Abstract
Background
Evidence is compelling for a positive correlation between climate change, urbanisation and prevalence of allergic sensitisation and diseases. The reason for this association is not clear to date. Some data point to a pro-allergenic effect of anthropogenic factors on susceptible individuals.
Objectives
To evaluate the impact of urbanisation and climate change on pollen allergenicity.
Methods
Catkins were sampled from birch trees from different sites across the greater area of Munich, pollen were isolated and an urbanisation index, NO2 and ozone exposure were determined. To estimate pollen allergenicity, allergen content and pollen-associated lipid mediators were measured in aqueous pollen extracts. Immune stimulatory and modulatory capacity of pollen was assessed by neutrophil migration assays and the potential of pollen to inhibit dendritic cell interleukin-12 response. In vivo allergenicity was assessed by skin prick tests.
Results
The study revealed ozone as a prominent environmental factor influencing the allergenicity of birch pollen. Enhanced allergenicity, as assessed in skin prick tests, was mirrored by enhanced allergen content. Beyond that, ozone induced changes in lipid composition and chemotactic and immune modulatory potential of the pollen. Higher ozone-exposed pollen was characterised by less immune modulatory but higher immune stimulatory potential.
Conclusion
It is likely that future climate change along with increasing urbanisation will lead to rising ozone concentrations in the next decades. Our study indicates that ozone is a crucial factor leading to clinically relevant enhanced allergenicity of birch pollen. Thus, with increasing temperatures and increasing ozone levels, also symptoms of pollen allergic patients may increase further.
Introduction
Epidemiological studies show an increasing trend in allergies, leading to a major health problem. Reasons discussed for this trend include a westernized life style with diminished immune stimulation [1] and anthropogenic air pollution [2], [3]. Particularly, irritant gases and diesel exhaust particles have been shown to exert adjuvant or aggravating effects on sensitisation and elicitation phases of allergic immune responses [4], [5]. As underlying mechanisms, effects on cells of the immune system as well as epithelial barrier disruption are discussed [6]. However, pollutants in ambient air do not only impact humans but also the allergen-carrier itself, i.e. the plant and its pollen. Therefore, the question arises whether the observed increase in allergic diseases in the western world might in part be explained by modified allergenicity of pollen caused by urbanisation and paralleled climate change. These environmental changes − higher temperature, in combination with higher concentrations of specific anthropogenic pollutants − lead to higher tropospheric ozone concentrations. In this scenario UV-radiation delivers the energy for ozone generation, but besides this, higher temperatures can also lead to an increase in ozone formation promoted by emission of highly reactive hydrocarbons from vegetation and evaporation processes [7]. Climate extremes are often observed in cities, which function as heat islands and can be regarded as a mirror of future climate [8]. However, urbanisation is not only characterised by higher temperatures, but also by higher levels of pollutants like particulate matter, carbon dioxide (CO2) or nitrogen dioxide (NO2). In this respect, it has to be considered that ozone does not show the same distribution as other pollutants [7], [9]. Ozone is a secondary pollutant whose formation underlies complex interactions, depending on the presence of precursors, degrading substances, temperature and UV-radiation. The main precursors are nitrogen oxides (NOx) and volatile organic compounds (VOCs). Especially NOx can be found at high concentrations in urban areas. NO, in turn, rapidly degrades the ozone generated in urban areas, especially during night-time. In rural areas, in contrast, we observe an accumulation of ozone due to lower NO levels and higher biogenic emissions [7]. Some studies already addressed the question of how pollutants affect the allergen carrier, showing that single pollutants strongly differ in their effects [10], [11]. The current study expands these observations by analyzing one of the most relevant allergen producers – the birch tree – in its natural environment. Hereby, we work with real exposure conditions to analyse relevant factors under natural circumstances. We take into account the parallel occurrence of different environmental factors as well as mechanisms of plant adaptation. Recent studies showed that pollen do not only release allergens, but also non-allergenic compounds such as pollen-associated lipid mediators (PALMs) (reviewed in [12]), which have been shown to exert immune modulatory and stimulatory effects. Allergenicity, thus, was evaluated in a holistic approach also taking adjuvant factors into account.
This study aimed at understanding how long-term increases in urbanisation and concomitant increases in pollution might influence pollen allergenicity, and how this might translate into immune cell activation and symptoms of pollen-allergic patients.
Materials and Methods
Ethic Statement
The ethical committee of the Technical University of Munich approved the study, and volunteers were enrolled after written informed consent. The study was carried out on private land and the owners of the land gave permission to conduct the study on these sites.
Sampling of Birch Pollen
Catkins were collected from birch trees located in the greater area of Munich (n = 40; see Fig. S1). Sampling of catkins took place during the birch flowering season in spring 2010. Their developmental stages were assessed by an adopted and extended code of the BBCH (Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie) [13]. The code included 12 different developmental stages, starting with winter rest (BBCH 50) and ending with end of flowering (BBCH 69). The stages of collection were BBCH 60 (single catkins sporadically emit pollen) and BBCH 61 (10% of the catkins emit pollen). After collection, catkins were air-dried, counted and weighed and pollen was extracted by sieving.
