second segment, 1009.38 ± 169.9 mmHg cm s, third segment, 2255.40 ± 412.80 mmHg cm s). The ratio of pressure volumes from second to third segment were calculated and compared. The mean ratio was 0.98 ± 0.1 in normal controls indicating equivalence of second and third segments. Mean ratios were 0.52 ± 0.1 in functional obstruction, but 1.8 ± 0.2 in mechanical obstruction, significantly different from controls (P < 0.007), and highly discriminatory of the type of obstruction (P < 0.0001). The ratio exceeding 1 identified 14 of 16 subjects (87.5%) with mechanical obstruction, yet included none of the 13 subjects with functional obstruction, correctly segregating 93.1% of symptomatic subjects (Fig. 3, P < 0.0001). A threshold ratio of 0.8 performed even better, correctly segregating 96.5% of subjects (P < 0.0001).
Figure 4.
Ratios of peak pressure and pressure volume (segment 2 : segment 3) in mechanical and functional obstruction. A threshold ratio of 1 correctly identified 82.8% of obstruction using peak pressure, and 96.5% using pressure volume (P ≤ 0.0001).
These observations translated into distinctive patterns on Clouse plots in both mechanical and functional obstruction. With mechanical obstruction produced by fundoplication, there is a conspicuous shift in strength of contraction to the second segment compared to normal (Fig. 1B). The exact opposite is seen with functional obstruction (Fig. 1C). Increased gradients over the gastric baseline are seen as pressure increments preceding peristalsis and ending abruptly at the level of the LES.
DISCUSSION
In this study, we demonstrate using HRM that differentiation of mechanical and functional esophageal obstruction can be accomplished by visual and analytical inspection of Clouse plots. Similar to the opossum, the mechanically obstructed human esophagus demonstrates an increase in contraction vigor in the second segment. In contrast, the functionally obstructed esophagus is associated with a shift in the force of contraction to the third segment. These findings improve the diagnostic ability of HRM, by directing diagnosis of mechanical obstruction (in contrast to functional LES dysfunction) when not clinically apparent; this may help select best candidates for symptomatic treatment aimed at gradient reduction. Further, these findings lend credence to the notion that visual pattern recognition on HRM Clouse plots may play a significant role in the identification of motor mechanisms in symptomatic patients.19
Pressure volume measurements were utilized in assessing contractile vigor of each of the smooth muscle contraction segments. This measurement, originally described by Clouse et al. assesses the cumulative pressure over a defined esophageal length for a specified time duration, using a pressure plane of 10 mmHg above the esophageal baseline as the bottom surface of this measurement.8 Volume measurements take advantage of axial interpolations and have proved sensitive when measuring the effects of pharmacologic manipulation on esophageal contraction segments.20 Further, similar pressure volume measurements encompassing both the smooth muscle segments have been used to designate hypercontractility in the smooth muscle esophagus, when the measure is termed ‘distal contractile integral’.18 Therefore, pressure volume measurement is a valid technique in assessing contractile vigor, and constitutes a representative measure of contractile force over time and space in the esophagus.
