Abstract
Background
Respiratory isolation of inpatients during evaluation for TB is a slow and costly process in low-burden settings. Xpert MTB/RIF (Xpert) is a novel molecular test for tuberculosis (TB) that is faster and more sensitive but substantially more expensive than smear microscopy. No previous studies have examined the costs of molecular testing as a replacement for smear microscopy in this setting.
Methods
We conducted an incremental cost–benefit analysis comparing the use of a single negative Xpert versus two negative sputum smears to release consecutive adult inpatients with presumed TB from respiratory isolation at an urban public hospital in the United States. We estimated all health-system costs and patient outcomes related to Xpert implementation, diagnostic evaluation, isolation, hospitalization, and treatment. We performed sensitivity and probabilistic uncertainty analyses to determine at what threshold the Xpert strategy would become cost-saving.
Results
Among a hypothetical cohort of 234 individuals undergoing evaluation for presumed active TB annually, 6.4% had culture-positive TB. Compared to smear microscopy, Xpert reduced isolation bed utilization from an average of 2.7 to 1.4 days per patient, leading to a 48% reduction in total annual isolation bed usage from 632 to 328 bed-days. Xpert saved an average of $2,278 (95% uncertainty range $1582–4570) per admission, or $533,520 per year, compared with smear microscopy.
Conclusions
Molecular testing for TB could provide substantial savings to hospitals in high-income countries by reducing respiratory isolation usage and overall length of stay.
Introduction
Guidelines in high-income countries recommend initiating respiratory isolation of patients being evaluated for pulmonary tuberculosis (TB) pending the results of serial sputum smear microscopy. [1], [2] This multi-day process consumes a significant amount of patient time and hospital resources but has a limited yield, with only 4–10% of isolated inpatients generally found to have smear-positive TB. [3], [4], [5], [6], [7] An alternative to serial sputum examination is to use nucleic acid amplification tests (NAATs), which are more sensitive than microscopy and can be performed within hours. [8] The Centers for Disease Control and Prevention (CDC) has recommended that NAATs be incorporated routinely into TB diagnostic strategies including triaging inpatients out of respiratory isolation. [2] High-quality evidence shows that NAATs have high diagnostic accuracy [9] and rapid turn-around times [5], [10], and that they could have substantial clinical impact. [5], [10] Nevertheless, NAATs have not been widely adopted, particularly in the inpatient setting. One major concern about NAATs is that, until recently, no study had shown them to be cost-effective in high-income, low-incidence countries. [11], [12], [13], [14], [15], [16], [17], [18].
Xpert MTB/RIF (“Xpert”, Cepheid, Inc., Sunnyvale, CA) is a novel molecular diagnostic test with high sensitivity and specificity for pulmonary TB. [19] Xpert is simpler, faster, and less labor intensive than sputum smear microscopy and other commercial NAATs, and these characteristics have led to its increasing adoption in low- and middle-income countries where the burden of TB is high. [20], [21] Although Xpert is expensive, its high sensitivity and specificity for smear-positive TB on a single sputum sample make it attractive as a potential alternative to serial sputum smear microscopy to guide use of scarce and costly respiratory isolation rooms. The FDA recently approved the use of Xpert for detecting tuberculosis on July 25, 2013 [22]; however, there is limited information about its potential impact in various settings. Therefore, we conducted a cost-benefit analysis comparing Xpert to smear microscopy for guiding triage of inpatients being evaluated for TB at an urban public hospital in the U.S.
Methods
Patients
To inform development of the cost-benefit model, we reviewed medical records of consecutive patients undergoing evaluation for TB from January 1 through December 31, 2009, at San Francisco General Hospital, a university-affiliated urban public hospital. We defined any inpatient who underwent microbiologic testing for TB while in respiratory isolation as representative of the modeled population.
Standard Diagnostic Strategy
Infection control policies at San Francisco General Hospital require that inpatients being evaluated for TB be placed in a negative-pressure respiratory isolation room until two sputum samples collected at least eight hours apart have been examined and found negative for acid-fast bacilli (AFB). A clinical laboratory scientist (CLS) in the central microbiology lab performs smear examination and reports the results once daily, seven days a week. Specimens received after 4 pm are processed the following day. Finally, in order to complete the microbiologic evaluation for TB, each patient should provide a third sputum sample for smear examination before or after discharge, and all three sputum samples should undergo mycobacterial culture and speciation. [23].
