Abstract
Darwin’s frogs (Rhinoderma darwinii and R. rufum) are two species of mouth brooding frogs from Chile and Argentina that have experienced marked population declines. Rhinoderma rufum has not been found in the wild since 1980. We investigated historical and current evidence of Batrachochytrium dendrobatidis (Bd) infection in Rhinoderma spp. to determine whether chytridiomycosis is implicated in the population declines of these species. Archived and live specimens of Rhinoderma spp., sympatric amphibians and amphibians at sites where Rhinoderma sp. had recently gone extinct were examined for Bd infection using quantitative real-time PCR. Six (0.9%) of 662 archived anurans tested positive for Bd (4/289 R. darwinii; 1/266 R. rufum and 1/107 other anurans), all of which had been collected between 1970 and 1978. An overall Bd-infection prevalence of 12.5% was obtained from 797 swabs taken from 369 extant individuals of R. darwinii and 428 individuals representing 18 other species of anurans found at sites with current and recent presence of the two Rhinoderma species. In extant R. darwinii, Bd-infection prevalence (1.9%) was significantly lower than that found in other anurans (7.3%). The prevalence of infection (30%) in other amphibian species was significantly higher in sites where either Rhinoderma spp. had become extinct or was experiencing severe population declines than in sites where there had been no apparent decline (3.0%; x 2 = 106.407, P<0.001). This is the first report of widespread Bd presence in Chile and our results are consistent with Rhinoderma spp. declines being due to Bd infection, although additional field and laboratory investigations are required to investigate this further.
Introduction
There are two species of Darwin’s frogs, both of which inhabit the temperate forests of South America: the northern Darwin’s frog (Rhinoderma rufum), which is endemic to central Chile, and the southern Darwin’s frog (R. darwinii), which is found in south and southern Chile and also in adjacent areas of Argentina [1], [2]. The behaviour that sets these frogs apart from all other amphibians is that the males care for their young by incubating them in their vocal sacs for at least part of their development, a process known as neomelia [3], [4]. In recent decades, both species have undergone marked population declines and R. rufum has not been recorded since 1980 [5]. The reasons for these apparent disappearances remain poorly understood [6], [7]. Throughout the historical distribution of R. rufum, and within the northern range of R. darwinii, there has been extensive habitat degradation, due to the large-scale replacement of native forest with pine (Pinus radiata) and eucalyptus (Eucalyptus globulus) plantations, and land use change to agriculture [1], [2]. Habitat loss, however, does not fully explain the enigmatic disappearances of R. rufum from its entire historical range or of the declines of R. darwinii from undisturbed ecosystems, including National Parks [8]. In this context, it has been hypothesised that amphibian chytridiomycosis, an infectious disease caused by the nonhyphal zoosporic chytrid fungus, Batrachochytrium dendrobatidis (Bd), might be implicated in the disappearances of Darwin’s frogs [1], [2], [8].
Amphibian chytridiomycosis, a recently-described emerging disease of amphibians [9], [10], has been associated with amphibian epizootic mass mortalities, population declines and global extinctions in different regions of the world [11], [12], [13], [14], [15], [16], [17]. Different genotypes of the fungus have been described, with the most virulent being a recombinant lineage, termed the global panzootic lineage (BdGPL) [18]. Recently, Bd whole-genome sequencing has demonstrated a higher genetic differentiation than previously recognised (including within BdGPL) [18], [19] and a complex evolutionary history that predates contemporary amphibian declines [20]. This highly-pathogenic and readily-transmissible pathogen appears to be capable of infecting an entire class of organism (the Amphibia), with devastating effects [21]. It has been described as: “the worst infectious disease ever recorded among vertebrates in terms of the number of species impacted and its propensity to drive them to extinction” [22]. In 2007, chytridiomycosis was identified as the cause of death of a group of 30 wild-caught R. darwinii exported to Germany for captive breeding [23]. Infection with Bd has been reported in populations of the invasive African clawed frog, Xenopus laevis [24] in central Chile. Additionally, Bourke et al. [25], [26] recently described Bd infection in R. darwinii and two other native frog species in the south of the country. The impacts of this emerging disease on amphibian populations in Chile, including Darwin’s frogs, however, have not been studied.
Here, we investigate whether amphibian chytridiomycosis is implicated in the population declines of Darwin’s frogs. We looked for evidence of historical Bd infection in Rhinoderma spp. and amphibians at current and former Rhinoderma sp. sites prior to and post the onset of declines. Also, we determined how widespread Bd infection is both in contemporary populations of R. darwinii across its current range and in other anuran species at sites of Rhinoderma spp. population decline or recent extinction.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the guidelines for use of live amphibians and reptiles in field research compiled by the American Society of Ichthyologists and Herpetologists (ASIH). Research was approved by the ZSL Ethics Committee and was conducted following Chilean and Argentinian wildlife regulations and according to permits 1241/08, 7377/09, 7993/10 and 300/12 of the Livestock and Agriculture Service (SAG) and 20/09, XI-01/09, 28/11 and X-03/11 of the National Forestry Corporation (CONAF) both in Chile, and permit 1119/11 of the National Parks Administration (APN) in Argentina. Archived amphibians were examined in their museum of origin, by the authors with specific permission given by all 5 zoological institutions.
