Abstract
Purpose
We conducted a double-blind trial to determine whether a single intramuscular injection of fosphenytoin prevents seizures and neurologic sequelae in children with acute coma.
Methods
We conducted this study at Kilifi District Hospital in coastal Kenya and Kondele Children's Hospital in western Kenya. We recruited children (age, 9 months to 13 years) with acute nontraumatic coma. We administered fosphenytoin (20 phenytoin equivalents/kg) or placebo and examined the prevalence and frequency of clinical seizures and occurrence of neurocognitive sequelae.
Results
We recruited 173 children (median age, 2.6 [interquartile range, 1.7-3.7] years) into the study; 110 had cerebral malaria, 8 had bacterial meningitis, and 55 had encephalopathies of unknown etiology. Eighty-five children received fosphenytoin and 88 received placebo. Thirty-three (38%) children who received fosphenytoin had at least 1 seizure compared with 32 (36%) who received placebo (P = .733). Eighteen (21%) and 15 (17%) children died in the fosphenytoin and placebo arms, respectively (P = .489). At 3 months after discharge, 6 (10%) children in the fosphenytoin arm had neurologic sequelae compared with 6 (10%) in the placebo arm (P = .952).
Conclusion
A single intramuscular injection of fosphenytoin (20 phenytoin equivalents/kg) does not prevent seizures or neurologic deficits in childhood acute nontraumatic coma.
Keywords: Coma, Child, Seizure, Prophylaxis, Anticonvulsants
1. Introduction
Acute coma in children is associated with high mortality and significant neurocognitive sequelae among survivors[1,2]. In sub-Saharan Africa, the most common causes of acute nontraumatic coma are cerebral malaria (CM), acute bacterial meningitis (ABM), and viral encephalitides [3,4]. Seizures are common in these encephalopathies, and prolonged and multiple seizures are associated with death and neurologic sequelae [5,6]. Such poor outcomes associated with seizures could be caused by neuronal damage by excitotoxins released by repeated neuronal firing, inadequate cerebral blood flow for metabolic demand in the context of impaired cerebral-vascular autoregulation, raised intracranial pressure, abnormal respiration, and aspiration of gastric contents during seizures [7,8]. Alternatively, seizures may be a marker of neurologic damage rather than the cause. If seizures do cause neuronal damage, prevention of their occurrence may reduce mortality and neurocognitive sequelae.
In CM, up to 80% of children present with a history of seizures and 60% have seizures during admission [5]. A placebo-controlled trial of a single intramuscular (IM) injection of phenobarbital (20 mg/kg) as a prophylactic antiepileptic drug (AED) in children with CM showed more than 50% reduction in the incidence of seizures in those who received phenobarbital, with weak evidence for decrease in neurologic sequelae [9]. However, the mortality was more than double in those given phenobarbital due to respiratory depression in these unventilated children. Fosphenytoin is an effective AED, causes minimal cardiorespiratory depression, and being water soluble, can be given intramuscularly, a useful characteristic in resource-poor regions. It has been used for the prevention of seizures in patients with traumatic brain injury (TBI) and in other neurosurgery patients [10]. In a preliminary pharmacokinetic study among children with severe falciparum malaria, IM fosphenytoin (18 phenytoin equivalents (PE)/kg) rapidly (5-20 minutes) achieved unbound plasma phenytoin concentrations within the therapeutic range and controlled status epilepticus in 64% of patients [11]. In a second study in a similar group of children, a single dose of I.M. fosphenytoin (18 PE/kg) rapidly achieved and sustained plasma total phenytoin concentrations of more than 10 μg/mL for more than 72 hours and unbound concentrations of more than 1 μg/mL, appropriately within therapeutic range, for 24 hours [12]. Only 30% of the children had clinical seizures compared with the expected 60%, and no significant cardiorespiratory depression was observed. On the basis of these findings, we investigated whether a single IM injection of fosphenytoin would provide seizure prophylaxis for at least an initial 24 hours of admission, the crucial time when most children with acute encephalopathies die in this setting, and when timely appropriate management in a basic health facility may be lifesaving before referral to a higher-level facility if necessary. Pharmacokinetic modeling indicated that IM fosphenytoin at a dose of 20 PE/kg would provide appropriate prolonged therapeutic concentrations without any adverse effects.
2. Materials and methods
The main null hypothesis for this study was that a single IM injection of fosphenytoin (20 PE/kg) did not reduce the frequency or prevalence of seizures in children with acute nontraumatic coma. We used a randomized double-blind placebo-controlled trial study design (ISRCTN11862726) (http://www.controlled-trials.com/ISRCTN11862726). The study was approved by the Kenya Medical Research Institute Ethics Review Committee and was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients recruited into the study provided written informed consent.