Xpert Diagnostic Strategy
In comparison, we proposed an alternative strategy using Xpert testing of a single sputum sample to guide the triage decision. In this strategy, we considered a negative result on one sputum Xpert test to be equivalent to two negative sputum smear exams for the purpose of allowing discharge of a patient with possible TB from respiratory isolation, and, absent any ongoing indication for hospitalization, from the hospital. We assumed that any positive Xpert result would lead to continued respiratory isolation and initiation of anti-mycobacterial treatment, and that the time required for a clinician to discontinue respiratory isolation after a negative Xpert assay would be identical to the time required to discontinue respiratory isolation after a second negative smear examination. We further assumed that Xpert testing would be performed and reported once daily, seven days per week, on the same schedule as smear microscopy, and that three sputum samples would be obtained for mycobacterial culture and speciation to confirm the presence or absence of TB in all patients (after discharge if necessary). According to our model, patients assigned to the Xpert strategy would submit the sputum samples remaining after discharge directly to the laboratory, and would not require clinic visits for sputum collection. Thus, we assumed no difference in cost or health benefit between the Xpert and microscopy strategies with respect to the third smear examination and any of the mycobacterial cultures.
Decision Analysis
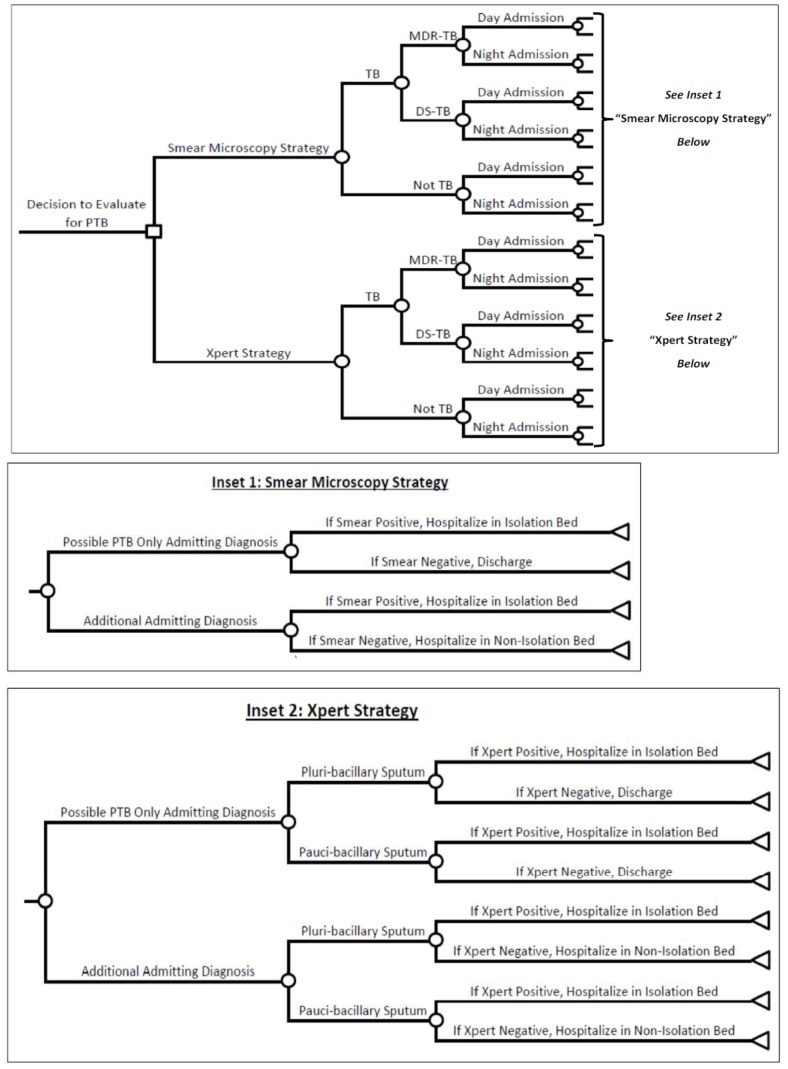
We developed a decision-analysis model comparing these two diagnostic strategies for guiding respiratory isolation decisions in inpatients being evaluated for active pulmonary TB (Figure 1). Our primary outcome was the incremental net monetary benefit of the Xpert strategy relative to the smear strategy. Since we expected incremental gains in health to be minimal in comparing these two approaches, we calculated incremental net monetary benefit as the net cost of the smear microscopy strategy minus the net cost of the Xpert strategy. We also considered savings in utilization costs as a secondary outcome, including reductions in (unnecessary) hospitalization and respiratory isolation. Within each strategy, we applied a cost penalty for false-positive screening results to reflect the unnecessary use of respiratory isolation, and for false-negative results to reflect the additional cost of contact investigation and treatment of transmitted TB (Online Supplement S1). We obtained base-case estimates of all epidemiologic and cost inputs (Table 1) through primary data collection and review of the literature (Online Supplement S1). We then aggregated the probabilities, utilization costs, and economic costs at each stage of evaluation and management to obtain unit individual and total annual estimates of cost, length of stay, and duration of respiratory isolation for each strategy. Finally, we calculated differences in economic and utilization costs between strategies. We adopted a health-system perspective, considering all economic costs of providing these health care services and the utilization costs of hospital beds and respiratory isolation rooms.