Study area
Archived amphibian specimens from museum collections in Europe and Chile were examined for evidence of Bd infection. Also, extensive surveys for Bd infection throughout the historical ranges of R. rufum and R. darwinii were conducted from October 2008 to March 2012. These ranges extended from Zapallar (32° 33’ 03’’S, 71° 26’ 37’’W) to Aysén (45° 24’ 24’’S, 72° 41’ 52’’W) in Chile, and included adjacent areas in the Andes in the Neuquén and Río Negro Provinces in Argentina (Figure 1).
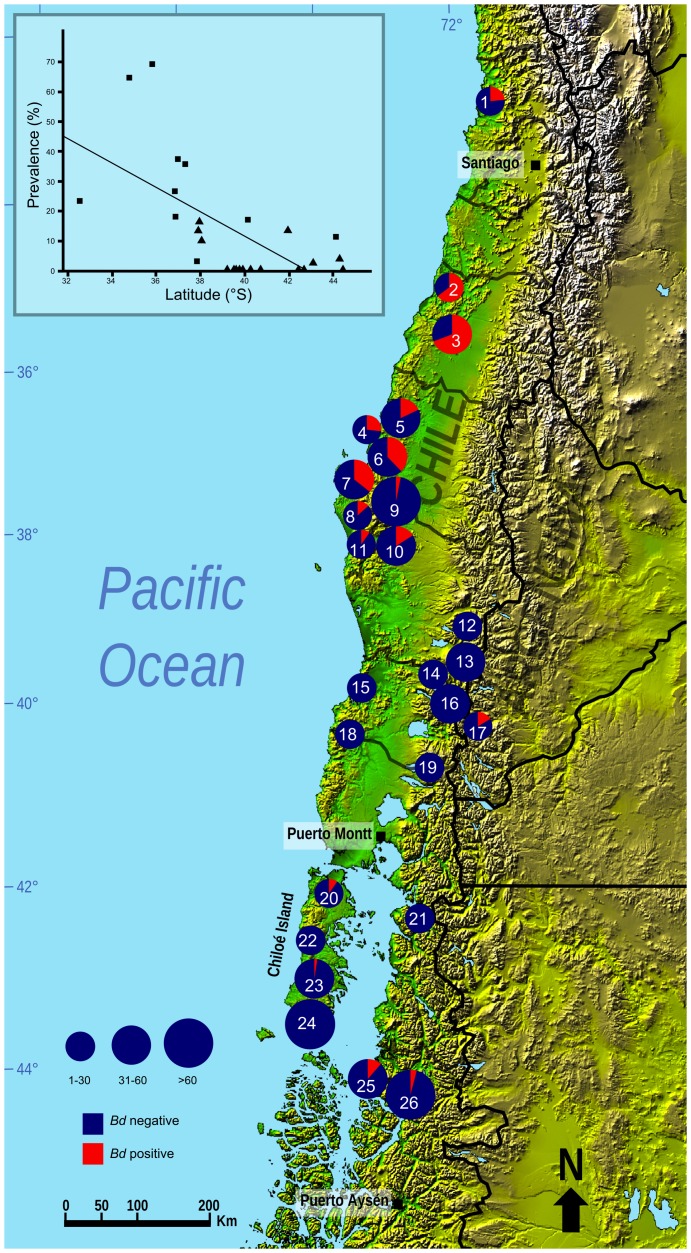
Figure 1. Batrachochytrium dendrobatidis infection prevalence at sites with extant or recently extinct Rhinoderma spp.
Map of central-south Chile and Argentina showing sites from which Rhinoderma spp. and sympatric anurans were sampled for Batrachochytrium dendrobatidis (Bd) detection between 2008 and 2012. Sample size is represented by the size of the circles, with Bd prevalence shown in the red segments. Inset: Graph showing the relationship between latitude and prevalence of Bd infection by site (R 2 = 0.405, P<0.001). Squares: sites with recent extinction or population decline of Rhinoderma spp. Triangles: sites with extant populations and no evidence of population decline of R. darwinii.
Archived anurans
A retrospective study was carried out by examining 555 postmetamorphic Rhinoderma spp. and 107 sympatric anuran specimens, from the collections of the Zoologisches Museum Hamburg (ZMH, n = 321); Natural History Museum, London (BMNH, n = 142); Museo de Zoología, Universidad de Concepción, Chile (MZUC, n = 121); Zoologisches Forschungsmuseum Alexander Koenig, Bonn (ZFMK, n = 46); and Centro de Investigaciones Zoológicas, Universidad de Chile (CIZ, n = 32). Specimens were preserved in 70% ethanol (or 70% industrial methylated spirits for BMNH amphibians) and had been collected in central and south Chile between 1835 and 1989 (Table 1) for purposes other than disease investigation.
Table 1. Archived Darwin’s frogs (Rhinoderma spp.) and sympatric amphibians from European and Chilean museums examined for Batrachochytrium dendrobatidis (Bd) infection.
| Species | Period of collection | No. examined | No. positives | Bd prevalence |
| Batrachyla leptopus | 1869−1932 | 6 | 0 | 0 |
| Batrachyla taeniata | 1845−1972 | 38 | 0 | 0 |
| Calyptocephalella gayi | 1871−1978 | 21 | 0 | 0 |
| Nannophryne variegata | 1845−1962 | 17 | 0 | 0 |
| Pleurodema bufonina | 1971 | 2 | 0 | 0 |
| Pleurodema thaul | 1970−1972 | 9 | 1 | 11.1 |
| Rhinella spinulosa | 1844−1860 | 13 | 0 | 0 |
| Rhinella arunco | 1972 | 1 | 0 | 0 |
| Rhinoderma darwinii | 1835−1989 | 289 | 4 | 1.4 |
| Rhinoderma rufum | 1904−1980 | 266 | 1 | 0.4 |
Living anurans
Cross-sectional studies were carried out at sites where R. darwinii was extant and at sites where Rhinoderma spp. had recently (since 1966) become extinct. Sites were delimited and a search effort of one hour by two researchers was conducted during daylight hours using a standardised methodology, as previously described [8].