Preparation of Aqueous Pollen Extracts for Bet v 1, LTB4 and PGE2 ELISA
Aqueous pollen extracts (APEs) were prepared in 0.1 M NH4HCO3, pH 8.1, as previously described [14]. For skin prick tests, APEs were prepared as described in [15]. APE concentrations given refer to the extraction of a given amount of pollen per mL of buffer before centrifugation (e. g. 1 mg pollen was extracted in 1 mL buffer), but do not refer to actual protein concentrations in the extracts.
Bet v 1 ELISA
Bet v 1 levels were determined by sandwich ELISA as described in Buters et al. [14]. Bet v 1-specific antibodies MAK 2E10G6G7 and 4B10D10F8 were kindly provided by Joachim Ganzer, Allergopharma, Hamburg, Germany.
PGE2 and LTB4 ELISA
Concentrations of the eicosanoid-like PALMs in APEs were measured by commercially available enzyme immunoassays for prostaglandin E2 and leukotriene B4 (GE Healthcare, Germany) according to the supplier’s protocol.
Passive Sampling Method for Ozone and Nitrogen Dioxide (NO2) Determination
Passive samplers for ozone were provided and analysed by PASSAM AG, Männedorf, Switzerland. Passive sampling for ozone and NO2 were done in parallel. Passive sampling was carried out during the one-week period from May 11th to 18th 2010, i.e. 2 weeks after the last catkins were sampled. Measurements for all 40 birch trees were done in the same time frame and directly at the tree. It is supposed that the traffic volume over the period of 1 week is a representative mean for this location. This data was used to characterize the tree for a relative ozone exposure.
The nitrogen dioxide concentration was measured at the 40 sites according to Palmes’ principle [16]. Briefly, stainless steel meshes were immersed in a triethanolamine-aceton mixture and were air-dried for 10 minutes. Three coated meshes were brought into an air-tight tube. NO2 binds to the coated meshes by forming a triethanolamine-NO2-complex. NO2 adsorption was determined photometrically after one week of exposure.
Calculation of Urbanisation Index
An urbanisation index (UI) based on CORINE Land Cover 2000 data (European Environment Agency 2000) was calculated using ArcGIS 9.3. This index reflects the proportion of predefined built up areas (e.g. continuous and discontinuous urban fabric, industrial or commercial units) within a radius of 2 km and thus can vary between 0 and 1; i.e. from a low (UI = 0) to a high (UI = 1) degree of urbanisation [17].
Temperature Measurements
15 birch trees were provided with data recording devices measuring air temperature (HOBO U23-001, Onset Computer Corporation, Southern MA, USA). The devices were fixed in a radiation shield at the northern side of the trees in 3 m height. Air temperatures were recorded every 10 minutes, and daily temperature means were calculated. Temperature data were acquired between 1st July 2009 and 5th May 2010. The mean temperature for this period for each location was calculated.
Blood Donors
Healthy, non-atopic blood donors without a history of allergic diseases were tested by RAST for sensitisation against common allergens including birch allergens. All subjects were tested negative and total IgE was <100 IU/ml. Volunteers did not take any medication for at least 15 days before blood sampling.
Isolation and Culture of Monocyte-derived Dendritic Cells
Monocyte-derived dendritic cells (moDCs) were cultured from human peripheral blood monocytes as described by Gilles et al. [18]. Immature DCs were harvested on day 5 followed by stimulation with LPS (100 ng/ml) plus APEs from the highest (pollenO3high: mean ozone = 85 µg/m3, n = 2) and lowest (pollenO3high: mean ozone = 54 µg/m3, n = 2) ozone-exposed birch trees included in the study (mean ozone: 54 µg/m3 versus 85 µg/m3). After 24 h, supernatants were collected, and IL-12p70 release was measured by ELISA (BD Pharmingen, Heidelberg, Germany). The IL-12 response to APEs alone was measured exemplary in four APEs and virtually no IL-12 was detected (unstimulated: 18.7 pg/ml; APEs: median (min.-max.): 15.4 (9.9–20.8) pg/ml, n = 4) (data not shown). Viability of the cells after 24 h of culture was tested by propidium iodide staining and subsequent FACS analysis (see Fig. S4, supplementary material). Viability was not decreased by any of the conditions.
Neutrophil Migration Assays
The chemotactic activity of APEs from the highest (pollenO3high: mean ozone = 85 µg/m3) and lowest (pollenO3high: mean ozone = 54 µg/m3) ozone-exposed birch trees included in the study was evaluated by measuring neutrophil migration through a 5 µm pore polycarbonate membrane (ChemoTx Disposable Chemotaxis System, NeuroProbe). Neutrophils were isolated from peripheral blood as described by Traidl-Hoffmann et al. [19]. APEs were pipetted into the bottom chambers and neutrophils (1×106 cells/ml) were added to the top of the membrane. After 1 h of incubation the cell suspension was removed and cells that had transmigrated into the lower chamber were recovered and counted with a FACSCalibur (Becton Dickinson, Heidelberg, Germany).
Skin Prick Tests
Birch allergic patients (n = 5) with a specific IgE >0.35 kU/l were pricked on their forearms with APE (10 mg/mL) prepared of pollen samples from the highest (pollenO3high: mean ozone = 85 µg/m3) and lowest (pollenO3high: mean ozone = 54 µg/m3) ozone-exposed birch trees of the study. Each sample was pricked in replicates of four (proximally and distally on the same arm, right and left arm), and the mean wheal and flare sizes were calculated out of these four measurements after 15 min.