2.1. Setting
We conducted this study at the high-dependency units (HDU) of Kilifi District Hospital (KDH) and Kondele Children's Hospital (KCH). Kilifi District Hospital is situated in the rural coast of Kenya in an area of stable malaria transmission. It has a 35-bed pediatric unit that admits more than 5000 children annually and is supported by a 7-bed HDU. Kondele Children's Hospital is a tertiary referral hospital located in Kisumu District, a region with stable malaria transmission. Kondele Children's Hospital has a 62-bed pediatric ward with a 7-bed HDU and admits approximately 6000 children annually.
2.2. Study population
We recruited children aged between 9 months and 13 years who were not able to localize a painful stimulus (Blantyre Coma Score ≤ 2), 30 minutes after treatment of a seizure or correction of hypoglycemia (blood glucose < 2.5 mmol/L). The Blantyre Coma Score is a simple score of coma status, based on assessment of motor, verbal response, and eye opening, and preferred in malaria endemic areas because it is more specific and has better interobserver agreement among health workers in this setting compared with the other coma scales [13]. We did not consider children younger than 9 months because at that developmental age, they cannot localize painful stimuli, a key criterion in our assessment of coma. We excluded children with a history of epilepsy, significant developmental delay, TBI, or sickle cell disease or those who had received phenytoin for treatment of seizures before presentation.
2.3. Study duration
We started enrollment of patients in December 2004 at KDH and in March 2008 at KCH after an application to add a second site in consideration of the low recruitment rate at the initial site. The study was interrupted twice, in April 2006 and July 2008, because of difficulty in obtaining stock of study drug. The study was terminated on March 31, 2009, as guided by a Data Safety and Monitoring Board (DSMB) interim analysis indicating futility in continued recruitment and in consideration of the continued slow recruitment rate.
2.4. Standard care
On admission, patients underwent initial resuscitation according to standard guidelines [14,15]. All children were initially treated with parenteral first-line antimicrobials and antimalarials until otherwise guided by the results of the initial 3 malaria blood slides taken 8 hours apart, blood, urine or cerebrospinal fluid (CSF) culture, and CSF microscopy and biochemistry results. Aciclovir was not available. Children who presented with seizures lasting at least 5 minutes even after correction of hypoglycemia received intravenous diazepam (0.3 mg/kg) or IM paraldehyde (0.4 mL/kg). Second-line agents were intravenous phenobarbital (15 mg/kg initial dose and 2.5-5 mg kg− 1 d− 1 maintenance dose for at least 3 days) and sodium valproate (25 mg/kg). Intravenous thiopental (0.4 mg/kg) or midazolam (initial dose 0.3 mg/kg and maintenance 0.1 mg kg− 1 h− 1) was given if the seizures were refractory to second-line drugs. A lumbar puncture was done when the patient was stable, and the CSF was examined for evidence of infection. Mechanical ventilatory support was not available other than bag and mask ventilation during resuscitation.
2.5. Study procedures
After initial resuscitation, the study compound (fosphenytoin or 0.9% saline) was administered intramuscularly on the upper lateral aspect of the right thigh. The site of injection was marked using a black marker pen to observe for local reactions. Thenceforth, vital signs were measured and documented every 10 to 20 minutes for the next 4 hours, after which the rate of monitoring was reduced to 2 hourly for 4 hours and then 4 hourly until discharge from the HDU. Monitoring was undertaken until the child regained consciousness. The following information was documented on the case report form during monitoring: episodes of clinical seizure episodes and abnormal motor posturing, anticonvulsant drug administration, clinical details of cardiorespiratory status, and any other significant clinical events. Adverse events (AEs) were observed for and serious AEs (SAEs) were reported to the DSMB and the Ethics Review Committee within 24 hours of occurrence.
Plasma samples collected at the time of clinical and laboratory review 4 hours after administration of the study drug and CSF samples collected at LP were stored at − 70°C until assayed for total phenytoin concentrations. The assay method has been described previously with 0.5 μg/mL as the lowest limit of detection [11].
Continuous electroencephalographic (EEG) monitoring was performed on alternate study subjects over the initial 72 hours of observation or until the child regained consciousness, whichever was earlier. An event was considered serious if it resulted in death, was life threatening, caused persistent or significant disability, or resulted in the prolongation of hospitalization. At discharge, neurologic deficits were examined for and cognitive assessment using event-related potentials (ERPs) was undertaken, as we have previously described [16]. Clinical review and ERP assessment were repeated at 3 months after discharge.
2.6. Treatment allocation and blinding
Randomization was computer generated in blocks of 50 and the drug ampoules containing either fosphenytoin (250 PE at 50 PE/mL) or placebo (5 mL of 0.9% saline) were numbered from 1 to 500 based on this randomization. The recruiting study investigators were blind to the treatment allocation. The list linking the study number to the intervention arm was retained by the DSMB. To ensure double blinding, the interventions, both colorless liquids, were presented in identical ampoules and were both administered at 0.4 mL/kg. Neurocognitive assessment at discharge and at 3-month review were conducted by study investigators (S.G., R.I., E.C., A.M., and M.K.) who were all blind to the study drug allocation.