Sampling
Archived anurans. The skin of the ventral pelvis and ventral hind limbs of each amphibian museum specimen was sampled by brushing with a tapered inter-dental brush (3.2 to 6.0 mm; Oral B Laboratories), following Soto-Azat et al. [27]. Where multiple specimens were held in a single jar, they were rinsed with running tap water prior to sampling to remove possible surface contamination with Bd. Each specimen was handled using a new pair of disposable nitrile or latex gloves.
Live anurans. Only post-metamorphic and adult anurans were sampled. Frogs were captured by hand, safely contained in individual sealed plastic bags and put back immediately after the capture session in the exact place of capture. Each individual was handled with the use of clean disposable nitrile gloves. A sterile dry, rayon-tipped swab (MW100, Medical & Wire Equipment Co.) was firmly run five times each over the ventral abdomen and pelvis, each ventral hind limb (femur and tibia) and the plantar surface of each hind foot, to complete a total of 35 strokes. Dorsal and ventral pattern photographs were taken of each Darwin’s frog sampled for identification purposes. In order to minimize any Bd contamination of samples or the spread of pathogens within or between study sites by researchers, equipment or materials, a strict field sampling and disinfection protocol was followed according to that recommended by the Amphibian and Reptile Groups, UK: ARG Advice Note 4 (http://www.arguk.org/advice-and-guidance/view-category). All samples were stored at −80 °C until processed.
Diagnostic analysis
Post sampling, whole interdental brushes and swab tips were deposited separately in 1.5 ml Eppendorf tubes containing 50 and 60 µl, respectively, of PrepMan Ultra (Applied Biosystems) and between 30 to 40 mg of Zirconium/silica beads of 0.5 mm diameter (Biospec Products). For each sample, DNA was extracted following the protocol of Boyle et al. [28]. Extracted DNA was diluted (1:10) in double-distilled water and analysed using a quantitative real-time polymerase chain reaction Taqman assay (qPCR) with primers specific for the ITS-1/5.8S ribosomal DNA region of Bd. In addition, bovine serum albumin (BSA) was included in the Taqman mastermix to minimise inhibition of the PCR [29]. For each sample, diagnostic assays were performed in duplicate, and standards of known zoospore concentration were included within each PCR plate, as were negative controls. A result was considered positive when: (1) amplification (i.e. a clearly sigmoid curve) occurred in both replicated PCR assays, (2) values higher than 0.1 genomic equivalents (GE) were obtained from both replicated reactions, and (3) average GE from both replicates were higher than its standard deviation. Extracted DNA from any positive sample was re-tested in duplicate and only determined to be positive for the purposes of this study if Bd DNA was clearly amplified in duplicate wells for a second time.
Data analysis
Areas with historical and current presence of Rhinoderma spp. > 2 km from each other were determined to be separate sites or populations [30]. Statistical analyses were performed using SPSS (v. 20.0) to detect any significant difference between: 1) Bd prevalence and time in archived R. darwinii (using Fisher’s exact test for small sample sizes), 2) Bd prevalence in extant R. darwinii and sympatric amphibians (using the chi-squared test), 3) Bd intensity in extant R. darwinii and all other amphibian species tested (using the Mann-Whitney U-test), and 4) Bd prevalence at sites with and without evidence of recent Rhinoderma spp. population decline in extant R. darwinii (using the chi-squared test). Data on Rhinoderma spp. abundance is scarce. To consider a population having evidence of recent decline, we used data from a previous study [8], which investigated population sizes and the extent of declines in Darwin’s frogs. Briefly, populations categorised as having declined comprised those known to have disappeared since 1966, or (in one case) known to have undergone a recent marked population decline. A relationship between Bd prevalence at sites with historical and current Rhinoderma spp. populations and latitude was also tested using a simple linear regression model.
Results
Batrachochytrium dendrobatidis in archived amphibians
Six (0.9%) of 662 archived anurans were positive for Bd (4/289 R. darwinii; 1/266 R. rufum; and 1/107 sympatric anurans, a four-eyed toad, Pleurodema thaul). Each Bd-positive sample was positive in duplicate when re-tested. Bd-infected specimens were not equally distributed across time of collection, with all Bd-positive animals having been collected in or since 1970 (1835−1969 = 0/347, 1970−1989 = 6/315; Fisher’s exact test, P = 0.011). Details of species sampled, periods of collection and Bd positive individuals are presented in Tables 1 and 2.
Table 2. Historical presence of Batrachochytrium dendrobatidis (Bd) infection in Rhinoderma spp. and sympatric amphibians.
| Year of Collection | Specimen reference no.a | Species | Origin | GE | SD |
| 1970 | BMNH 19.722.013 | P. thaul | Concepción | 0.2 | 0.0 |
| 1971 | MZUC A36870 | R. darwinii | PN V. Perez Rosales | 0.1 | 0.1 |
| 1975 | ZMH A04604 | R. rufum | Chiguayante | 0.4 | 0.1 |
| 1978 | ZFMK 32088 | R. darwinii | Valdivia | 0.5 | 0.1 |
| 1978 | ZFMK 32089 | R. darwinii | Valdivia | 0.6 | 0.0 |
| 1978 | ZFMK 32091 | R. darwinii | Valdivia | 0.5 | 0.0 |
Details of Bd positive archived amphibians with number of genomic equivalents (GE) detected using a Bd-specific quantitative real-time PCR Taqman assay.