Figure 1. Decision analysis model comparing Xpert MTB/RIF and smear diagnostic strategies guiding respiratory isolation decisions.
Definition of abbreviations: DS-TB = drug-sensitive TB. MDR-TB = multi-drug resistant TB. PTB = pulmonary TB. TB = tuberculosis. Legend: The large square represents a decision node, each circle a probabilistic node; and each triangle a terminal node. The two insets display the detailed distal branches leading to the terminal nodes for the two strategies being compared in the master tree. Applying the inputs in Tables 1 and 2, we used this decision analysis to generate the outcome data on economic and utilization costs presented in Tables 3 and 4. Note that for the Xpert strategy, the diagnostic performance of Xpert was estimated using the smear-positive sensitivity and specificity data for pluri-bacillary sputa and using the smear-negative sensitivity and specificity data for pauci-bacillary sputa. [19].
Table 1. Input variables for epidemiologic and diagnostic parameters used in the base-case and sensitivity analyses.
| Variable | Base Value | Range | Reference |
| Epidemiologic Parameters | |||
| TB prevalence among patients evaluated for TB (%) | 6.4 | 0.5–15 | Clinical data† |
| Proportion admitted during the daytime* (%) | 24 | 10–50 | Clinical data‡ |
| No indication for admission except TB evaluation (%) | 13 | 0–25 | Clinical data‡ |
| Number of annual inpatient TB evaluations | 234 | 50–1000 | Clinical data† |
| Diagnostic Parameters | |||
| Sensitivity of Xpert (%) | |||
| Smear-positive specimens | 98 | 97–99 | [19] |
| Smear-negative specimens | 68 | 59–75 | [19] |
| Specificity of Xpert (%) | |||
| Smear-positive specimens | 98 | 92–100 | [19] |
| Smear-negative specimens | 98 | 97–99 | [19] |
| Sensitivity of smear microscopy (%) | 78.5 | 65–92 | [24] |
| Specificity of smear microscopy (%) | 98 | 97–99 | [24] |
Definition of abbreviations: TB = tuberculosis.
Legend:
Admitted between 9 am–4 pm.
Microbiology laboratory database and patient chart review.
SFGH inpatient admission database.
Sensitivity and Uncertainty Analyses
To test the robustness of our model and identify the inputs that most contributed to costs and benefits, we performed one- and two-way sensitivity analyses for all parameters, using the ranges shown in Tables 1 and 2. We then performed probabilistic uncertainty analyses in which values from all parameters were simultaneously drawn from triangular distributions bounded by the highest and lowest plausible input values for each variable. We defined the 95% uncertainty range (UR) as the 2.5th and 97.5th percentiles of 10,000 such Monte Carlo simulations. We used TreeAge Pro Edition 2012 (TreeAge Software, Inc. Williamstown, MA) for all economic analyses.
Table 2. Input variables for cost parameters used in the base-case and sensitivity analyses.
| Variable | BaseValue | Range | Reference |
| Cost Parameters * | |||
| Xpert cost per test | $218 | $10–$1,000 | |
| Device (annualized over10 years) | $59 | – | Cost data‡ |
| Maintenance† | $64 | – | Cost data‡ |
| Cartridge | $60 | – | Cost data‡ |
| Labor | $35 | ||
| Cost per hour (salary andbenefits) | $60 | – | Cost data§ |
| Minutes per test | 35 | – | Observation |
| Smear cost per test | $10 | $1–$100 | |
| Materials | $2.50 | – | [14], [25] |
| Labor | $7.50 | ||
| Cost per hour (salary andbenefits) | $60 | – | Cost data‖ |
| Minutes per test | 7.5 | – | Observation |
| Hospital bed cost per day | $2,292 | 500–5,000 | Cost data‖ |
| Marginal cost respiratoryisolation per day | $1,527 | 0–2,000 | Cost data‖ |
| Cost of four-drug anti-TBtherapy per day | $4.55 | – | Cost data** |
Definition of abbreviations: TB = tuberculosis.