Statistics
Unpaired t-test was used for Gaussian distributed samples to determine statistically significant differences between groups. For non-Gaussian populations, the non-parametric Mann-Whitney U test was applied. The correlation coefficients (r) and 95% confidence intervals were calculated using the Pearson’s approach for Gaussian distributions and the Spearman’s approach for non-parametric correlation. Additionally, we applied linear multiple regression analyses based on stepwise variable selection to test which environmental factor is most important for pollen allergenicity. To analyse cell assays, the area under the curve (AUC) was determined and the Wilcoxon matched-pairs signed-ranks test was applied. Prick tests were also analysed by the Wilcoxon matched-pairs signed-ranks test. P values of <0.05 were considered significant (*). **: p<0.01, ***: p<0.001. Statistics were done with Graph Pad Prism 5, San Diego, CA, USA.
Results
Correlation Analysis of Urbanisation Related Factors and Relationship to Pollen Allergenic Potential
No significant correlation was detected between Bet v 1 content of the pollen specimens and urbanisation index (UI) or NO2 concentration (Fig. 1A, C; n = 40). However, a negative correlation could be observed for Bet v 1 content and temperature (Fig. 1B; r = -0.51; p = 0.042; n = 16). In contrast, ozone was positively correlated with Bet v 1 content. (r = 0.37; p = 0.017; Fig. 1D; n = 40).
Figure 1. Bet v 1 content of birch pollen in trees against different, urbanisation-related environmental conditions.
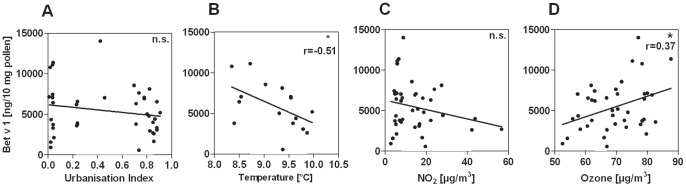
No significant correlation could be observed between Bet v 1 content and urbanisation index (A; n = 40) or NO2 concentration (B; n = 40). Bet v 1 showed a significant and negative correlation with temperature (C; n = 16) and was positively correlated with site-specific ozone levels (D; n = 40). *: p<0.05.
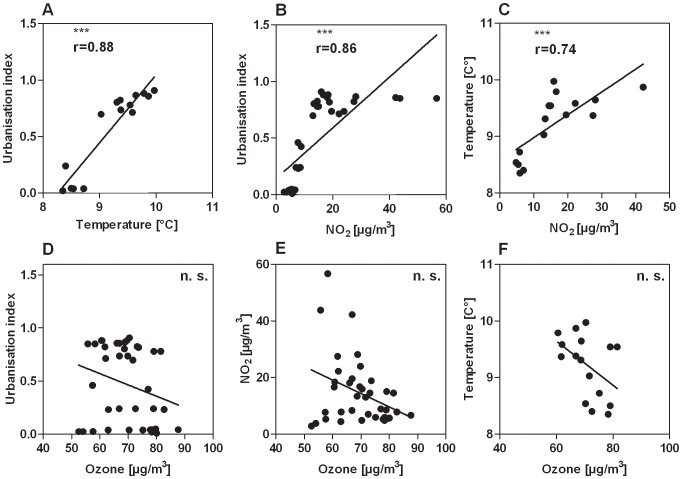
Sites of birch trees were analyzed for environmental parameters. By correlation analyses the relationship of the determined parameters was investigated. A highly significant and positive correlation of the variables UI (n = 40), temperature (n = 16) and NO2-concentration (n = 40) could be observed (Fig. 2 A, B, C). In contrast, ozone (n = 40) was not statistically associated with any of these parameters (Fig. 2 D, E, F).
Figure 2. Scatter plots of different environmental parameters.
A significant correlation was observed between the parameters urbanisation index (UI) and temperature (A; n = 16), (B) UI and NO2 concentration (n = 40) and (C) temperature and NO2 concentration (n = 16). Ozone was not correlated with either (D) UI (n = 40), (E) NO2 (n = 40) or (F) temperature (n = 16); ***: p<0.001.
To investigate whether ozone or temperature is the factor influencing pollen Bet v 1 content, we applied multiple regression analyses based on stepwise variable selection. Indeed, the role of the independent variable ozone for predicting Bet v 1 content was superior, since temperature has been excluded in the linear model, yielding in an R2 of 27.6% (data not shown).