2.7. Analysis
Sample size determination of 250 in each study arm was based on a desired ability to be able to detect a 50% reduction (from 27% of prolonged seizures observed in a previous study to 13.5%) in patients with at least 1 seizure, lasting longer than 5 minutes, allowing for a 20% loss to follow-up and 90% power at the conventional 5% significance level [5]. We recorded the study data on paper-based case report forms, which were then double entered onto a Visual Foxpro (Visual Foxpro Developing Centre, Microsoft, Wash) (version 9, x) database. We analyzed the data using STATA (version 11.0; StataCorp LP, College Station., Tex). We verified that there were no significant differences in the baseline clinical and laboratory parameters between the 2 study sites before pooling the data. We analyzed the data as intention to treat. The primary outcome variables were the number and prevalence of clinical seizures lasting at least 5 minutes and the prevalence of neurocognitive sequelae at 3-month review postdischarge. The secondary outcome variables were the prevalence of seizures of any duration, the prevalence of seizures detected by EEG monitoring, the prevalence of abnormal motor posturing, the prevalence of neurocognitive deficits at discharge, in-hospital mortality, and the frequency of hypotension and respiratory depression. We measured plasma phenytoin concentrations to explore the relationship between phenytoin concentrations and the frequency of seizures. We examined differences between the 2 arms using either the χ2 test or Fisher exact test for categorical variables, and the unpaired t test or Wilcoxon rank sum test for continuous variables, the choice of test being guided by the distribution of the data. To determine cognitive outcomes, the latencies and amplitudes of the ERP components were compared using repeated-measures analysis of variance with age, treatment group, and diagnosis as fixed variables. The ERP data were analyzed using SPSS (PASW version 18 [Chicago, Ill]) software. We described the association between the study drugs and the outcomes using odds ratios and 95% confidence intervals (95% CIs).
3. Results
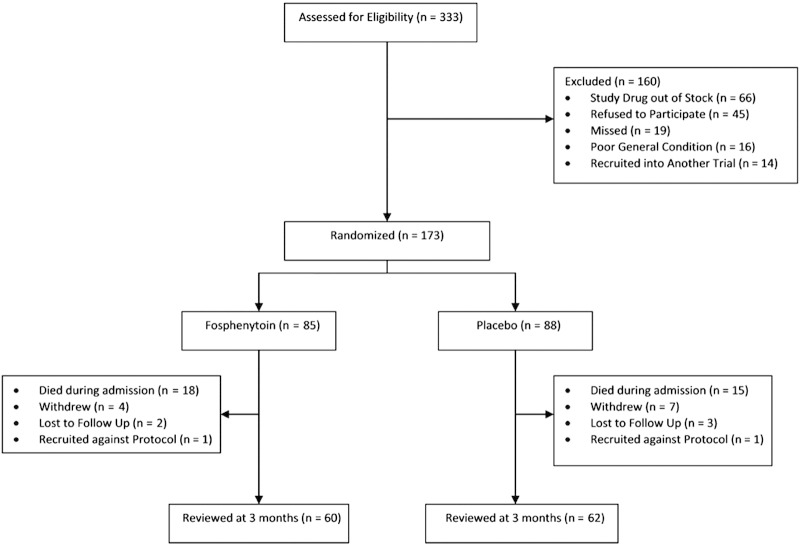
Over the study period, we recruited 173 children of an eligible 333 children (Fig. 1): 157 at the Kilifi site and 16 at the Kisumu site. They were of a median age of 2.6 (interquartile range [IQR], 1.8-3.6) years, and 72 (42%) were female. One hundred ten (64%) children had CM, 55 (32%) had encephalopathies of unknown etiology (EUA), and 8 (5%) had ABM. Children with ABM (75%) had higher case fatality than did those with EUA (20%) and CM (15%) (P < .001). Children with EUA were more likely to have neurologic deficits at discharge compared with those with CM. Eighty-five (49%) children received fosphenytoin, and 88 (51%) received placebo. Baseline characteristics and diagnoses were similar across the 2 study arms (Table 1). The duration of time between admission and actual administration of study drug was also the same between the 2 groups. None of the children had respiratory depression requiring bag and mask ventilation at the point of initial resuscitation before administration of the study drug. Thirty-one (36%) children who would later receive fosphenytoin had seizures during admission requiring first-line AED compared with 35 (40%) who would receive placebo (P = .66). Six children (7%) in the fosphenytoin group went on to require phenobarbital during this initial period before administration of study drug compared with 8 (9%) who would receive placebo (P = .62).
Fig. 1.
Study flowchart. Children who were recruited against protocol were not followed up on discharge from hospital.
Table 1.