BMNH = Natural History Museum, London; MZUC = Museo de Zoología, Universidad de Concepción, Chile; ZMH = Zoologisches Museum Hamburg; ZFMK = Zoologisches Forschungsmuseum Alexander Koenig, Bonn.
Batrachochytrium dendrobatidis in extant amphibians
Twenty-six sites with current or past presence of Rhinoderma spp. were surveyed. No R. rufum was found. A total of 797 skin swabs were obtained from R. darwinii (n = 369) and other amphibians (428), including areas with current presence of R. darwinii (16 sites, 144 sympatric amphibians) and 284 amphibians from sites where Rhinoderma spp. had gone extinct since 1966 (10 sites). Details of Bd positive individuals by site were: (1) Zapallar, 4/17 (No. amphibian positive/No. tested); (2) Lago Vichuquén, 11/17; (3) Río Longaví, 25/36; (4) Chiguayante, 4/15; (5) San Pedro, 8/44; (6) Hualqui, 12/32; (7) Ramadillas, 14/39; (8) Butamalal, 2/15; (9) PN Nahuelbuta, 2/63; (10) El Natre, 5/31; (11) Contulmo, 2/20; (12) PN Huerquehue, 0/11; (13) PN Villarrica, 0/38; (14) Coñaripe, 0/14; (15) Oncol, 0/8; (16) Huilo Huilo, 0/37; (17) PN Lanín, 1/6; (18) PN Alerce Costero, 0/7; (19) PN Puyehue, 0/22; (20) Senda Darwin, 2/15; (21) Huinay, 0/15; (22) Los Alerzales, 0/13; (23) Tantauco Norte, 1/40; (24) Tantauco Sur, 0/130; (25) Melimoyu, 4/35; and (26) PN Queulat, 3/77 (Figure 1). We found Bd to be widespread in central-south Chile, from the region of Valparaiso to the region of Aysén, and also to be present in Argentina, covering an area of 1,305 km in length, with an estimated overall infection prevalence of 12.5%, varying by site from 0 to 69.4%. The prevalence of Bd infection varied amongst species, from 0 to 100% of individuals tested, although sample sizes for many species were small and distributed across multiple sites (Table 3).
Table 3. Batrachochytrium dendrobatidis (Bd) infection in 19 amphibian species at 26 sites with historical and current presence of Darwin’s frogs (Rhinoderma spp.), sampled during the period 2008−2012 in central and south Chile and south-western Argentina.
| Species | No. Sampled | No. Positives | Infection prevalence (%) | Mean GE | GE Range |
| Alsodes australis | 2 | 0 | 0 | - | - |
| Alsodes barrioi | 12 | 0 | 0 | - | - |
| Alsodes nodosus | 12 | 4 | 33.3 | 17.7 | 1.7−53.1 |
| Alsodes verrucosus | 2 | 0 | 0 | - | - |
| Batrachyla antartandica | 34 | 6 | 17.6 | 177.6 | 1.4−656.3 |
| Batrachyla leptopus | 16 | 0 | 0 | - | - |
| Batrachyla taeniata | 68 | 3 | 4.4 | 180.5 | 26.5−408.9 |
| Calyptocephalella gayi | 18 | 18 | 100 | 997.0 | 79.3−3,355.0 |
| Eupsophus altor | 1 | 0 | 0 | - | - |
| Eupsophus calcaratus | 32 | 1 | 3.1 | 5.4 | - |
| Eupsophus contulmoensis | 15 | 4 | 26.7 | 5.0 | 0.1−14.0 |
| Eupophus emiliopugini | 4 | 0 | 0 | - | - |
| Eupsophus nahuelbutensis | 59 | 3 | 5.1 | 15.1 | 0.4−40.1 |
| Eupsophus roseus | 10 | 2 | 20.0 | 6.4 | 2,6−11,7 |
| Eupsophus vertebralis | 1 | 0 | 0 | - | - |
| Hylorina sylvatica | 3 | 1 | 33.3 | 593.3 | - |
| Pleurodema thaul | 137 | 51 | 37.2 | 149.5 | 0.2−4,481.0 |
| Rhinoderma darwinii | 369 | 7 | 1.9 | 1,221.4 | 6.7−7,059.1 |
| Telmatobufo bullocki | 2 | 0 | 0 | - | - |
Genomic equivalents (GE) detected using a Bd-specific quantitative real-time PCR Taqman assay are expressed in means and ranges.
Of the 369 R. darwinii tested, seven frogs from four different populations were positive for Bd (Table 4). Overall, the Bd prevalence in R. darwinii (1.9%) was significantly lower to that in sympatric amphibians tested (n = 109, 7.3%; x 2 = 8.200, P = 0.004). Contemporary R. darwinii populations sampled in south and southern Chile and their Bd prevalences are shown in Table 5. Although R. darwinii had the highest infection intensities (median: 127.1; range: 6.7−7,059.1 GE) when compared with all other infected species (13.9; 0.1−4,481.0 GE) they were not significantly different (Mann-Whitney U-test; U = 188.0, P = 0.063). Of particular interest were two R. darwinii from which GE counts over 1,000 were detected. Both frogs belonged to the northernmost known populations. Of these, one individual (NATRE74/12; 1,020 GE) was found dead at the capture site and subsequent histopathological examination revealed chytridiomycosis as the cause of death (Figure 2). The prevalence of Bd infection was significantly higher at sites with either Rhinoderma spp. extinction or severe population decline (30.0%) than at sites with no apparent Rhinoderma spp. declines (3.0%; x 2 = 106.407, P<0.001). Additionally, when Bd prevalence by site and geographical location were analysed, a linear regression revealed an inverse relationship between Bd prevalence and latitude (R 2 = 0.405, P<0.001; Figure 1).