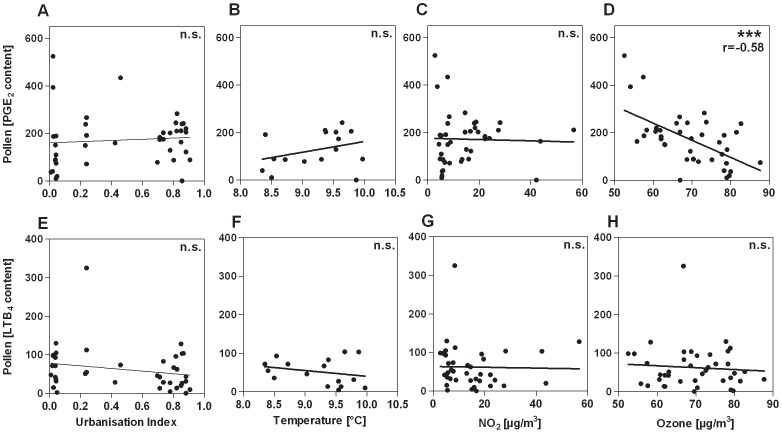
To determine immune stimulatory and modulatory potential of the birch pollen specimens, we analysed the content of PALMs in the different pollen extracts. Prostaglandin E2-like PALMs, termed PALMPGE2, harbour the immune modulatory PALMs [20], [21]. No statistically significant correlation was observed between PALMPGE2 contents and UI (n = 40), temperature (n = 16) or ambient NO2 (n = 40) (Fig. 3A, B, C). Instead, PALMPGE2 content was negatively correlated with ambient ozone (n = 40) levels (r = -0.58; p<0.0001; Fig. 3D). The LTB4-like PALMs harbour the chemotactic, immune stimulatory PALMs [19]. PALMLTB4 contents were not correlated with either UI (n = 40), temperature (n = 16), NO2 (n = 40) or ozone (n = 40) levels (Fig. 3E, F, G, H).
Figure 3. Content of PALMs in pollen samples plotted against different urbanisation-related environmental conditions.
No correlation was seen between PALMPGE2 and UI (A; n = 40), temperature (B; n = 16) or NO2 concentration (C; n = 40). A significant association of high ozone concentrations and low PALMPGE2 contents was observed (D; n = 40). PALMLTB4 did not show any significant correlation. Neither UI (E; n = 40), nor temperature (F; n = 16), NO2- (G; n = 40) and ozone (H; n = 40) were related to the content of PALMLTB4. ***: p<0.001.
Immune-stimulatory and Immune-regulatory Capacity of Pollen Samples
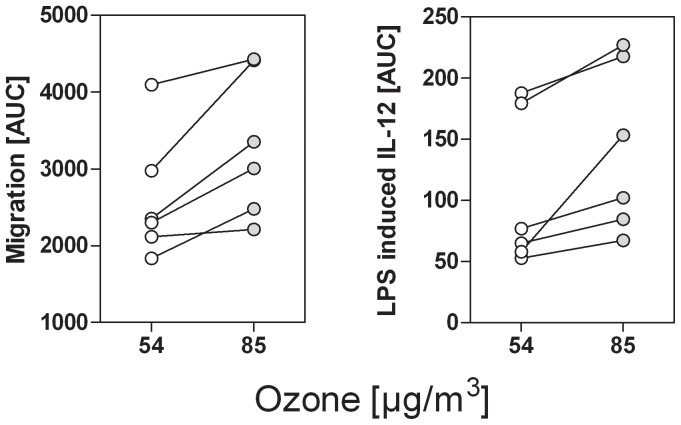
To assess whether the altered lipid compositions related to higher and lower ozone concentrations go along with differences in immune stimulatory and –modulatory potential, pollen from differentially ozone-exposed birch trees (mean ozone: 85 µg/m3 (pollenO3high) versus 54 µg/m3 (pollenO3low)) were chosen and analysed for induction of neutrophil chemotaxis and modulation of dendritic cell (moDC) cytokine secretion. Migration to pollenO3high and pollenO3low was tested in 3 concentrations (Fig. S4) and the AUC was calculated. PollenO3high induced significantly stronger neutrophil chemotaxis than pollenO3low. Neutrophil migration towards pollen extracts (median AUC (min.-max.)) were 3181 (2214–4429) for pollenO3high versus 2327 (1839–4095) for pollenO3low; p = 0.03 (Fig. 4A). To test the immune modulatory capacity of differentially ozone-exposed pollen, moDCs were stimulated with LPS in the presence and absence of APE, and IL-12 was measured in the supernatants (LPS induced IL-12 response: median (min.-max.): 14000 (8928–14500) pg/ml). APEs were applied in 3 concentrations (Fig. S3) and AUC was calculated. As shown in Fig. 4B, pollenO3high were less potent inhibitors of the moDC’s IL-12 response than pollenO3low. IL-12 release of dendritic cells (% of LPS; median AUC (min-max)) after stimulation with APE was 127.7 (67.3–227.0) for pollenO3high versus 71.0 (52.8–187.7) for pollenO3low; p = 0.02.
Figure 4. Immune stimulatory versus immune modulatory potential of pollen samples from higher- and lower-ozone-exposed birch trees.
Aqueous extracts (APEs) of birch pollen sampled from high and low ozone exposed trees were chosen for neutrophil migration assays and stimulation of monocyte derived dendritic cells. APEs were applied in 3 concentrations and the AUC was calculated. Higher ozone-exposed pollen induced stronger neutrophil chemotaxis compared to pollen samples from lower ozone–exposed trees (A). In contrast, birch pollen from lower ozone-exposed trees were more potent in inhibiting the LPS-induced release of IL-12p70 from human monocyte-derived dendritic cells (B). APEs were prepared from birch pollen sampled from higher ozone-exposed trees (n = 2; mean ozone: 85 µg/m3) and from lower ozone-exposed trees (n = 2; mean ozone: 54 µg/m3). All APEs were tested in n = 3 patients. *: p<0.05 (Wilcoxon matched-pairs signed-ranks test).