The closest animal model of the human esophagus is the opossum, and many physiologic characteristics of the human esophagus have been elucidated using the opossum esophagus.21,22 In the setting of distal esophageal obstruction in the opossum, contractile amplitudes decrease in the distal esophagus; while they increase in the proximal esophageal smooth muscle.9,23 A similar augmentation of contractile amplitude following mechanical obstruction has also been demonstrated in the human esophagus following fundoplication.24,25 Using conventional esophageal manometry Heider et al. demonstrated that esophageal peristalsis ‘normalizes’ following fundoplication in a majority of patients, with a significant increase in postoperative contractile amplitude.26 These findings corroborate our observation of increased contractile strength in the second segment postfundoplication, and likely represents a ‘rallying effect’ of proximal smooth muscle contraction in an attempt to overcome mechanical obstruction at the level of the gastro-esophageal junction. Similar augmentation of contraction amplitudes is noted in many hollow viscus upstream of an obstructive process.27,28 We believe our findings demonstrate the application of these physiologic observations in human esophageal pathophysiology. However, these observations do not completely explain the weaker third segment in the mechanically obstructed subjects. Since all the mechanically obstructed subjects had either reflux disease or underwent fundoplication for management of reflux disease, it is plausible that the weaker third segment represents a hypomotile pattern that can sometimes be associated with reflux disease.29 Other potential mechanisms could include impaired esophageal shortening associated with reflux disease, or prestenotic dilation seen upstream of obstructed viscus – the latter mechanism can dampen contractile amplitudes and result in lower measured pressure, and consequently, lower pressure volume. Alternatively, an overly long wrap may diminish contractile strength of the third segment in the postfundoplication state by making it part of the LES.30
Functional obstruction manifesting as incomplete LES relaxation is recognized as part of the spastic disorder spectrum, from aberrant inhibitory mechanisms.3,5,31,32 The presence of these aberrant inhibitory influences have been linked to esophageal hypersensitivity, and we have preliminarily reported a similar shift in contraction vigor to the third segment in subjects with acid sensitivity.33 These reports and our current findings suggest that the HRM features on Clouse plots in functional obstruction are probably features of the motor disorder rather than secondary changes from the obstruction itself. Additionally, there is limited evidence that botulinum toxin injection into the LES may result in improvement of dysphagia.34 However, despite the concordance of findings between functional LES obstruction and non-specific spastic disorders, alternate etiologies could be responsible for obstructive features at the gastroesophageal junction. It is well recognized that diaphragmatic crural contraction, especially in the presence of a small hiatus hernia, can result in an obstructed appearance at the gastroesophageal junction.35 In the current study cohort, however, careful evaluation of the HRM plots did not suggest a hiatus hernia as the cause for obstruction.
Our study does have some limitations. Firstly, the sample size was small, and the mechanical obstruction group was dominated by postfundoplication dysphagia, which can alter contractile function in the esophagus. Subjects with clearly defined obstructive symptoms from alternate causes of obstruction were infrequent during the time period of the study; we had to limit the study because our motility experience transitioned to solid state high-resolution systems which have different capabilities. Further, esophageal biopsies were not uniformly performed on the study cohort, and etiologies such as eosinophilic esophagitis were not systematically excluded. Nevertheless, we believe these findings are an attempt, albeit preliminary, to correlate segmental contraction abnormalities in patients to those previously reported in the obstructed opossum esophagus; the findings reported do appear to be concordant with previous descriptions of the obstructed esophagus. Secondly, given the retrospective nature of the study, the project was not geared to determine outcome data; therefore the impact of management directed by HRM is unavailable. Investigators were not blinded to the etiology of dysphagia, which is a potential source of bias. Further, we do not have data on prefundoplication manometry studies on the subjects with postfundoplication dysphagia, or post therapy manometry on any of the subjects; we realize that this could have strengthened our observations. Finally, our observation of a greater delay in LES relaxation in subjects with mechanical obstruction is hindered by the limitations of the prototype HRM system used in this study, without the ‘esleeve’ function. While we believe the findings may help direct clinical impression regarding the cause of obstructive phenomena at the esophagogastric junction, clinical presentation and alternate evaluations (including endoscopy and barium studies) need to be factored in while deciding further management. Hence, at this stage, it is fair to say that these segmental findings offer support for clinical impressions when concordant with alternate testing and clinical impressions. Therefore, the full clinical relevance of our findings is difficult to determine.
In conclusion, visual and analytical inspection of Clouse plots on HRM demonstrate discriminating segmental changes between mechanical and functional obstruction at the esophagogastric junction. These findings demonstrate the importance of evaluating for aberrations in individual contraction segments while analyzing HRM studies despite the fact that endoscopy and radiologic studies will almost always have findings to identify the mechanically obstructed esophagus. Further prospective and blinded studies are needed to replicate our results in larger patient cohorts, and to determine if patient outcome can be predicted by these segmental changes.
Acknowledgments
The authors wish to acknowledge the contributions of the late Ray E. Clouse, Professor of Medicine and Psychiatry at Washington University School of Medicine, to the fields of esophagology and HRM in general and to this project in particular. The concepts evaluated in this report were conceived in collaboration with Dr. Clouse, but he passed away before the manuscript could be completed. This paper is therefore dedicated to his memory.