Table 4. Details of Batrachochytrium dendrobatidis positive Southern Darwin’s frogs (Rhinoderma darwinii) sampled during the period 2008−2012 in south Chile with number of genomic equivalents (GE) detected using a Bd-specific quantitative real-time PCR Taqman assay.
| Reference | Site | Animal | GE | SDa |
| NATRE74/12 | El Natre | subadult | 1,019.5 | 90.3 |
| NATRE151/12 | El Natre | brooding male | 249.0 | 40.4 |
| CON123/10 | Contulmo | adult female | 7,059.1 | 777.4 |
| CON224/11 | Contulmo | Juvenile | 21.0 | 0.5 |
| SD08/11 | Senda Darwin | adult male | 127.1 | 9.5 |
| SD03/11 | Senda Darwin | adult male | 67.9 | 3.7 |
| YAL45/12 | Yaldad | adult female | 6.7 | 0.3 |
Standard deviation.
Table 5. Batrachochytrium dendrobatidis infection in 14 extant populations of the southern Darwin’s frog (Rhinoderma darwinii) sampled during the period 2009−2012 in south Chile.
| Site | Coordinates (lat, long) | altitude (m) | No. sampled | No. positives | Infection prevalence (%) |
| Butamalal | 37° 49' S, 73° 09' W | 560 | 6 | 0 | 0 |
| El Natre | 37° 53' S, 73° 16' W | 433 | 15 | 2 | 13,3 |
| Contulmo | 38° 01' S, 73° 11' W | 370 | 13 | 2 | 15,4 |
| PN Huerquehue | 39° 08' S, 71° 42' W | 1,239 | 1 | 0 | 0 |
| PN Villarrica | 39° 29' S, 71° 51' W | 1,114 | 32 | 0 | 0 |
| Coñaripe | 39° 33' S, 71° 59' W | 371 | 14 | 0 | 0 |
| Oncol | 39° 42' S, 73° 18' W | 523 | 5 | 0 | 0 |
| Huilo Huilo | 39° 52' S, 71° 54' W | 619 | 36 | 0 | 0 |
| PN Alerce Costero | 40° 12' S, 73° 26' W | 912 | 7 | 0 | 0 |
| PN Puyehue | 40° 39' S, 72° 11' W | 352 | 22 | 0 | 0 |
| Senda Darwin | 41° 53' S, 73° 41' W | 9 | 14 | 2 | 14,3 |
| Los Alerzales | 42° 35' S, 74° 05' W | 169 | 13 | 0 | 0 |
| Tantauco Norte | 43° 02' S, 73° 48' W | 146 | 27 | 1 | 3,7 |
| Tantauco Sur | 43° 22' S, 74° 07' W | 5 | 97 | 0 | 0 |
| PN Queulat | 44° 14' S, 72° 30' W | 143 | 67 | 0 | 0 |
Figure 2. Skin histological section of a wild southern Darwin’s frog (Rhinoderma darwinii) with cutaneous chytridiomycosis.
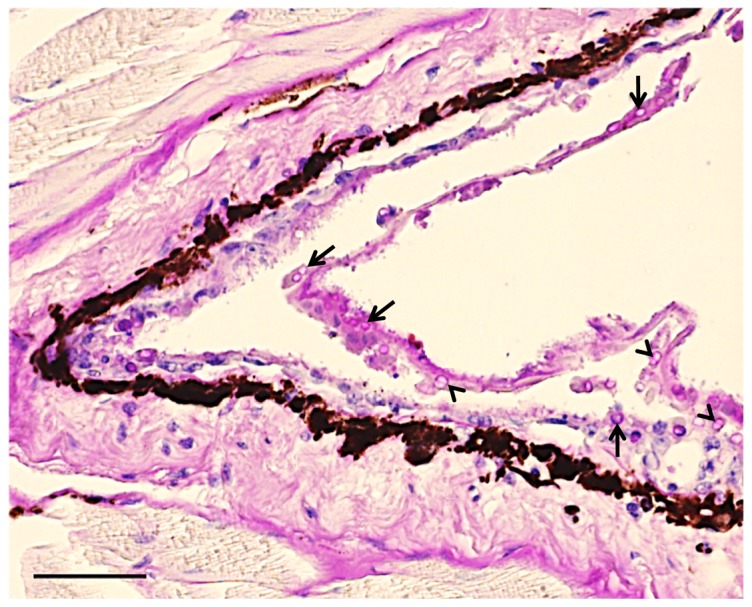
Note multiple empty zoosporangia (arrows) within the superficial keratinised layer of the epidermis. Several zoosporangia with an internal septum can be seen (arrowheads), morphologically typical of Batrachochytrium dendrobatidis. Stained with Periodic Acid-Shiff (PAS). Bar = 20 µm.