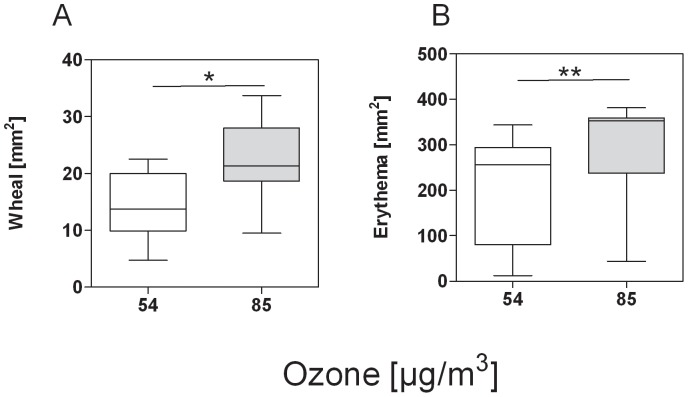
To test for clinical relevance of enhanced Bet v 1 levels, birch pollen allergic patients were subjected to skin prick tests with APEs prepared from differently exposed pollen. Wheal and flare sizes were significantly larger when patients were pricked with APEs prepared from pollenO3high. Wheal sizes (in mm2; median (min.-max.)) were 21.3 (9.5–33.8) for pollenO3low and 13.8 (4.7–22.5) for pollenO3high; p = 0.02. Flare sizes (in mm2; median (min.-max.)) were 256.3 (12.4–343.8) for pollenO3low and 353.1 (43.8–381.3) for pollenO3high; p = 0.005 (Fig. 5A, B). This is in line with higher Bet v 1 content of pollenO3high.
Figure 5. Cutaneous immune response towards pollen from higher and lower ozone-exposed birch trees.
APEs were prepared from pollen sampled from higher ozone-exposed trees (n = 2; mean ozone: 85 µg/m3) and from lower ozone-exposed trees (n = 2; mean ozone: 54 µg/m3). All APEs were tested in n = 5 patients. Higher ozone-exposed pollen induced larger wheals (A) and flares (B) in skin prick tests compared to lower ozone-exposed pollen. *: p<0.05; **: p<0.01 (Wilcoxon matched-pairs signed-ranks test).
Discussion
The present study reveals ozone exposure as important factor enhancing the allergenicity of birch pollen, with clinical relevance for susceptible individuals. Besides a positive relationship of the Bet v 1 content with ozone, we observed a negative association with temperature. Results of studies analysing the influence of temperature on Bet v 1 expression, however, are inconsistent. While Ahlholm et al. [22] reported a positive effect of high temperatures on Bet v 1 content, Helander et al. [23] indicated a negative association. The effect of temperature on the expression of Bet v 1 was also investigated by Tashpulatov et al. [24], using transgenic tobacco plants carrying a Bet v 1a-pomoter-reporter gene fusion. This study showed that warm temperatures positively regulate the activity of the Bet v 1 promoter. Our data indicate the opposite as we observed a negative correlation of temperature with Bet v 1. However, temperature and UI as well as NO2 concentration were highly and significantly related, while UI and NO2 did not correlate with Bet v 1. The number of entities for NO2 and UI were 40, while merely 16 data points for temperature were available. Considering the strong relationship of the parameters UI and NO2 with temperature it can be assumed that the correlation of temperature and Bet v 1 is observed by chance. Moreover when having a detailed look at the interrelationship of the environmental parameters of this study we also observed a non-significant negative association of ozone and temperature (Fig. 2F; p = 0.07; r = -0,46). This seems to be contradictory as ozone formation is associated with UV radiation and temperature [25]. However, ozone formation also depends on the presence of precursors (e.g. biogenic emissions) and degrading substances (e.g. NO). Rural areas are associated with lower temperatures. At the same time, the composition of ozone forming and -degrading factors in rural areas favor ozone accumulation. This might explain why we observe a negative correlation of ozone and temperature.
It might be hypothesized that a positive influence of ozone on Bet v 1 and the relationship of ozone and temperature implied a negative correlation of temperature and Bet v 1.
To confirm our suggestion, we applied multiple regression analyses based on stepwise variable selection. Indeed, the role of the independent variable ozone for predicting Bet v 1 content was superior, since temperature has been excluded in the linear model (data not shown).
Besides, Tashpulatov et al. [24] showed that abscisic acid, a stress- and development-related plant hormone, positively regulates the activity of the Bet v 1a promoter. This goes in line with our study showing that ozone, a major stress factor for plants, is associated with a higher Bet v 1 expression. Besides, also former studies in ragweed and grass species gave evidence that ozone impacts on the allergen transcript and content [26], [27]. In these studies, plants were exposed to defined ozone concentrations. In our study, trees were subjected to pollen sampling in their natural environment and under natural exposure conditions.
In a holistic approach analysing the allergenicity of birch pollen, we showed that elevated ozone exposure of birch trees was not only associated with increased allergen content in pollen but also with an altered composition of adjuvant PALMs. No relation of PALMLTB4 and PALMPGE2 content and the degree of urbanisation (UI), temperature or NO2 concentration was observed. This seems to be in contrast to a recent study [28]. However, this discrepancy is most likely explained by the fact that in the present study, pollen sampling was carried out at defined, distinct maturation stages. As demonstrated in Fig. S2, the content of PALMs in pollen grains differs profoundly depending on the maturation stage of pollen, as does the Bet v 1 content, confirming former results [29].