Legend:
In 2009 US Dollars.
Includes full-service coverage after expiration of one-year warranty.
Cepheid Schedule of Fees (July 2011).
State of California Employee Salary Database.
San Francisco General Hospital (SFGH) Department of Finance Charge Database.
SFGH Department of Pharmacy Drug Database.
Human Subjects Considerations
The study was approved by the Committee on Human Research at the University of California, San Francisco. The Committee exempted the investigators from obtaining written informed consent on grounds that data collection for study inputs posed minimal risk to subjects.
Results
Patient Population
Between January and December, 2009, 234 patients were admitted and placed in respiratory isolation at San Francisco General Hospital for evaluation of pulmonary TB. TB evaluation was the sole reason for admission in 30 (13%) patients. The majority of admissions (76%) occurred during evening or nighttime lab hours (4 pm–9 am). Fifteen of the 234 (6.4%) patients were AFB smear-positive, and none had MDR-TB. If this cohort had hypothetically undergone Xpert testing, we estimated that these 15 and four additional patients without TB would have tested positive.
Cost-benefit Analysis
Tables 1 and 2 show the key input variables for the decision analysis model, including base-case estimates and the ranges of values considered in one-way sensitivity analyses. After applying all costs of start-up, diagnostic evaluation, hospitalization, overhead, and treatment, the model predicted an average savings of $2,278 (95% UR $1582–4569) per inpatient admission for the Xpert strategy ($15,503 per admission) compared to the smear microscopy strategy ($17,783 per admission; Table 3) in the base-case scenario. As expected, these savings differed by TB status, with the Xpert strategy costing $205 more (95% UR $75–237) than the smear microscopy strategy among TB patients and $2,450 less (95% UR $964–2570) among non-TB patients. When we aggregated net costs for each strategy for all patients in the hypothetical cohort over an entire year, we projected substantial savings of $533,520 annually using the Xpert strategy compared to the smear strategy (Table 4).
Table 3. Average utilization and economic costs per patient.
| Outcome | SmearStrategy | XpertStrategy | Difference | 95% UncertaintyRange‡ |
| Length of Stay * | ||||
| Isolation room | 2.7 | 1.4 | 1.3 | 1.2, 1.3 |
| Standard room | 3.2 | 4.4 | −1.2 | −1.2, −1.1 |
| Total | 5.9 | 5.8 | 0.1 | −0.1, 0.2 |
| Costs † | ||||
| Isolation room | $10,483 | $5,305 | $5,178 | $4,234, $5,200 |
| Standard room | $7,285 | $9,980 | −$2,695 | −$2,750, −$2,504 |
| Diagnostictesting | $15 | $218 | −$203 | −$699, −$66 |
| Total | $17,783 | $15,503 | $2,278 | $1,582, $4,570 |
Legend:
In days;
In 2009 US Dollars;
2.5–97.5% uncertainty range based on simultaneous sampling of all parameters of simultaneously drawn from triangular distributions bounded by the highest and lowest plausible input values for each variable in Tables 1 and 2.
Note that numbers displayed were subject to rounding.
Table 4. Total annual utilization and economic costs for all patients.
| Outcome | SmearStrategy | XpertStrategy | Difference | 95% UncertaintyRange‡ |
| Length of Stay * | ||||
| Isolation room | 632 | 328 | 304 | 281, 304 |
| Standard room | 749 | 1030 | −281 | −281, −257 |
| Total | 1,381 | 1,358 | 23 | −23, 47 |
| Costs † | ||||
| Isolation room | $2,453,022 | $1,241,370 | $1,211,652 | $990,756, $1,216,800 |
| Standard room | $1,704,690 | $2,335,320 | −$630,630 | −$645,500, −$585,936 |
| Diagnostictesting | $3,510 | $51,012 | −$47,502 | −$163,655, −$15,444 |
| Total | $4,161,222 | $3,627,702 | $533,520 | $370,188, $1,069,380 |
Legend:
In days;
In 2009 US Dollars.