Discussion
Museum amphibian specimens have been increasingly recognised as a valuable source of information for retrospective epidemiological studies [31], [32], [33], [34], [35]. Using such specimens, we demonstrated historical evidence of Bd infection in three species of native frogs from south Chile (R. darwinii, R. rufum and P. thaul). Although we examined similar numbers of frogs that had been collected prior to 1970 and post-1970, all six Bd-positive archived amphibians were collected from 1970 to 1978 inclusive: a time coincident with the onset of the global amphibian population decline phenomenon, including the disappearance of R. rufum, and the occurrence of the first amphibian global extinctions subsequently associated with Bd [16], [35], [36]. The only R. rufum Bd-positive animal was an individual kept in a jar with 179 other R. rufum specimens, all of which had been collected from Chiguayante (Biobío Region, near Concepción) during a two-day collection session in December 1975.
As the fixation history of the examined archived amphibians is not known, the overall infection prevalence (0.9%) and intensity of infection (GE values 0.1−0.6) obtained are likely an underestimation of the true situation. For example, although all of the archived specimens examined were preserved in alcohol, it is highly possible that many had been initially fixed in formalin, a chemical known to degrade DNA, reducing the likelihood of Bd detection [27]. Also, the fixative, IMS, can inhibit PCR. A previous study, however, was successful in detecting Bd DNA from the skin of amphibian specimens fixed in IMS [35] and in the current study we incorporated BSA to the PCR protocol to minimize the effect of any PCR inhibiters present [29].
Our field surveys failed to detect R. rufum, but infection with Bd was found in extant R. darwinii, but at a lower prevalence (1.9%) than in the other sympatric amphibian species tested (prevalence 7.3%), possibly as a consequence of different habitat use by the studied species (e.g. dependence of water for breeding). If highly susceptible to chytridiomycosis, however, it is possible that R. darwinii die soon after infection. This also would result in a low infection prevalence and might explain the disappearance of Rhinoderma spp. from many of the sites where Bd was found, especially if other amphibians act as reservoirs of infection, as might be predicted from their higher Bd prevalences [17], [37].
Amphibian chytridiomycosis is thought to have caused 100% mortality of 30 wild-caught R. darwinii exported to Germany in 2007 [23], [25]. According to these authors [25], travel stress and lack of isolation between individuals during transportation might have contributed to this high mortality rate. In the current study, two of seven Bd-positive wild R. darwinii had infection loads > 1,000 GE; including an individual found dead with chytridiomycosis. Disease and mortality caused by chytridiomycosis have been associated with infections higher than 1,000 GE in experimentally-infected green tree frogs (Litoria caerulea) [38], [39]. Experimental Bd infection trials in R. darwinii, similar to those performed with the Critically Endangered New Zealand Archey’s frog (Leiopelma archeyi) [40], [41] and with the Panamanian golden frog (Atelopus zeteki) [42], should be considered to further investigate the susceptibility of R. darwinii to chytridiomycosis. As the outcomes of Bd infection often are highly context-specific, experimental infection studies using R. darwinii under different hydric environments could help to infer the likely effects of Bd infection on R. darwinii under different climate and land-use change scenarios [43], [44], [45]. In a declining species like R. darwinii, however, promoting the survival of the species has to take priority: the use of animals in experiments should be internationally justifiable and only surplus captive-bred animals not suitable for conservation programmes should be used.
Batrachochytrium dendrobatidis is a waterborne pathogen and stream-living has been identified as a risk factor for Bd-associated declines [46]. Rhinoderma darwinii has evolved to develop an extreme case of parental care in which the species does not depend on water bodies for tadpole development [47]. In contrast, while R. rufum tadpoles spend their first two weeks of development in the vocal sacs of their male parents, they are then released into water as larvae where they live for the next approximately 120 days until metamorphosis takes place [48]. This association of R. rufum with streams in central Chile could render this species even more susceptible to population declines and extinction due to chytridiomycosis. Although found in only a single archived specimen, evidence of Bd infection was found in possibly the largest known R. rufum population [8] five years before the species was last recorded [5]. This, along with a positive association between Bd prevalence and Rhinoderma spp. population extinction/decline, suggests a possible association between chytridiomycosis and the disappearance of R. rufum.
We detected an inverse relationship between Bd prevalence and latitude, similar to that found by Kriger et al. [49] in the stony creek frog (Litoria lesueuri) in eastern Australia. Whether this is a reflection of the historical introduction and spread of Bd in Chile, with the organism not yet having reached the south of the country, or if it is due to environmental factors (e.g. temperature) is not yet clear. Longitudinal sampling of sites across the gradient would help to answer this question. That such a gradient exists, however, indicates that northern populations of R. darwinii are likely to be under a greater threat from chytridiomycosis than those in the south. It also suggests that the instigation of biosecurity measures might decrease the rate of spread of the disease to the southern populations of R. darwinii (assuming that Bd has not already reached this region and is less readily detected due to the low temperatures there limiting its growth).
It is not known if the Bd detected in the archived or extant specimens in the current study is the hypervirulent BdGPL, a BdGPL-hybrid, or perhaps an endemic lineage (or lineages) of the fungus. If BdGPL is present in Chile, its spread to the country might have occurred via the introduction of X. laevis [32]. Feral populations of this invasive species, which have been established in central Chile since the 1970s, are known to be Bd-positive, although other mechanisms of pathogen introduction cannot be excluded [24].