Notably, PALMPGE2 was significantly negatively associated to ozone concentrations. In our in vitro assays, pollenO3high were significantly more chemotactic for neutrophils than extracts of pollenO3low. Since PALMLTB4 did not significantly differ in differently exposed pollen we hypothesize that other, up to now unknown, substances besides PALMLTB4 account for this effect. Moreover, Armstrong [30] could show that PGE2 is able to inhibit the chemotaxis of neutrophils in a concentration dependent manner. Consequently, lower concentrations of PALMPGE2 in pollenO3high could also have contributed to enhanced neutrophil chemotaxis. In contrast, APEs prepared from pollenO3low were significantly more efficient in inhibiting dendritic cell IL-12 secretion, in line with higher levels of immune modulatory PALMPGE2. Among the immune modulatory PALMs are plant isoprostanes identified as E1-phytoprostanes. E1-phytoprostanes inhibit the IL-12 response in maturing DCs [31], [32], finally licensing DCs to differentiate naïve CD4+ T cells into Th2 cells [32]. Transferring our data to allergy mechanisms, we hypothesize that higher PALMPGE2 concentrations in low-ozone areas might facilitate de novo sensitisation by providing Th2 promoting signals. Higher Bet v 1 concentration and less anti-inflammatory PALMs in pollenO3high might in turn lead to pronounced allergic symptoms in already sensitized individuals.
In summary, urbanisation-related, anthropogenic environmental factors can influence birch trees to produce pollen with altered allergenic potential. Our study emphasizes the correlation of ozone exposure to the pollen content of allergen and non-allergenic, adjuvant factors. It is likely that future climate change with more frequent and intense warm spells [33] as well as increases in urbanisation and anthropogenic air pollutants such as NOx will further enhance the local accumulation of tropospheric ozone. As indicated by this study, ozone might – apart from direct adverse impacts on human health – lead to increased allergic symptoms via its impact on the allergen carrier.
Supporting Information
Locations of pollen sampling. Birch pollen were sampled during the birch flowering season of 2010. Red dots represent urban trees, green dots rural trees. Background: CORINE Land Cover 2000 (EEA 2000), major classes: red = urban fabric, green = forest and pastures, yellow = arable land, blue = rivers, lakes (see www.eea.europa.eu/themes/landuse/interactive/clc-download for a complete legend).
(TIFF)
Catkin maturation and allergenic potential of pollen. A: Catkins of different maturation stages were collected at different time points from the same trees (BBCH-Code 51–52: n = 1; BBCH-Code 55–65: n = 5) and classified according to a BBCH-Code. Pollen were isolated from the catkins and aqueous pollen extracts were prepared. APEs were then analyzed for the presence of Bet v 1 and PALMs. The content of Bet v 1 peaked at maturation stages 60–61. Inversely to Bet v 1, levels of PALMLTB4 and PALMPGE2 were high in pollen from immature catkins and decreased during maturation. A concentration minimum of PALMs corresponded to a maximum in Bet v 1. BBCH-Code: 52: catkins increase in length and show green expansion cracks; 55: enhanced expansion cracks through further increase in length; 60: first catkins emit pollen (sporadically); 61: beginning of flowering: few catkins emit pollen; 65: full flowering: more than 50% of the catkins emit pollen; 67: flowering finishing: just a few catkins still emit pollen.
(TIFF)
Immune stimulatory versus immune modulatory potential of high versus low ozone-exposed pollen samples. Aqueous extracts (APEs) of birch pollen sampled from high and low ozone exposed trees were chosen for neutrophil migration assays and stimulation of monocyte derived dendritic cells. APEs were applied in 3 concentrations. Higher ozone-exposed pollen induced stronger neutrophil chemotaxis compared to pollen samples from lower ozone–exposed trees (A). In contrast, birch pollen from lower ozone-exposed trees were more potent in inhibiting the LPS-induced release of IL-12p70 from human monocyte-derived dendritic cells (B). APEs were prepared from birch pollen sampled from higher ozone-exposed trees (n = 2; mean ozone: 85 µg/m3) and from lower ozone-exposed trees (n = 2; mean ozone: 54 µg/m3). All APEs were tested in n = 3 patients. *: p<0.05 (Wilcoxon matched-pairs signed-ranks test).
(TIFF)
Viability of moDCs after stimulation with LPS plus APEs from high- and low ozone-exposed pollen. Viability of monocyte-derived dendritic cells (moDcs) after 24 h of stimulation with LPS (100 ng/ml) and APEs (1, 3, 10 mg/ml) was tested by propidium iodide staining and subsequent FACS analysis. APEs were prepared from birch pollen sampled from higher ozone-exposed trees (n = 2; mean ozone: 85 µg/m3) and from lower ozone-exposed trees (n = 2; mean ozone: 54 µg/m3). All APEs were tested in n = 3 patients.