2.5–97.5% uncertainty range based on simultaneous sampling of all parameters of simultaneously drawn from triangular distributions bounded by the highest and lowest plausible input values for each variable in Tables 1 and 2.
Note that numbers displayed were subject to rounding.
The majority of the cost savings arose from reductions in length of stay in respiratory isolation. We projected that the Xpert strategy would on average reduce time spent in respiratory isolation by 1.3 days (95% UR 1.2–1.3 days; 1.4 isolation days with the Xpert strategy versus 2.7 days with the microscopy strategy), although average time in hospital would not necessarily decrease (5.8 hospital days with Xpert versus 5.9 with microscopy; Difference 0.1 days, 95% UR −0.1 to 0.2 days). Not surprisingly, projected length of stay also differed by TB status. TB patients were estimated to remain in hospital in respiratory isolation on average for 12.1 days after initiating treatment regardless of testing strategy, a length of stay driven by the severity of illness and by hospital infection-control guidelines for patients from congregate settings. Among non-TB patients, we projected an average stay in isolation of 0.7 days with the Xpert strategy and 2.1 days with the smear microscopy strategy, for a savings of 1.4 hospital days (95% UR 1.1–1.6) with the Xpert strategy. The average length of stay in hospital for non-TB patients did differ by evaluation strategy but to a lesser degree: 5.5 days with Xpert and 5.3 days with smear microscopy (Difference 0.2 days, 95% UR 0.2–0.3 days).
Based on these individual findings, our model also projected substantial reductions in total annual utilization of respiratory isolation beds among all patients (Table 4). Patients spent a total of 632 days in isolation when evaluated with the smear strategy versus an expected 328 days with Xpert (Difference 304 isolation days, 95% UR 281–304 days), a projected 48% decrease. Thus, as further shown in Tables 3 and 4, the number of days spent in respiratory isolation was the primary factor influencing average and total costs.
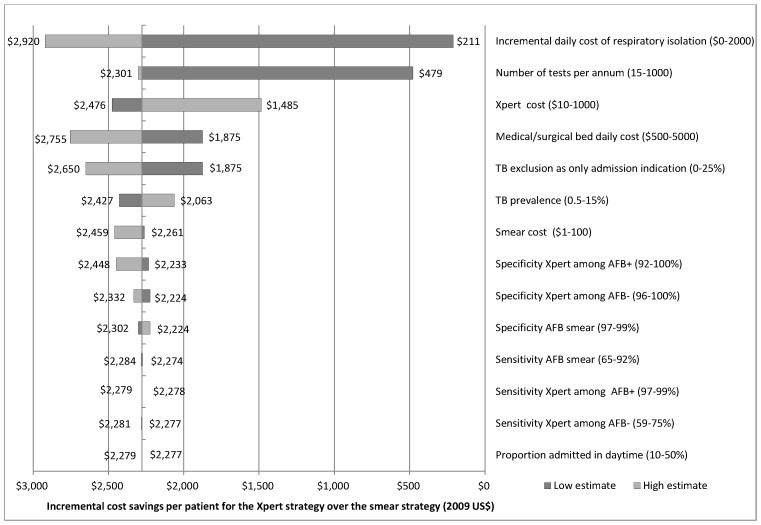
Sensitivity Analyses
As shown in the one-way sensitivity analyses in Figure 2, the Xpert strategy was cost-saving relative to the serial smear strategy across a broad range of assumptions about the expenses of hospitalization, respiratory isolation, and diagnostic testing, including Xpert cost. In addition, our findings were robust to changes in Xpert sensitivity and specificity across the range of summary estimates reported in the literature, and to volume of tests performed, with the smear strategy favored only when the number of TB tests performed annually was less than 14. Finally, a two-way sensitivity analysis around the two variables found to be most influential – incremental daily cost of respiratory isolation and the number of tests performed per year – showed the Xpert strategy to be cost saving for most combinations of these two inputs. However, as the cost of isolation decreased, more tests needed to be performed annually for the Xpert strategy to achieve cost savings relative to the smear strategy (Figure S1). In a second two-way sensitivity analysis incorporating the cost of hospitalization and the incremental cost of an isolation room, we found that even if there were no additional cost for a respiratory isolation room, the cost of a regular acute hospital bed would have to decrease by over 50% - from $2292 to $1095 per day – to favor the smear microscopy strategy over the Xpert strategy.