Clinical features at admission
| Fosphenytoin (n = 85) | Placebo (n = 88) | |
|---|---|---|
| Age (median; y) | 2.6 (IQR, 1.7-3.7) | 2.6 (IQR, 1.8-3.5) |
| Sex | ||
| Male | 52 | 49 |
| Female | 33 | 39 |
| History of seizures on admission | 76 | 77 |
| Duration (h) of unconsciousness at admission (median) | 4 (IQR, 2-8) | 3 (IQR, 2-6) |
| Coma status; Blantyre Coma Score | ||
| 0 | 16 | 15 |
| 1 | 30 | 41 |
| 2 | 39 | 32 |
| Received first-line AED before study drug | 31 | 35 |
| Received second-line AED before study drug | 6 | 8 |
| Time (h) between admission and administration of study drug (median) | 2.2 (IQR, 1.6-3.9) | 2.4 (IQR, 1.4-4.2) |
| Mean admission sodium (mmol/L) | 135 (95% CI, 133-136) | 136 (95% CI, 134-137) |
| Mean admission potassium (mmol/L) | 4.2 (95% CI, 3.9-4.4) | 4.0 (95% CI, 3.8-4.3) |
| Mean admission blood glucose (mmol/L) | 5.6 (95% CI, 4.9-6.4) | 6.8 (95% CI, 5.8-7.8) |
| Mean admission hemoglobin (g/dL) | 7.8 (95% CI, 7.3-8.4) | 8.4 (95% CI, 7.9-8.9) |
| Mean admission white cell count (count/10− 9 L) | 16.4 (95% CI, 13.7-19.1) | 16.9 (95% CI, 14.8-19.0) |
| Mean admission platelets (count/10− 9 L) | 256 (95% CI, 206-305) | 260 (95% CI, 216-304) |
| Diagnosis | ||
| CM | 54 | 56 |
| Unknown encephalopathy | 27 | 28 |
| Acute bacterial meningitis | 4 | 4 |
Children who received fosphenytoin had a 4-hour median plasma phenytoin concentration of 16.8 (IQR, 13.0-19.0) μg/mL compared with insignificant concentrations of 0.2 (IQR, 0.0-0.3) μg/mL, in those who received placebo (P < .01). Only 2 (2%) of the children who received fosphenytoin had 4-hour phenytoin plasma concentrations below the therapeutic value of 10 μg/mL: 9.4 and 3.2 μg/mL. There was no difference in plasma phenytoin concentrations among those who received fosphenytoin between those who had CM (median concentration, 17.19 [IQR, 13.37-18.98] μg/mL), ABM (median concentration, 17.67 [IQR, 12.44-23.92] μg/mL), and EUA (median concentration, 15.56 [IQR, 12.81-18.44] μg/mL; P = .843). Lumbar puncture was performed at varied times after administration of the study drug depending on the clinical progress of the patient. Children who received fosphenytoin had a median CSF phenytoin concentration of 2.1 (IQR, 1.6-2.6) μg/mL compared with 0.1 (IQR, 0.0-0.3) μg/mL in those who received placebo (P < .01).
3.1. Seizures, AED use, and abnormal motor posturing
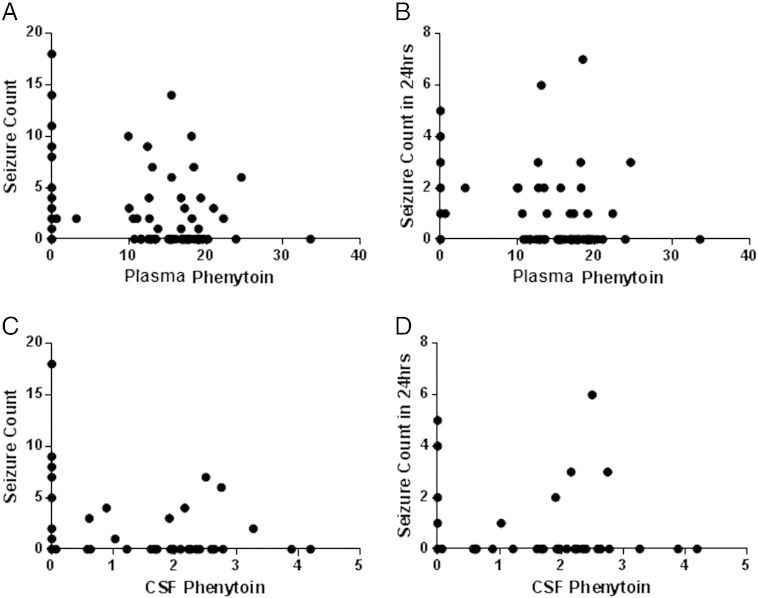
Most (n = 108; 62%) of the children did not have any seizures after administration of the study drug. Overall, there were no differences in frequency or prevalence of clinical and EEG seizures between the 2 study arms (Table 2). Among children with CM (n = 110), there was no difference in the prevalence of seizures between the fosphenytoin (n = 20; 37%) and the placebo (n = 21; 38%) (P = .980) arms of the study. The need for AED for treatment of seizures during the duration of study monitoring was similar between the 2 study arms, except for use of second-line AED over the whole duration of the study period, which was significantly lower in the placebo arm. The frequency of seizures in children who suffered seizures was not affected by plasma or CSF concentration of phenytoin (Supplementary Fig. 1).