The authors also acknowledge the Mentors in Medicine program, Department of Medicine, Washington University School of Medicine for providing time and support for VMK to participate in this project.
Footnotes
AUTHOR CONTRIBUTIONS
CPG was involved in study concept and design, data collection, analysis and interpretation, manuscript preparation and revision; VMK was involved in additional data collection, supervision of data analysis, subgroup analysis, manuscript preparation and revision.
COMPETING INTERESTS
The authors have no competing interests.
References
- 1.Pandolfino JE, Ghosh SK, Lodhia N, Kahrilas PJ. Utilizing intraluminal pressure gradients to predict esophageal clearance: a validation study. Am J Gastroenterol. 2008;103:1898–905. doi: 10.1111/j.1572-0241.2008.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identified clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–42. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 3.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13:2219–25. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staiano A, Clouse RE. Detection of incomplete lower esophageal sphincter relaxation with conventional point-pressure sensors. Am J Gastroenterol. 2001;96:3258–67. doi: 10.1111/j.1572-0241.2001.05323.x. [DOI] [PubMed] [Google Scholar]
- 5.Aliperti G, Clouse RE. Incomplete lower esophageal sphincter relaxation in subjects with peristalsis: prevalence and clinical outcome. Am J Gastroenterol. 1991;86:609–14. [PubMed] [Google Scholar]
- 6.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677–84. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 7.Clouse RE, Staiano A. Topography of normal and high-amplitude esophageal peristalsis. Am J Physiol. 1993;265:G1098–107. doi: 10.1152/ajpgi.1993.265.6.G1098. [DOI] [PubMed] [Google Scholar]
- 8.Clouse RE, Alrakawi A, Staiano A. Intersubject and interswallow variability in topography of esophageal motility. Dig Dis Sci. 1998;43:1978–85. doi: 10.1023/a:1018838710214. [DOI] [PubMed] [Google Scholar]
- 9.Shirazi S, Schulze-Delrieu K. Role of altered responsiveness of hypertrophic smooth muscle in manometric abnormalities of the obstructed opossum oesophagus. Neurogastroenterol Motil. 1996;8:111–9. doi: 10.1111/j.1365-2982.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 10.Prakash C, Underwood RA, Haroian L, Soper NJ, Clouse RE. Topographic manometric analysis following fundoplication in the opossum identifies a potentially protective response against postoperative peristaltic dysfunction. Gastroenterology. 1999;114:A973. [Google Scholar]
- 11.Clouse RE, Staiano A, Alrakawi A. Topographic analysis of esophageal double-peaked waves. Gastroenterology. 2000;118:469–76. doi: 10.1016/s0016-5085(00)70252-6. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878–85. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 13.Clouse RE, Staiano A, Alrakawi A. Development of a topographic analysis system for manometric studies in the gastrointestinal tract. Gastrointest Endosc. 1998;48:395–401. doi: 10.1016/s0016-5107(98)70010-0. [DOI] [PubMed] [Google Scholar]
- 14.Clouse RE, Staiano A. Contraction abnormalities of the esophageal body in patients referred to manometry. A new approach to manometric classification. Dig Dis Sci. 1983;28:784–91. doi: 10.1007/BF01296900. [DOI] [PubMed] [Google Scholar]
- 15.Richter JE, Wu WC, Johns DN, et al. Esophageal manometry in 95 healthy adult volunteers. Variability of pressures with age and frequency of ‘‘abnormal’’ contractions. Dig Dis Sci. 1987;32:583–92. doi: 10.1007/BF01296157. [DOI] [PubMed] [Google Scholar]
- 16.Clouse RE, Staiano A. Manometric patterns using esophageal body and lower sphincter characteristics. Findings in 1013 patients. Dig Dis Sci. 1992;37:289–96. doi: 10.1007/BF01308186. [DOI] [PubMed] [Google Scholar]