Figure 2. One-way sensitivity analyses comparing Xpert MTB/RIF and smear diagnostic strategies. Definition of abbreviations: AFB+, sputum acid-fast bacli smear-positive; AFB−, sputum acid-fast bacilli smear-negative.
Legend: †Ranges for sensitivity analyses are shown in parentheses. The vertical line at $2278 corresponds to the incremental savings in total costs per patient as shown in Table 3, dark grey bars show the estimated incremental savings of Xpert versus sputum smear when using the lowest value of the parameter range shown, and the light grey bars show the corresponding estimate when using the highest parameter value.
Discussion
Active pulmonary disease and other infectious forms of TB are infrequent among inpatients in the U.S. and other high-income countries, yet excluding these conditions consumes a substantial amount of patient time and hospital resources. Although molecular testing has the potential to speed such evaluations, concerns about the high costs of implementing these tests have prevented them from being used widely. [15], [16] This analysis suggests that the routine adoption of Xpert, a novel automated NAAT, to guide triage of inpatients undergoing evaluation for presumed pulmonary TB could reduce the cost of respiratory isolation by $2,278 per inpatient admission. This would save a medium-sized urban public hospital like ours approximately $533,520 per year.
This is the first study to find that using a NAAT for TB evaluation is cost-saving in a low-burden, high-income setting. Dowdy and colleagues examined how the GenProbe MTD test could be used as an add-on test for confirming M. tuberculosis complex in smear-positive specimens in a university hospital in the U.S., and found that the technical complexity of the manual GenProbe assay contributed to high labor costs and slow turn-around times, thereby blunting the potential benefit of their NAAT strategy. [13] Similarly, Rajalahti et al evaluated the cost-effectiveness of Cobas Amplicor PCR assay as an add-on test for evaluating isolation and treatment decisions for patients with presumptive TB in a Finnish hospital. [26] Although they found NAAT inexpensive in this scenario, they only performed NAATs twice weekly and on average waited four days for results; sensitivity analyses found that the strategy would have been cost-saving had testing been performed daily.
The key advantage of Xpert over prior NAATs is that its simplicity and automated nature enables real-time instead of batched testing, leading to more rapid availability of results. Indeed, we found that Xpert was cost-saving even though the costs of Xpert testing were similar to those reported for NAATs in previous studies [13], [14], [25], [26], because it reduced the number of respiratory isolation days. Because the incremental cost of respiratory isolation is high at our institution, Xpert’s ability to reduce respiratory isolation time by 1.4 days led to a 14% reduction in the overall costs of hospitalization compared with the standard smear strategy. In contrast, previous studies found that NAATs increased costs by 13–51% [13], [14], [26], primarily because of the high costs of testing and the low costs of isolation and hospitalization. Although the incremental cost of a respiratory isolation bed may vary widely between institutions, we found that even if respiratory isolation were free, the Xpert strategy would still be favored until the cost of a hospital bed day fell by over 50%. Although our sensitivity analyses show that the Xpert strategy is cost-saving across a range of inputs, the cost savings do depend on a minimal volume of testing at the hospital, primarily because the capital and maintenance costs of Xpert are spread across the total number of tests.
In addition to being cost-saving, Xpert has several other advantages over smear microscopy as a tool to guide triage of inpatients being evaluated for infectious TB. Xpert detects a large proportion of sputum smear-negative TB cases, and these contribute to disease transmission if undetected. [27] This is of particular concern in the hospital setting where exposure to TB may adversely affect a large number of health care workers and susceptible patients. Xpert is also highly specific for TB, which is potentially advantageous in low TB-burden settings where non-tuberculous mycobacteria may be more common. Finally, the use of Xpert also provides significant unmeasured individual benefits to patients in low prevalence settings by allowing earlier discharge from respiratory isolation and potentially earlier discharge from the hospital. These benefits include increased contact with staff while in the hospital, potentially decreased risk of healthcare-associated complications while hospitalized because of decreased time in the hospital, and faster return to work and family. Although the explicit inclusion of patient costs is beyond the scope of this analysis, we would expect shorter hospitalizations to directly reduce costs to uninsured, self-paying patients, while a faster return to work would reduce lost earnings from absenteeism. In low-burden settings such as ours, the vast majority of patients do not have TB and therefore stand to benefit from earlier exclusion of TB.