Conclusions
This is the first report of widespread Bd presence in Chile and our results provide evidence of an association between the presence of Bd and mortality in wild R. darwinii. Although, assessing the role of pathogens in extinctions remains problematic and infectious diseases are probably an underestimated cause of biodiversity loss [16], retrospective and prospective epidemiological data provide evidence that Bd infection is probably implicated in the enigmatic disappearance of R. rufum and the declines of R. darwinii, particularly from the northern part of their historical range. Nevertheless, further studies, such as the isolation and DNA sequencing of Bd in Chile, are required to further investigate the possible role of Bd in Rhinoderma spp. declines.
Acknowledgments
We thank J. Reardon, H. Meredith, S. Wren, R. Monsalve, A. Toro, C. Espinoza, R. Sánchez, G. Harding and E. Flores for their important fieldwork support. We also thank S. Sarmiento for laboratory assistance. We are very grateful to Parque Tantauco, Fundación Huilo Huilo, Parque Oncol and Parque Pumalín. This study was carried out as part fulfilment of the Conservation Medicine Ph. D. degree (by CSA) at the Faculty of Ecology and Natural Resources, Universidad Andres Bello, Chile.
Funding Statement
This research was funded by the Institute of Zoology, Zoological Society of London (ZSL) and the ZSL EDGE Fellowship Programme; the Dirección General de Investigación y Doctorados, Universidad Andres Bello; the Field Veterinary Programme, Wildlife Health Fund, Wildlife Conservation Society; and a Fundación Futuro Scholarship. AAC is supported by a Royal Society Wolfson Research Merit Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Veloso A, Núñez H, Diaz-Páez H, Formas R (2010) Rhinoderma rufum IUCN Red List of Threatened Species. Version 2012.1. Available: http://www.iucnredlist.org. Accessed 2013 Jan 25.
- 2.Úbeda C, Veloso A, Núñez H, Lavilla E (2010) Rhinoderma darwinii IUCN Red List of Threatened Species. Version 2012.1. Available: http://www.iucnredlist.org. Accessed 2013 Jan 25.
- 3. Jiménez de la Espada DM (1872) Sobre la reproducción de Rhinoderma darwinii . Anales de la Sociedad de Historia Natural de Madrid 1: 139–151. [Google Scholar]
- 4.Bürger O (1905) La neomelia de la Rhindoerma darwinii D & B. Santiago: Imprenta Cervantes. 23 p. [Google Scholar]
- 5. Penna M, Veloso A (1990) Vocal diversity in frogs of the South American temperate forest. J Herpetol 24: 23–33. [Google Scholar]
- 6. Bourke J, Busse K, Bohme W (2012) Searching for a lost frog (Rhinoderma rufum): identification of the most promising areas for future surveys and possible reasons of its enigmatic decline. North-West J Zool 8: 99–106. [Google Scholar]
- 7.Crump ML, Veloso A (2005) El aporte de observaciones de terreno y del análisis genético para la conservación de Rhinoderma darwinii en Chile. In: Smith-Ramirez C, Armesto JJ, Valdovinos C, editors. Historia, biodiversidad y ecología de los bosques costeros de Chile. Santiago: Editorial Universitaria. pp. 452−455.
- 8. Soto-Azat C, Valenzuela-Sánchez A, Collen B, Rowcliffe JM, Veloso A, et al. (2013) The population decline and extinction of Darwin's frogs. Plos One 8: e66957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger L, Speare R, Daszak P, Green DE, Cunningham AA, et al. (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. P Natl Acad Sci U S A 95: 9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91: 219–227. [Google Scholar]
- 11. Bosch J, Martínez-Solano I, García-París M (2001) Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol Conserv 97: 331–337. [Google Scholar]
- 12. Lips KR (1999) Mass mortality and population declines of anurans at an upland site in Western Panama. Conserv Biol 13: 117–125. [Google Scholar]
- 13. Bradley GA, Rosen PC, Sredl MJ, Jones TR, Longcore JE (2002) Chytridiomycosis in native Arizona frogs. J Wildlife Dis 38: 206–212. [DOI] [PubMed] [Google Scholar]
- 14. Green DE, Converse KA, Schrader AK (2002) Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996−2001. Ann N Y Acad Sci 969: 323–339. [DOI] [PubMed] [Google Scholar]
- 15. Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, et al. (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4: 125–134. [Google Scholar]
- 16. Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, et al. (2006) The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? EcoHealth 3: 35–40. [Google Scholar]
- 17. Daszak P, Berger L, Cunningham AA, Hyatt AD, Green DE, et al. (1999) Emerging infectious diseases and amphibian population declines. Emerg Infect Dis 5: 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farrer RA, Weinert LA, Bielby J, Garner TWJ, Balloux F, et al. (2011) Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. P Natl Acad Sci U S A 108: 18732–18736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schloegel LM, Toledo LF, Longcore JE, Greenspan SE, Vieira CA, et al. (2012) Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol Ecol 21: 5162–5177. [DOI] [PubMed] [Google Scholar]