- 17.Leite LP, Johnston BT, Barrett J, Castell JA, Dastell DO. Ineffective esophageal motility (IEM): the primary finding in patients with nonspecific esophageal motility disorder. Dig Dis Sci. 1997;42:1859–65. doi: 10.1023/a:1018802908358. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 19.Grübel C, Hiscock R, Hebbard G. Value of spatiotemporal representation of manometric data. Clin Gastroenterol Hepatol. 2008;6:525–30. doi: 10.1016/j.cgh.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Clouse RE, Staiano A. The effects of cisapride on the topography of oesophageal peristalsis. Aliment Pharmacol Ther. 1996;10:875–82. doi: 10.1046/j.1365-2036.1996.94266000.x. [DOI] [PubMed] [Google Scholar]
- 21.Weisbrodt NW, Christensen J. Gradients of contractions in the opossum esophagus. Gastroenterology. 1972;62:1159–66. [PubMed] [Google Scholar]
- 22.Underwood RA, Prakash C, Yarakeshi K, et al. Didelphis virginiana, the North American Opossum: a new-old model for the study of upper-gut motility. Surg Endosc. 1999;13:S83. [Google Scholar]
- 23.O’Rourke RW, Seltman AK, Chang EY, et al. A model for gastric banding in the treatment of morbid obesity: the effect of chronic partial gastric outlet obstruction on esophageal physiology. Ann Surg. 2006;244:723–33. doi: 10.1097/01.sla.0000218082.12999.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbella FA, Tedesco P, Nipomnick I, Fisichella PM, Patti MG. Effect of partial and total laparoscopic fundoplication on esophageal body motility. Surg Endosc. 2004;21:285–8. doi: 10.1007/s00464-006-0108-2. [DOI] [PubMed] [Google Scholar]
- 25.Tsereteli Z, Sporn E, Astudillo JA, Miedema B, Eubanks WS, Thaler K. Laparoscopic Nissen fundoplication is a good option in patients with abnormal esophageal motility. Surg Endosc. 2009;23:2292–5. doi: 10.1007/s00464-008-0314-1. [DOI] [PubMed] [Google Scholar]
- 26.Heider TR, Behrns KE, Koruda MJ, et al. Fundoplication improves disordered esophageal motility. J Gastrointest Surg. 2003;7:159–63. doi: 10.1016/s1091-255x(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 27.Hillemeier C, Biancani P. Mechanical properties of obstructed colon in a Hirschsprung’s model. Gastroenterology. 1990;99:995–1000. doi: 10.1016/0016-5085(90)90618-b. [DOI] [PubMed] [Google Scholar]
- 28.Lu C, Schulze-Delrieu K, Shirazi S, Cram M, Raab J. Dynamic imaging of obstructed opossum esophagus. From altered load to altered contractility. Dig Dis Sci. 1994;39:1377–88. doi: 10.1007/BF02088037. [DOI] [PubMed] [Google Scholar]
- 29.Ang D, Blondeau K, Sifrim D, Tack J. The spectrum of motor function abnormalities in gastroesophageal reflux disease and Barrett’s esophagus. Digestion. 2009;79:158–68. doi: 10.1159/000210265. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh SK, Kahrilas PJ, Zaki T, Pandolfino JE, Joehl RJ, Brasseur JG. The mechanical basis of impaired esophageal emptying postfundoplication. Am J Physiol Gastrointest Liver Physiol. 2005;289:G21–35. doi: 10.1152/ajpgi.00235.2004. [DOI] [PubMed] [Google Scholar]
- 31.Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology. 2008;135:756–69. doi: 10.1053/j.gastro.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clouse RE. Spastic disorders of the esophagus. Gastroenterologist. 1997;5:112–27. [PubMed] [Google Scholar]
- 33.Kushnir VM, Gyawali CP. High resolution manometry differentiates acid sensitivity from reflux disease in non-cardiac chest pain. Gastroenterology. 2009;136:A152–3. [Google Scholar]
- 34.Porter R, Gyawali CP. Botulinum toxin in dysphagia syndromes with preserved esophageal peristalsis and incomplete lower esophageal sphincter relaxation. Neurogastroenterol Motil. 2011;23:139–e28. doi: 10.1111/j.1365-2982.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- 35.Pandolfino JE, Kwiatek MA, Ho K, Kahrilas PJ. Unique features of esophagogastric junction pressure topography in hiatus hernia patients with dysphagia. Surgery. 2010;147:57–64. doi: 10.1016/j.surg.2009.05.011. Epub 2009 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]