Abstract
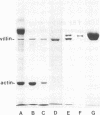
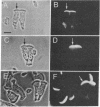
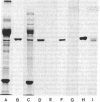
The major protein associated with actin in the microfilament core of intestinal microvilli has been purified. This protein, for which we propose the name villin, has a polypeptide molecular weight of approximately 95,000. Two arguments suggest that villin may be the microvillus crossfilament protein that links the microfilament core laterally down its length to the cytoplasmic side of the plasma membrane. First, electron microscopy shows that crossfilaments stay attached to isolated membrane-free microvillus cores. Calculation of the expected abundance of the crossfilament protein shows that only villin is present in sufficient quantity to account for these structures. Second, decoration of microvillus cores by antibodies to either actin or villin, followed by ferritin-labeled second antibody in a sandwich procedure, results in specific labeling of the cores in both cases. The antivillin decoration, however, gives rise to a greater increase in diameter, in agreement with a model in which villin projects from the F-actin microfilament core. Villin is distinct from α-actinin, a protein suggested to be involved in membrane anchorage of microfilaments in nonmuscle cells. The two proteins differ in molecular weight. Specific antibodies against villin and α-actinin show no immunological crossreactivity. Immunofluorescence microscopy reveals that villin is located in the microvilli of the brush border whereas α-actinin is absent from the microvilli but is found in the terminal web. In addition, villin is not found in microfilament bundles of tissue culture cells, which are rich in α-actinin. Thus, villin and α-actinin appear to be immunologically and functionally different proteins.
Keywords: brush border, membrane attachment, α-actinin, immunofluorescence microscopy, immunoferritin label
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa N., Robson R. M., Goll D. E. An improved method for the preparation of alpha-actinin from rabbit striated muscle. Biochim Biophys Acta. 1970 Feb 17;200(2):284–295. doi: 10.1016/0005-2795(70)90172-8. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Localization of actin and microfilament-associated proteins in the microvilli and terminal web of the intestinal brush border by immunofluorescence microscopy. J Cell Biol. 1978 Dec;79(3):839–845. doi: 10.1083/jcb.79.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Purification of microvilli and an analysis of the protein components of the microfilament core bundle. Exp Cell Res. 1978 Oct 15;116(2):397–407. doi: 10.1016/0014-4827(78)90463-9. [DOI] [PubMed] [Google Scholar]
- Brunser O., Luft H. J. Fine structure of the apex of absorptive cell from rat small intestine. J Ultrastruct Res. 1970 May;31(3):291–311. doi: 10.1016/s0022-5320(70)90133-4. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Pardo J. V. alpha-Actinin localization in the junctional complex of intestinal epithelial cells. J Cell Biol. 1979 Jan;80(1):203–210. doi: 10.1083/jcb.80.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. The enteric surface coat on cat intestinal microvilli. J Cell Biol. 1965 Dec;27(3):475–491. doi: 10.1083/jcb.27.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B. M., Burger M. M., DaPrada M., Richards J. G., Chaponnier C., Gabbiani G. alpha-Actinin attached to membranes of secretory vesicles. Nature. 1977 Dec 15;270(5638):628–629. doi: 10.1038/270628a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D., Semeriva M., Maroux S. The brush-border intestinal aminopeptidase, a transmembrane protein as probed by macromolecular photolabelling. J Mol Biol. 1976 Oct 5;106(4):1023–1035. doi: 10.1016/0022-2836(76)90350-8. [DOI] [PubMed] [Google Scholar]
- Maestracci D., Schmitz J., Preiser H., Crane R. K. Proteins and glycoproteins of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):113–124. doi: 10.1016/0005-2736(73)90435-5. [DOI] [PubMed] [Google Scholar]
- Masaki T., Endo M., Ebashi S. Localization of 6S component of a alpha-actinin at Z-band. J Biochem. 1967 Nov;62(5):630–632. doi: 10.1093/oxfordjournals.jbchem.a128717. [DOI] [PubMed] [Google Scholar]
- Mooseker M. S. Brush border motility. Microvillar contraction in triton-treated brush borders isolated from intestinal epithelium. J Cell Biol. 1976 Nov;71(2):417–433. doi: 10.1083/jcb.71.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Tilney L. G. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975 Dec;67(3):725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T. M., Staehelin L. A. The fine-structural organization of the brush border of intestinal epithelial cells. J Cell Sci. 1971 May;8(3):573–599. doi: 10.1242/jcs.8.3.573. [DOI] [PubMed] [Google Scholar]
- Osborn M., Webster R. E., Weber K. Individual microtubules viewed by immunofluorescence and electron microscopy in the same PtK2 cell. J Cell Biol. 1978 Jun;77(3):R27–R34. doi: 10.1083/jcb.77.3.r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlubnaya Z. A., Tskhovrebova L. A., Zaalishtsbvili M. M., Stefanenko G. A. Electron microscopic study of alpha-actinin. J Mol Biol. 1975 Feb 25;92(2):357–359. doi: 10.1016/0022-2836(75)90234-x. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]
- Tilney L. G., Mooseker M. S. Actin filament-membrane attachment: are membrane particles involved? J Cell Biol. 1976 Nov;71(2):402–416. doi: 10.1083/jcb.71.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Mooseker M. Actin in the brush-border of epithelial cells of the chicken intestine. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2611–2615. doi: 10.1073/pnas.68.10.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Wehland J., Herzog W. Griseofulvin interacts with microtubules both in vivo and in vitro. J Mol Biol. 1976 Apr 25;102(4):817–829. doi: 10.1016/0022-2836(76)90293-x. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Osborn M., Weber K. Visualization of the same PtK2 cytoskeletons by both immunofluorescence and low power electron microscopy. Exp Cell Res. 1978 Nov;117(1):47–61. doi: 10.1016/0014-4827(78)90426-3. [DOI] [PubMed] [Google Scholar]
- Wieland T. Modification of actins by phallotoxins. Naturwissenschaften. 1977 Jun;64(6):303–309. doi: 10.1007/BF00446784. [DOI] [PubMed] [Google Scholar]