Abstract
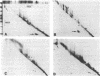
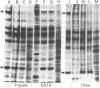
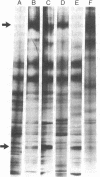
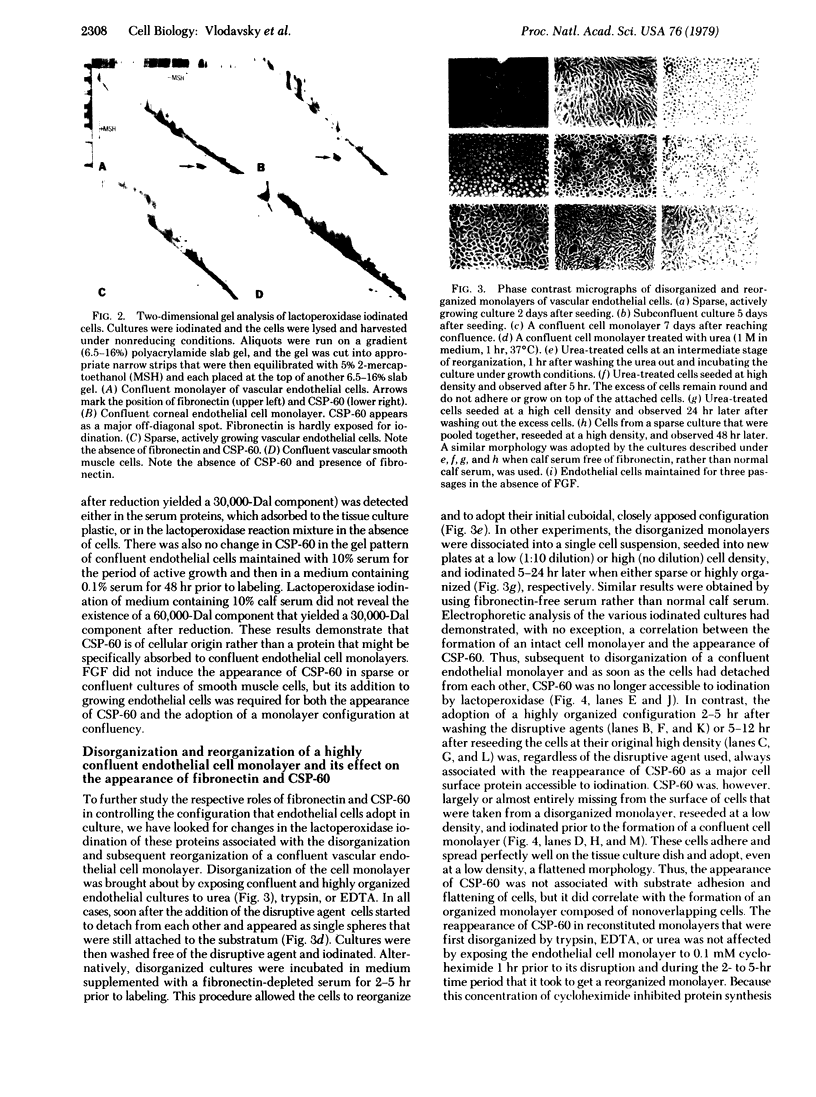
The formation of a highly organized vascular and corneal endothelial cell monolayer is associated with the appearance of a 60,000-dalton cell surface protein (CSP-60) (30,000 daltons after reduction with dithiothreitol) which is not detectable in rapidly growing endothelial cells and in subconfluent cultures that do not yet exhibit the strict morphology of a confluent monolayer. It is also absent from vascular smooth muscle cells and from endothelial cultures that are maintained in the absence of fibroblast growth factor and grow on top of each other at confluence. After disorganization of cells in a confluent endothelial monolayer by urea, EDTA, or trypsin, CPS-60 is no longer exposed on the cell surface, but it reappears as soon as the cells readopt their characteristic two-dimensional configuration. This reorganization can be achieved in the presence of cycloheximide and despite removal of fibronectin by urea, EDTA, or trypsin. Maximal amounts of fibronectin and no CSP-60 are detected in subconfluent, but not yet organized, endothelial cultures or in endothelial cells that no longer form a monolayer of nonoverlapping cells at confluence. Likewise, cultures of vascular smooth muscle cells contain fibronectin but no CSP-60. These results suggest that CSP-60, rather than fibronectin, could be involved in the adoption of a monolayer configuration by confluent endothelial cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Mautner V., Lanza R., Hynes R. O. Restoration of normal morphology, adhesion and cytoskeleton in transformed cells by addition of a transformation-sensitive surface protein. Cell. 1977 May;11(1):115–126. doi: 10.1016/0092-8674(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Gospodarowicz D., Nicolson G. L. Identification, localization, and role of fibronectin in cultured bovine endothelial cells. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3273–3277. doi: 10.1073/pnas.75.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fielding P. E., Vlodavsky I., Gospodarowicz D., Fielding C. J. Effect of contact inhibition on the regulation of cholesterol metabolism in cultured vascular endothelial cells. J Biol Chem. 1979 Feb 10;254(3):749–755. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Mescher A. L., Birdwell C. R. Stimulation of corneal endothelial cell proliferations in vitro by fibroblast and epidermal growth factors. Exp Eye Res. 1977 Jul;25(1):75–89. doi: 10.1016/0014-4835(77)90248-2. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macarak E. J., Kirby E., Kirk T., Kefalides N. A. Synthesis of cold-insoluble globulin by cultured calf endothelial cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2621–2625. doi: 10.1073/pnas.75.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Lieberman I., Lansing A. I. Synthesis of the plasma membrane of the liver cell. Biochem Biophys Res Commun. 1968 Apr 5;31(1):54–58. doi: 10.1016/0006-291x(68)90030-2. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Chen L. B. Thrombin-sensitive surface protein of cultured chick embryo cells. Nature. 1976 Feb 19;259(5544):578–580. doi: 10.1038/259578a0. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Fielding P. E., Fielding C. J., Gospodarowicz D. Role of contact inhibition in the regulation of receptor-mediated uptake of low density lipoprotein in cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):356–360. doi: 10.1073/pnas.75.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Richards F. M. An approach to nearest neighbor analysis of membrane proteins. Application to the human erythrocyte membrane of a method employing cleavable cross-linkages. J Biol Chem. 1974 Dec 25;249(24):8005–8018. [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]