(TIFF)
Funding Statement
The study was supported by a grant of the German Research Council (Deutsche Forschunsgemeinschaft, DFG, TR467/5-1 and ME 179/3-1) (CTH and AM), the HWP grant from of the Technische Universität München (SG), and CK Care, Christine Kühne Center for Allergy Research and Education. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Strachan DP (1999) Lifestyle and atopy. Lancet 353: 1457–1458. [DOI] [PubMed] [Google Scholar]
- 2. Behrendt H, Friedrichs KH, Kramer U, Hitzfeld B, Becker WM, et al. (1995) The role of indoor and outdoor air pollution in allergic diseases. Progress in Allergy and Clinical Immunology Volume 3, Stockholm 3: 83–89. [Google Scholar]
- 3. Kramer U, Koch T, Ranft U, Ring J, Behrendt H (2000) Traffic-related air pollution is associated with atopy in children living in urban areas. Epidemiology 11: 64–70. [DOI] [PubMed] [Google Scholar]
- 4. Diaz-Sanchez D, Riedl M (2005) Diesel effects on human health: a question of stress? American journal of physiology Lung cellular and molecular physiology 289: L722–723. [DOI] [PubMed] [Google Scholar]
- 5. Heinrich J, Wichmann HE (2004) Traffic related pollutants in Europe and their effect on allergic disease. Current opinion in allergy and clinical immunology 4: 341–348. [DOI] [PubMed] [Google Scholar]
- 6. Ring J, Eberlein-Koenig B, Behrendt H (2001) Environmental pollution and allergy. Ann Allergy Asthma Immunol 87: 2–6. [DOI] [PubMed] [Google Scholar]
- 7.Sillman S (1999) The relation between ozone, NOx and hydrocarbons in urban and polluted rural environments. Atmospheric Environment 33.
- 8. Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, et al. (2003) Cities as harbingers of climate change: common ragweed, urbanization, and public health. The Journal of allergy and clinical immunology 111: 290–295. [DOI] [PubMed] [Google Scholar]
- 9.Health Effects Institute B, MA (2010) HEI Panel on the Health Effects of Traffic-Related Air Pollution. 2010. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. HEI Special Report 17.
- 10. Behrendt H, Becker WM, Fritzsche C, Sliwa-Tomczok W, Tomczok J, et al. (1997) Air pollution and allergy: experimental studies on modulation of allergen release from pollen by air pollutants. Int Arch Allergy Immunol 113: 69–74. [DOI] [PubMed] [Google Scholar]
- 11. Darbah JN, Kubiske ME, Nelson N, Oksanen E, Vaapavuori E, et al. (2007) Impacts of elevated atmospheric CO2 and O3 on paper birch (Betula papyrifera): reproductive fitness. ScientificWorldJournal 7 Suppl 1240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilles S, Behrendt H, Ring J, Traidl-Hoffmann C (2012) The Pollen Enigma: Modulation of the Allergic Immune Response by Non-Allergenic, Pollen-Derived Compounds. Current pharmaceutical design. [DOI] [PubMed]
- 13.Meier U (2001) Entwicklungsstadien mono- und dikotyler Pflanzen. BBCH-Monography. In: Forstwirtschaft BBfLu, editor. Berlin, Braunschweig. 165.
- 14. Buters JTM, Kasche A, Weichenmeier I, Schober W, Klaus S, et al. (2008) Year-to-year variation in release of Bet v1 allergen from birch pollen: Evidence for geographical differences between west and south Germany. International Archives of Allergy and Immunology 145: 122–130. [DOI] [PubMed] [Google Scholar]
- 15.Gilles S, Fekete A, Zhang X, Beck I, Blume C, et al.. (2011) Pollen metabolome analysis reveals adenosine as a major regulator of dendritic cell-primed T(H) cell responses. J Allergy Clin Immunol 127: 454–461 e451–459. [DOI] [PubMed]
- 16. Palmes ED, Gunnison AF, Dimattio J, Tomczyk C (1976) Personal Sampler for Nitrogen-Dioxide. American Industrial Hygiene Association Journal 37: 570–577. [DOI] [PubMed] [Google Scholar]
- 17. Jochner SC, Beck I, Behrendt H, Traidl-Hoffmann C, Menzel A (2011) Effects of extreme spring temperatures on urban phenology and pollen production: a case study in Munich and Ingolstadt. Climate Research 49: 101–112. [Google Scholar]
- 18. Gilles S, Mariani V, Bryce M, Mueller MJ, Ring J, et al. (2009) Pollen-derived E1-phytoprostanes signal via PPAR-gamma and NF-kappaB-dependent mechanisms. J Immunol 182: 6653–6658. [DOI] [PubMed] [Google Scholar]
- 19. Traidl-Hoffmann C, Kasche A, Jakob T, Huger M, Plotz S, et al. (2002) Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. Journal of Allergy and Clinical Immunology 109: 831–838. [DOI] [PubMed] [Google Scholar]
- 20. Traidl-Hoffmann C, Jakob T, Behrendt H (2009) Determinants of allergenicity. J Allergy Clin Immunol 123: 558–566. [DOI] [PubMed] [Google Scholar]
- 21. Behrendt H, Kasche A, von Eschenbach CE, Risse U, Huss-Marp J, et al. (2001) Secretion of proinflammatory eicosanoid-like substances precedes allergen release from pollen grains in the initiation of allergic sensitization. International Archives of Allergy and Immunology 124: 121–125. [DOI] [PubMed] [Google Scholar]
- 22.Hjelmroos M, Schumacher MJ, van Hage-Hamsten M (1995) Heterogeneity of pollen proteins within individual. Betula pendula trees. International Archives of Allergy and Applied Immunology 108. [DOI] [PubMed]