- 20. Rosenblum EB, James TY, Zamudio KR, Poorten TJ, Ilut D, et al. (2013) Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. P Natl Acad Sci U S A 110: 9385–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gower DJ, Doherty-Bone T, Loader SP, Wilkinson M, Kouete MT, et al. (2013) Batrachochytrium dendrobatidis infection and lethal chytridiomycosis in caecilian amphibians (Gymnophiona). Ecohealth 10: 173–183. [DOI] [PubMed] [Google Scholar]
- 22.Gascon C, Collins JP, Moore RD, Church DR, McKay JE, et al.. (2007) Amphibian conservation action plan. Proceedings: IUCN/SSC Amphibian Conservation Summit 2005. GlandSwitzerland and CambridgeUK: IUCN. 68 p. [Google Scholar]
- 23. Werning H (2009) From Darwin's treasure chest: Rhinoderma . IRCF Reptiles and Amphibians 16: 247–255. [Google Scholar]
- 24. Solís R, Lobos G, Walker SF, Fisher M, Bosch J (2010) Presence of Batrachochytrium dendrobatidis in feral populations of Xenopus laevis in Chile. Biol Invasions 12: 1641–1646. [Google Scholar]
- 25. Bourke J, Mutschmann F, Ohst T, Ulmer P, Gutsche A, et al. (2010) Batrachochytrium dendrobatidis in Darwin's frog Rhinoderma spp. in Chile. Dis Aquat Organ 92: 217–221. [DOI] [PubMed] [Google Scholar]
- 26. Bourke J, Ohst T, Graser Y, Bohme W, Plotner J (2011) New records of Batrachochytrium dendrobatidis in Chilean frogs. Dis Aquat Organ 95: 259–261. [DOI] [PubMed] [Google Scholar]
- 27. Soto-Azat C, Clarke BT, Fisher MC, Walker SF, Cunningham AA (2009) Non-invasive sampling methods for the detection of Batrachochytrium dendrobatidis in archived amphibians. Dis Aquat Organ 84: 163–166. [DOI] [PubMed] [Google Scholar]
- 28. Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ 60: 141–148. [DOI] [PubMed] [Google Scholar]
- 29. Garland S, Baker A, Phillott AD, Skerratt LF (2010) BSA reduces inhibition in a TaqMan (R) assay for the detection of Batrachochytrium dendrobatidis . Dis Aquat Organ 92: 113–116. [DOI] [PubMed] [Google Scholar]
- 30. Scribner KT, Arntzen JW, Cruddace N, Oldham RS, Burke T (2001) Environmental correlates of toad abundance and population genetic diversity. Biol Conserv 98: 201–210. [Google Scholar]
- 31. Weldon C, du Preez LH, Hyatt AD, Muller R, Speare R (2004) Origin of the amphibian chytrid fungus. Emerg Infect Dis 10: 2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soto-Azat C, Clarke BT, Poynton JC, Cunningham AA (2010) Widespread historical presence of Batrachochytrium dendrobatidis in African pipid frogs. Divers Distrib 16: 126–131. [Google Scholar]
- 33. Ouellet M, Mikaelian I, Pauli BD, Rodrigue J, Green DM (2005) Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv Biol 19: 1431–1440. [Google Scholar]
- 34. Suarez AV, Tsutsui ND (2004) The value of museum collections for research and society. Bioscience 54: 66–74. [Google Scholar]
- 35. Cheng TL, Rovito SM, Wake DB, Vredenburg VT (2011) Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis . P Natl Acad Sci U S A 108: 9502–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, et al. (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- 37. Daszak P, Cunningham AA, Hyatt AD (2003) Infectious disease and amphibian population declines. Divers Distrib 9: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voyles J, Berger L, Young S, Speare R, Webb R, et al. (2007) Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Organ 77: 113–118. [DOI] [PubMed] [Google Scholar]
- 39. Voyles J, Young S, Berger L, Campbell C, Voyles WF, et al. (2009) Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326: 582–585. [DOI] [PubMed] [Google Scholar]
- 40. Bishop PJ, Speare R, Poulter R, Butler M, Speare BJ, et al. (2009) Elimination of the amphibian chytrid fungus Batrachochytrium dendrobatidis by Archey's frog Leiopelma archeyi . Dis Aquat Organ 84: 9–15. [DOI] [PubMed] [Google Scholar]
- 41. Shaw SD, Bishop PJ, Berger L, Skerratt LF, Garland S, et al. (2010) Experimental infection of self-cured Leiopelma archeyi with the amphibian chytrid Batrachochytrium dendrobatidis . Dis Aquat Organ 92: 159–163. [DOI] [PubMed] [Google Scholar]
- 42. Bustamante HM, Livo LJ, Carey C (2010) Effects of temperature and hydric environment on survival of the Panamanian Golden Frog infected with a pathogenic chytrid fungus. Integr Zool 5: 143–153. [DOI] [PubMed] [Google Scholar]
- 43. Murphy PJ, St-Hilaire S, Corn PS (2011) Temperature, hydric environment, and prior pathogen exposure alter the experimental severity of chytridiomycosis in boreal toads. Dis Aquat Organ 95: 31–42. [DOI] [PubMed] [Google Scholar]
- 44. Rowley JJL, Alford RA (2013) Hot bodies protect amphibians against chytrid infection in nature. Sci Rep 3: 1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, et al. (2013) Disease and thermal acclimation in a more variable and unpredictable climate. Nat Clim Change 3: 146–151. [Google Scholar]
- 46. Bielby J, Cooper N, Cunningham AA, Garner TWJ, Purvis A (2008) Predicting susceptibility to future declines in the world's frogs. Conserv Lett 1: 82–90. [Google Scholar]
- 47. Goicoechea O, Garrido O, Jorquera B (1986) Evidence for a trophic paternal-larval relationship in the frog Rhinoderma darwinii . J Herpetol 20: 168–178. [Google Scholar]
- 48. Jorquera B, Pugin E, Garrido O, Goicoechea O, Formas R (1981) Procedimiento de desarrollo en dos especies del género Rhinoderma . Medio Ambiente 5: 58–71. [Google Scholar]
- 49. Kriger KM, Pereoglou F, Hero JM (2007) Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conserv Biol 21: 1280–1290. [DOI] [PubMed] [Google Scholar]