Table 2.
Prevalence of seizures, AED use, and abnormal motor postures after administration of study drug
| Fosphenytoin (n = 85) | Placebo (n = 88) | Odds ratio (95% CI) |
P | |
|---|---|---|---|---|
| Prevalence of clinical seizures | 33 (38%) | 32 (36%) | 0.90 (0.48-1.67) | .733 |
| Prevalence of clinical seizures within 24 h | 24 (28%) | 27 (31%) | 1.13 (0.58-2.18) | .726 |
| Prevalence of clinical seizures ≥ 5 min during the whole duration of monitoring | 17 (20%) | 22 (25%) | 1.33 (0.65-2.75) | .432 |
| Prevalence of clinical seizures ≥ 5 min within 24 h | 11 (13%) | 10 (11%) | 1.86 (0.82-4.22) | .133 |
| Prevalence of clinical seizures ≥ 30 min during the whole duration of monitoring | 4 (5%) | 5 (6%) | 1.23 (0.32-4.79) | .773 |
| Prevalence of clinical seizures ≥ 30 min within 24 h | 2 (2%) | 4 (5%) | 2.00 (0.35-11.33) | .431 |
| Number of clinical seizures per patient within 24 h of monitoring, median (IQR) | 0 (0-2); range, 0-22 | 0 (0-2); range, 0-30 | – | .666 |
| Prevalence of seizures on EEG | 6 (7%; n = 21) | 10 (4%; n = 25) | – | .418 |
| Received AED after administration of study drug | 36 (43%) | 35 (41%) | 1.12 (0.60-2.06) | .725 |
| Received AED within 24 h after administration of study drug | 28 (34%) | 32 (36%) | 0.86 (0.46-1.62) | .638 |
| Received first-line AED after study drug | 24 (28%) | 26 (30%) | 1.07 (0.55-2.06) | .850 |
| Received first-line AED within 24 h after study drug | 20 (24%) | 23 (26%) | 0.87 (0.43-1.74) | .693 |
| Received second-line AED within 24 h after study drug | 19 (22%) | 16 (18%) | 1.30 (0.61-2.74) | .496 |
| Received second-line AED after study drug | 30 (35%) | 18 (20%) | 2.12 (1.06-4.25) | .029 |
| Prevalence of abnormal motor posturing | 39 (46%) | 32 (36%) | 1.50 (0.81-2.78) | .199 |
| Type of abnormal motor posturing | ||||
| i. Decorticate | 20 | 13 | 1.78 (0.81-3.90) | .142 |
| ii. Decerebrate | 21 | 13 | 1.90 (0.87-4.14) | .100 |
| iii. Opisthotonous | 5 | 4 | 1.31 (0.34-5.10) | .691 |
| CSF phenytoin concentration (μg/mL) | 2.1 (IQR, 1.6-2.6) | 0.1 (IQR, 0.0-0.3) | < .001 | |
| Plasma phenytoin concentration 4 h after administration (μg/mL) | 16.8 (IQR, 13.0-19.0) | 0.2 (IQR, 0.0-0.3) | < .001 |
3.2. Outcome
Nine children (14%; n = 65) in the fosphenytoin arm of the study were found to have motor deficits at discharge compared with 14 (19%; n = 73) in the placebo arm (P = .359; Table 3). Among children with CM, 5 children (11%) in the fosphenytoin arm had gross motor deficits at discharge compared with 9 (19%) in the placebo arm (P = .283). There were no differences between the 2 arms of the study in mortality, time to localize painful stimulus, and time to regain full consciousness. At 3 months after discharge, 7 children in the fosphenytoin arm and 11 children in the placebo arm were not followed up, having been lost to follow-up, withdrawn, or earlier recruited against protocol (Fig. 1). At this assessment, 10 children who had motor deficits at discharge were found to be normal. Two children, 1 in each study arm, had developed seizures after discharge. Thus, 6 (10%) children in the fosphenytoin arm had neurologic sequelae at 3 months compared with 6 (10%) in the placebo arm (P = .952).
Table 3.
Outcome at discharge and at 3 months after admission
| Fosphenytoin (n = 85) | Placebo (n = 88) | P | |
|---|---|---|---|
| Outcome at discharge | |||
| Died | 18 (21%) | 15 (17%) | .489 |
| Sequelae | 9 (13%; n = 67) | 14 (19%; n = 73) | .359a |
| Time to localize pain (h)b | 18 (8-28) | 14 (6-33) | .378a |
| Time to regain full consciousness (h)b | 21.5 (15.5-32.5) | 24 (10-48) | .875 |
| Outcome at 3 mo after discharge | |||
| Lost to follow-up/withdrew/not followed up | 7 (12%) | 11 (18%) | .415 |
| Neurologic deficits | 6 (10%) | 6 (10%) | .952 |
Differences in proportions examined using the χ2 test.