- 23. Helander ML, Savolainen J, Ahlholm J (1997) Effects of air pollution and other environmental factors on birch pollen allergens. Allergy 52: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 24. Tashpulatov AS, Clement P, Akimcheva SA, Belogradova KA, Barinova I, et al. (2004) A model system to study the environment-dependent expression of the Bet v 1a gene encoding the major birch pollen allergen. Int Arch Allergy Immunol 134: 1–9. [DOI] [PubMed] [Google Scholar]
- 25. Stathopoulou E, Mihalakakou G, Santamouris M, Bagiorgas HS (2008) On the impact of temperature on tropospheric ozone concentration levels in urban environments. J Earth Syst Sci 117: 227–236. [Google Scholar]
- 26. Eckl-Dorna J, Klein B, Reichenauer TG, Niederberger V, Valenta R (2010) Exposure of rye (Secale cereale) cultivars to elevated ozone levels increases the allergen content in pollen. J Allergy Clin Immunol 126: 1315–1317. [DOI] [PubMed] [Google Scholar]
- 27. Kanter U, Heller W, Durner J, Winkler JB, Engel M, et al. Molecular and Immunological Characterization of Ragweed (Ambrosia artemisiifolia L.) Pollen after Exposure of the Plants to Elevated Ozone over a Whole Growing Season. PLoS One 8: e61518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behrendt H, Kasche A, Traidl C, Plotz S, Huss-Marp J, et al. (2002) Pollen grains contain and release not only allergens, but also eicosanoid-like substances with neutrophil chemotactic activity: A new step in the initiation of allergic sensitization? New Trends in Allergy V: 3–8. [Google Scholar]
- 29. Buters JT, Weichenmeier I, Ochs S, Pusch G, Kreyling W, et al. (2010) The allergen Bet v 1 in fractions of ambient air deviates from birch pollen counts. Allergy 65: 850–858. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong RA (1995) Investigation of the inhibitory effects of PGE2 and selective EP agonists on chemotaxis of human neutrophils. Br J Pharmacol 116: 2903–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilles S, Mariani V, Bryce M, Mueller MJ, Ring J, et al. (2009) Pollen allergens do not come alone: pollen associated lipid mediators (PALMS) shift the human immune systems towards a T(H)2-dominated response. Allergy Asthma Clin Immunol 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, et al. (2005) Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med 201: 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schar C, Vidale PL, Luthi D, Frei C, Haberli C, et al. (2004) The role of increasing temperature variability in European summer heatwaves. Nature 427: 332–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Locations of pollen sampling. Birch pollen were sampled during the birch flowering season of 2010. Red dots represent urban trees, green dots rural trees. Background: CORINE Land Cover 2000 (EEA 2000), major classes: red = urban fabric, green = forest and pastures, yellow = arable land, blue = rivers, lakes (see www.eea.europa.eu/themes/landuse/interactive/clc-download for a complete legend).
(TIFF)
Catkin maturation and allergenic potential of pollen. A: Catkins of different maturation stages were collected at different time points from the same trees (BBCH-Code 51–52: n = 1; BBCH-Code 55–65: n = 5) and classified according to a BBCH-Code. Pollen were isolated from the catkins and aqueous pollen extracts were prepared. APEs were then analyzed for the presence of Bet v 1 and PALMs. The content of Bet v 1 peaked at maturation stages 60–61. Inversely to Bet v 1, levels of PALMLTB4 and PALMPGE2 were high in pollen from immature catkins and decreased during maturation. A concentration minimum of PALMs corresponded to a maximum in Bet v 1. BBCH-Code: 52: catkins increase in length and show green expansion cracks; 55: enhanced expansion cracks through further increase in length; 60: first catkins emit pollen (sporadically); 61: beginning of flowering: few catkins emit pollen; 65: full flowering: more than 50% of the catkins emit pollen; 67: flowering finishing: just a few catkins still emit pollen.
(TIFF)
Immune stimulatory versus immune modulatory potential of high versus low ozone-exposed pollen samples. Aqueous extracts (APEs) of birch pollen sampled from high and low ozone exposed trees were chosen for neutrophil migration assays and stimulation of monocyte derived dendritic cells. APEs were applied in 3 concentrations. Higher ozone-exposed pollen induced stronger neutrophil chemotaxis compared to pollen samples from lower ozone–exposed trees (A). In contrast, birch pollen from lower ozone-exposed trees were more potent in inhibiting the LPS-induced release of IL-12p70 from human monocyte-derived dendritic cells (B). APEs were prepared from birch pollen sampled from higher ozone-exposed trees (n = 2; mean ozone: 85 µg/m3) and from lower ozone-exposed trees (n = 2; mean ozone: 54 µg/m3). All APEs were tested in n = 3 patients. *: p<0.05 (Wilcoxon matched-pairs signed-ranks test).
(TIFF)
Viability of moDCs after stimulation with LPS plus APEs from high- and low ozone-exposed pollen. Viability of monocyte-derived dendritic cells (moDcs) after 24 h of stimulation with LPS (100 ng/ml) and APEs (1, 3, 10 mg/ml) was tested by propidium iodide staining and subsequent FACS analysis. APEs were prepared from birch pollen sampled from higher ozone-exposed trees (n = 2; mean ozone: 85 µg/m3) and from lower ozone-exposed trees (n = 2; mean ozone: 54 µg/m3). All APEs were tested in n = 3 patients.
(TIFF)