Our analysis has several limitations. We collected much of our input data from a single institution, which may limit the generalizability of our model, although sensitivity analyses showed the Xpert strategy to be cost-saving across a range of plausible inputs. Another limitation is that our institution requires only two negative sputa to discontinue respiratory isolation, whereas many other hospitals may require three negative sputa. However, Xpert would likely result in further cost savings for those hospitals because it would be able to reduce respiratory isolation time even more than predicted in our model. Finally, our model does not account for the fact that the same Xpert platform used for TB testing can also be used (with different modular cartridges) for U.S. Food and Drug Administration (FDA) - approved testing of other infections including influenza, C. difficile colitis, and methicillin-resistant Staphylococcus aureus (MRSA). In institutions that already utilize this platform, the incremental per-test cost of machine procurement and maintenance for TB evaluation could drop substantially.
In conclusion, we used a decision analysis model, coupled with primary data on costs and outcomes, to demonstrate that using Xpert to guide respiratory isolation decisions for patients undergoing evaluation for TB in an urban public hospital is likely to reduce health care costs and improve the patient experience by shortening the amount of time spent in respiratory isolation. The World Health Organization recently endorsed the use of Xpert for incremental case finding in low- and middle-income countries where TB incidence is high [20]; our analysis suggests that Xpert could also confer large benefits in low TB-incidence, high-income settings for the qualitatively different purpose of excluding TB. Given the robustness of our results to very wide parameter variations, implementation of Xpert is likely to reduce costs associated with respiratory isolation of patients being evaluated for TB at most hospitals in low-burden settings. Now that Xpert is FDA-approved for TB testing in the US, implementation data on actual costs and impact can further inform clinical and public health practice and future hospital infection-control guidelines on appropriate TB evaluation strategies.
Supporting Information
Two-way sensitivity analysis of the incremental cost per day of respiratory isolation and the number of TB tests per year. Legend: The two-way sensitivity analysis on the incremental cost per day of respiratory isolation and the number of TB test per year. The area in blue is cost saving for the Xpert strategy and the area in pink is cost saving for the smear strategy for the estimates applied in the sensitivity analysis.
(TIFF)
(DOC)
Acknowledgments
We would like to acknowledge Barbara Haller, Marguerite Roemer, and David Cantu in the San Francisco General Hospital Microbiology Division for assistance arranging observations of laboratory procedures and collection of data related to the timing of smear microscopy. We would also like to thank the UCSF Library for supporting the article publication charge.
Funding Statement
These authors have received support from the American Lung Association (CG-197164-N to JLD), the UCSF-GIVI Center for AIDS Research (to JLD), and the National Institutes of Health (K23 AI080147 to JLD; K23 HL094141 to AC). This work was also supported by the National Center for Research Resources (KL2 RR024130 to the UCSF Clinical and Translational Sciences Institute). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lawn SD, Zumla AI (2011) Tuberculosis. Lancet 378: 57–72. [DOI] [PubMed] [Google Scholar]
- 2. Jensen P, Lambert L, Iademarco M, Ridzon R (2005) Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 54: 1–141. [PubMed] [Google Scholar]
- 3. Pavelchak N, DePersis RP, London M, Stricof R, Oxtoby M, et al. (2000) Identification of factors that disrupt negative air pressurization of respiratory isolation rooms. Infect Control Hosp Epidemiol 21: 191–195. [DOI] [PubMed] [Google Scholar]
- 4. Wilmer A, Bryce E, Grant J (2011) The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: A controversial topic revisited. Can J Infect Dis Med Microbiol 22: E1–E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore D, Guzman J, Mikhail L (2005) Reduction in turnaround time for laboratory diagnosis of pulmonary tuberculosis by routine use of a nucleic acid amplification test. Diagn Microbiol Infect Dis: 247–252. [DOI] [PubMed]
- 6. Tokars JI, McKinley GF, Otten J, Woodley C, Sordillo EM, et al. (2001) Use and efficacy of tuberculosis infection control practices at hospitals with previous outbreaks of multidrug-resistant tuberculosis. Infect Control Hosp Epidemiol 22: 449–455. [DOI] [PubMed] [Google Scholar]