Kruskal-Wallis equality of population test.
Time to localize painful stimulus and time to regain full consciousness considered only for those who survived without motor neurologic deficits and presented as median (IQR).
3.3. Event-related potentials
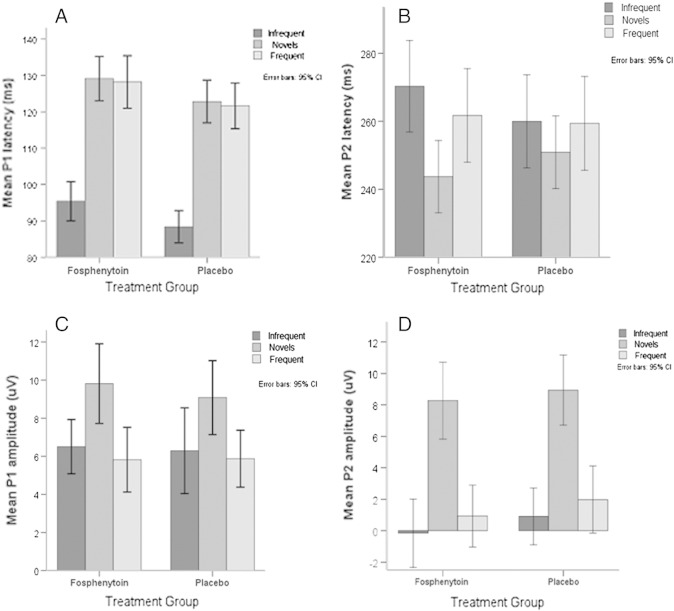
Ninety-five children (68% of those who survived by hospital discharge) had ERP measurements recorded at 3 months after discharge; 45 had received fosphenytoin and 50 had received placebo. There was no significant difference in age and diagnosis between the 2 treatment groups. Children with CM had significantly longer ERP latencies (both P1 and P2) and larger amplitudes compared with those with unknown encephalopathies. The P1 and P2 latencies decreased with age. Correcting for age and diagnosis, children who received placebo had significantly shorter P1 latency compared with those who received fosphenytoin (Fig. 2). However, there was no difference in P2 latency and in P1 and P2 amplitudes between the 2 trial groups.
Fig. 2.
Event-related potential profiles between the 2 study groups. A, Mean P1 latencies of treatment groups. B, Mean P1 amplitudes of treatment groups. C, Mean P2 latencies of treatment groups. D, Mean P2 amplitudes of treatment groups. There were observed decreasing P1 and P2 latencies with increasing age. Correcting for age and diagnosis, children who received placebo had significantly shorter P1 latency compared with those who received fosphenytoin. There was no difference in P2 latency and in P1 and P2 amplitudes between the 2 trial groups. Supplementary Fig. 1: Plasma and CSF phenytoin concentration vs seizure count in children who had seizures after administration of the study drug. A, Plasma phenytoin concentration vs seizure count during the whole duration of in-hospital monitoring. B, Plasma phenytoin concentration vs seizure count within the first 24 hours after administration of the study drug. C, CSF phenytoin concentration vs seizure count during the whole duration of in-hospital monitoring. D, CSF phenytoin concentration vs seizure count during the whole duration of in-hospital monitoring. Most of the children did not have any seizures after administration of the study drug and are therefore not included in these plots. Children who had more than 20 seizures within the first 24 hours (n = 2) and during the whole duration of monitoring (n = 6) are also not included in the plot to facilitate easy interpretation of the graphs.
3.4. Serious AEs
Ten (12%) children in the fosphenytoin group had respiratory depression compared with 13 (15%) children in the placebo arm (P = .560). Twenty-nine children had hypotension during the study monitoring, 15 in the fosphenytoin group and 14 in the placebo group (P = .760). Only 2 children were observed to have bradycardia; both were in the placebo arm of the study. Two children in the fosphenytoin arm had local reactions at the site of injection: 1 a swelling and the second, induration.
4. Discussion
In this study, we investigated the efficacy of a single IM injection of fosphenytoin for preventing seizures in children with acute nontraumatic coma and in improving outcome. We observed no difference between the 2 arms in the prevalence and frequency of seizures. Occurrence of neurocognitive sequelae and mortality were similar between the 2 groups. Event-related potentials at 3 months review after discharge were similar between the 2 groups, except for P1 latency, which was shorter in children who received placebo, suggesting better cognitive outcome in this group [17,18].