- 7. Wisnivesky J, Henschke C, Balentine J, Willner C, Deloire A, et al. (2005) Prospective validation of a prediction model for isolating inpatients with suspected pulmonary tuberculosis. Arch Intern Med 165: 453–457. [DOI] [PubMed] [Google Scholar]
- 8. Catanzaro A, Perry S, Clarridge JE, Dunbar S, Goodnight-White S, et al. (2000) The role of clinical suspicion in evaluating a new diagnostic test for active tuberculosis: Results of a multicenter prospective trial. JAMA 283: 639–645. [DOI] [PubMed] [Google Scholar]
- 9. Greco S, Girardi E, Navarra A, Saltini C (2006) Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis. Thorax 61: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campos M, Quartin A, Mendes E, Abreu A, Gurevich S, et al. (2008) Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am J Respir Crit Care Med 178: 300–305. [DOI] [PubMed] [Google Scholar]
- 11. Laraque F, Griggs A, Slopen M, Munsiff S (2009) Performance of nucleic acid amplification tests for diagnosis of tuberculosis in a large urban setting. Clin Infect Dis 49: 46–54. [DOI] [PubMed] [Google Scholar]
- 12. Drobniewski FA, Watt B, Smith EG, Magee JG, Williams R, et al. (1999) A national audit of the laboratory diagnosis of tuberculosis and other mycobacterial diseases within the United Kingdom. J Clin Pathol 52: 334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dowdy DW, Maters A, Parrish N, Breyer C, Dorman S (2003) Cost-effectiveness analysis of the Gen-Probe Amplified Mycobacterium tuberculosis direct test as used routinely on smear-positive respiratory specimens. J Clin Micro 41: 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes R, Wonderling D, Li B, Higgens B (2012) The cost effectiveness of nucleic acid amplification techniques for the diagnosis of tuberculosis. Respir Med 106: 300–307. [DOI] [PubMed] [Google Scholar]
- 15. Dorman S (2009) Editorial commentary: Coming of age of nucleic acid amplification tests for the diagnosis of tuberculosis. Clin Infect Dis 49: 55–57. [DOI] [PubMed] [Google Scholar]
- 16. Dylewski J (2009) Nucleic acid amplification testing for the diagnosis of tuberculosis: Not for all. Clin Infect Dis 49: 1456–1457. [DOI] [PubMed] [Google Scholar]
- 17. Marks SM, Cronin W, Venkatappa T, Maltas G, Chon S, et al. (2013) The health system benefits and cost-effectiveness of using Mycobacterium tuberculosis direct nucleic acid amplification testing to diagnose tuberculosis disease in the United States. Clin Infect Dis 5: 532–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi HW, Miele K, Dowdy D, Shah M (2013) Cost-effectiveness of Xpert ® MTB/RIF for diagnosing pulmonary tuberculosis in the United States. Int J Tuberc Lung Dis 17: 1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, et al. (2013) Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 1: CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (2011) Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampin resistance: Xpert MTB/RIF system. A policy statement. Available: http://whqlibdoc.who.int/publications/2011/9789241501545_eng.pdf. Accessed 2013 Oct 30. [PubMed]
- 21. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FDA permits marketing of first US test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin. FDA News Release July 25th 2013. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm362602.htm. Accessed on 2013 Aug 26. [PubMed]
- 23. American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America (2000) Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 161(4 Pt 1): 1376–95. [DOI] [PubMed] [Google Scholar]
- 24. Steingart KR, Henry M, Ng V, Hopewell PC, Ramsay A, et al. (2006) Fluorescence versus conventional sputum smear microscopy for tuberculosis: A systematic review. Lancet Infect Dis 6: 570–581. [DOI] [PubMed] [Google Scholar]
- 25. Dowdy D, O’Brien M, Bishai D (2008) Cost effectiveness of novel diagnostic tools for the diagnosis of tuberculosis. Int J Tuberc Lung Dis 12: 1021–1029. [PubMed] [Google Scholar]
- 26. Rajalahti I, Ruokonen E, Kotomäki T, Sintonen H, Nieminen MM (2004) Economic evaluation of the use of PCR assay in diagnosing pulmonary TB in a low-incidence area. European Respiratory Journal 23: 446–451. [DOI] [PubMed] [Google Scholar]
- 27. Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, et al. (1999) Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353: 444–449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-way sensitivity analysis of the incremental cost per day of respiratory isolation and the number of TB tests per year. Legend: The two-way sensitivity analysis on the incremental cost per day of respiratory isolation and the number of TB test per year. The area in blue is cost saving for the Xpert strategy and the area in pink is cost saving for the smear strategy for the estimates applied in the sensitivity analysis.
(TIFF)
(DOC)