This was a randomized double-blind trial, with the investigators blinded to the treatment allocation until after the analysis. The baseline clinical characteristics between the 2 groups were similar. The median time to peak unbound plasma phenytoin concentration in a similar group of children receiving antibiotics has previously been shown to be 4 hours [12]. Except for 2 children, those who received fosphenytoin achieved optimal phenytoin concentration as indicated by blood samples taken 4 hours after administration of the study drug. A previous pharmacokinetic study indicated that a single IM injection of a slightly lower dose of fosphenytoin quickly achieved optimal levels and sustained plasma concentrations of unbound phenytoin within therapeutic range for at least 24 hours [12]. Albumin and protein levels were not consistently determined in our patients, but low levels would have increased the free levels of phenytoin further. It would have been useful to correct measured phenytoin levels for the determined protein levels and relate this to seizure prevention. Lumbar puncture was done at varied times after administration of the study drug, depending on the clinical course. In patients who died shortly after admission or who remained critically ill for a prolonged period during their acute illness, an opportunity for a lumbar puncture did not present. Thus, it was difficult to discern the relationship between seizure prevention and the plasma/CSF phenytoin ratio. In our study, an interim analysis suggested futility in continuation of the trial. That there is no clear benefit in the prophylactic use of fosphenytoin over placebo in preventing seizures or improving outcome in our group of children with nontraumatic coma, including CM specifically, is an unexpected finding.
The design of our trial allowed for initial resuscitation and treatment of any seizures observed at admission before recruitment. Thirty-eight percent of the children recruited into the study received at least 1 AED during resuscitation before administration of the study drug. Lack of initial seizure control in acute encephalopathies begets more seizures, and conversely, appropriate initial seizure treatment prevents more seizures. Thus, efficient seizure treatment during initial emergency resuscitation could have diminished the apparent effect of prophylactic AED. Even so, further analysis of our data did not suggest a difference in risk of seizures between the 2 groups even among patients who did not receive AED treatment during resuscitation at admission.
The need for second-line AED after administration of the study drug was greater in the fosphenytoin group compared with the placebo group. However, narrowing to the first 24 hours of monitoring during which period therapeutic levels of unbound phenytoin are likely to be sustained, this difference is not apparent. It is possible that this difference in need for AED, apparent after lapse of a period when the therapeutic window is likely to have occurred, may be caused by rebound seizures caused by a rapid drop in phenytoin levels not sustained further by maintenance dosages, a phenomenon well described in epilepsy in relation to acute AED withdrawal [19].
The role of fosphenytoin in seizure prophylaxis among patients with TBI and neurosurgery patients is well established in developed country settings [10]. Admittedly, such use entails repeat maintenance dosages, a practice that we would likely uphold with continued management beyond the initial 24 hours of resuscitation and acute management. It is possible that a higher dose of fosphenytoin may be more effective in reducing seizures and improving outcome, but patients may need more intensive care support than is available in most health facilities in sub-Saharan Africa. There is a clearer understanding of the pathophysiology of TBI, and perhaps, the ability to determine the time of neurologic insult provides a beneficial intervention during an appropriate window of opportunity. Among patients with nontraumatic encephalopathy, it is difficult to determine the time of onset of neurologic insult. Seizures may be a consequence rather than cause of already existent neurologic damage and, perhaps, less amenable to prevention. Prolonged seizures associated with fever have been shown to be less responsive to phenytoin and other AED treatment [20]. Sodium channel mutations are associated with prolonged seizures in the context of fever, altering mechanisms of stopping seizures and the affinity of sodium-channel blockers such as phenytoin [21]. Thus, it may be that strategies to prevent seizures may require greater insight into pathophysiological processes of nontraumatic encephalopathies. It will be useful to investigate other newer AEDs like levetiracetam, which is more efficacious for prevention of seizures among neurosurgical patients when compared with phenytoin and yet has minimal adverse effects [22]. The overall incidence of seizures lasting at least 5 minutes within the first 24 hours after administration of study drug was 12%. This is a lower incidence than the 27% anticipated during the design of the study. However, this observation was in a setting where seizure management during initial resuscitation was optimal, thus limiting seizure recurrence during the subsequent inpatient stay. The need for readjustment of effect parameters in calculating sample sizes for similar future trials may therefore need to be considered.
From the results of our study, there appears to be no benefit in using IM fosphenytoin at 20 PE/kg for seizure prophylaxis in children with acute nontraumatic coma.
The following are the supplementary materials related to this article.
Supplementary Figure 1_Fosphenytoin Trial.tif.
Acknowledgment
Recruitment of patients for the study was facilitated by the clinical teams in KDH and KCH. Judy Tumaini (KDH) assisted in the follow-up of patients. Rachel Odhiambo (KDH) developed and maintained the study database. Dr Juliana Otieno (KCH) assisted with the recruitment of patients. This manuscript is published with the permission of the Director of Kenya Medical Research Institute.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Setting for trial: Kilifi District Hospital, Kilifi, Coast, Kenya, and Kondele Children's Hospital, Kisumu, Nyanza, Kenya.
Study sponsorship: This study was sponsored through a Wellcome Trust fellowship award (to C.N.; 070114). We thank Professor P.N. Patsalos for commenting on the manuscript. The sponsor did not play any role in the design, data collection, analysis and interpretation of results, and approval of the manuscript.
Conflict of interest. On behalf of all authors, the corresponding author states that there are no conflicts of interest.
References
- 1.Pelkonen T., Roine I., Monteiro L. Risk factors for death and severe neurological sequelae in childhood bacterial meningitis in sub-Saharan Africa. Clin Infect Dis. 2009;48(8):1107–1110. doi: 10.1086/597463. [DOI] [PubMed] [Google Scholar]
- 2.Birbeck G.L., Molyneux M.E., Kaplan P.W. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9(12):1173–1181. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibekwe R.C., Ibekwe M.U., Onwe O.E. Non-traumatic childhood coma in Ebonyi State University Teaching Hospital, Abakaliki, South Eastern Nigeria. Niger J Clin Pract. 2011;14(1):43–46. doi: 10.4103/1119-3077.79239. [DOI] [PubMed] [Google Scholar]
- 4.Anga G., Barnabas R., Kaminiel O. The aetiology, clinical presentations and outcome of febrile encephalopathy in children in Papua New Guinea. Ann Trop Paediatr. 2010;30(2):109–118. doi: 10.1179/146532810X12703902243818. [DOI] [PubMed] [Google Scholar]
- 5.Crawley J., Smith S., Kirkham F. Seizures and status epilepticus in childhood cerebral malaria. Qjm. 1996;89(8):591–597. doi: 10.1093/qjmed/89.8.591. [DOI] [PubMed] [Google Scholar]
- 6.Idro R., Carter J.A., Fegan G. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2006;91(2):142–148. doi: 10.1136/adc.2005.077784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton C.R., Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79(1):1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 8.Mishra S.K., Newton C.R. Diagnosis and management of the neurological complications of falciparum malaria. Nat Rev Neurol. 2009;5(4):189–198. doi: 10.1038/nrneurol.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawley J., Waruiru C., Mithwani S. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000;355(9205):701–706. doi: 10.1016/S0140-6736(99)07148-2. [DOI] [PubMed] [Google Scholar]
- 10.Boucher B.A., Feler C.A., Dean J.C. The safety, tolerability, and pharmacokinetics of fosphenytoin after intramuscular and intravenous administration in neurosurgery patients. Pharmacotherapy. 1996;16(4):638–645. [PubMed] [Google Scholar]
- 11.Ogutu B.R., Newton C.R., Muchohi S.N. Pharmacokinetics and clinical effects of phenytoin and fosphenytoin in children with severe malaria and status epilepticus. Br J Clin Pharmacol. 2003;56(1):112–119. doi: 10.1046/j.1365-2125.2003.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogutu B.R., Newton C.R., Muchohi S.N. Phenytoin pharmacokinetics and clinical effects in African children following fosphenytoin and chloramphenicol coadministration. Br J Clin Pharmacol. 2002;54(6):635–642. doi: 10.1046/j.1365-2125.2002.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton C.R., Chokwe T., Schellenberg J.A. Coma scales for children with severe falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91(2):161–165. doi: 10.1016/s0035-9203(97)90207-8. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Pocket book of hospital care for children—guidelines for the management of common illnesses with limited resources. Geneva: WHO; 2005. [PubMed]
- 15.Gwer S., Thuo N., Idro R. Changing trends in incidence and aetiology of childhood acute non-traumatic coma over a period of changing malaria transmission in rural coastal Kenya: a retrospective analysis. BMJ Open. 2012;2(2):e000475. doi: 10.1136/bmjopen-2011-000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kihara M., Hogan A.M., Newton C.R. Auditory and visual novelty processing in normally-developing Kenyan children. Clin Neurophysiol. 2010;121(4):564–576. doi: 10.1016/j.clinph.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller C.A., Brown C.J., Abbas P.J. The clinical application of potentials evoked from the peripheral auditory system. Hear Res. 2008;242(1–2):184–197. doi: 10.1016/j.heares.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 18.deRegnier RA. Neurophysiologic evaluation of early cognitive development in high-risk infants and toddlers. Ment Retard Dev Disabil Res Rev 2005, 11(4):317–324. [DOI] [PubMed]
- 19.Azar N.J., Wang L., Song Y. Temporal pattern of oxcarbazepine and phenytoin withdrawal seizures during epilepsy monitoring. Epilepsy Res. 2008;79(1):78–83. doi: 10.1016/j.eplepsyres.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Ismail S, Levy A, Tikkanen H et al. Lack of efficacy of phenytoin in children presenting with febrile status epilepticus. Am J Emerg Med. 2012. [DOI] [PubMed]
- 21.Wallace R.H., Wang D.W., Singh R. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nat Genet. 1998;19(4):366–370. doi: 10.1038/1252. [DOI] [PubMed] [Google Scholar]
- 22.Kern K., Schebesch K.M., Schlaier J. Levetiracetam compared to phenytoin for the prevention of postoperative seizures after craniotomy for intracranial tumours in patients without epilepsy. J Clin Neurosci. 2012;19(1):99–100. doi: 10.1016/j.jocn.2011.07.021. [DOI] [PubMed] [Google Scholar]