SUMMARY
Targeting of type III secretion proteins at the injectisome is an important process in bacterial virulence. Nevertheless, how the injectisome specifically recognizes TTS substrates among all bacterial proteins is unknown. A TTS peripheral membrane ATPase protein located at the base of the injectisome has been implicated in the targeting process. We have investigated the targeting of the EspA filament protein and its cognate chaperone CesAB to the EscN ATPase of the enteropathogenic E. coli (EPEC). We show that EscN selectively engages the EspA-loaded CesAB, but not the unliganded CesAB. Structure analysis revealed that the targeting signal is encoded in a disorder-order structural transition in CesAB that is elicited only upon binding of its physiological substrate, EspA. Abrogation of the interaction between the CesAB–EspA complex and EscN resulted in severe secretion and infection defects. We further show that the targeting and secretion signals are distinct and the two processes are likely regulated by different mechanisms.
INTRODUCTION
The type III secretion (TTS) system is a multi-protein machinery that has evolved to deliver bacterial virulence proteins directly into eukaryotic cells, through an organelle termed the injectisome (Cornelis, 2006; Galán and Wolf-Watz, 2006). The TTS substrates (needle-forming proteins, effectors, and translocators) are targeted to the cytoplasmic base of the injectisome and hierarchically secreted through the channel (Izoré et al., 2011). In the cytosol, TTS substrates are typically found as complexes with their cognate chaperones (Birtalan et al., 2002; Page and Parsot, 2002; Feldman and Cornelis, 2003; Francis, 2010), which have established roles as anti-aggregation and stabilizing factors for TTS substrates. It has been hypothesized that chaperones may also act as signals for targeting and hierarchy-determining factors (Birtalan et al., 2002; Lilic et al., 2006; Rodgers et al., 2010; Lara-Tejero et al., 2011).
A key protein in TTS systems is the ATPase (Woestyn et al., 1994; Pallen et al., 2005), a peripheral membrane protein located at the entrance of the injectisome. Biochemical experiments have provided evidence that the TTS ATPase protein, which is ubiquitous to all TTS systems, may serve to recognize and engage the TTS proteins at the injectisome (Gauthier and Finlay, 2003; Akeda and Galán, 2005; Thomas et al., 2005; Boonyom et al., 2010; Cooper et al., 2010). The ATPase is located at the cytoplasmic base of the injectisome and forms a ring structure (Müller et al., 2006) that resembles the F1F0-ATPase (Pallen et al., 2006; Imada et al., 2007; Zarivach et al., 2007). The molecular basis for the targeting of TTS substrates to the ATPase remains completely unknown.
We studied the targeting process in EPEC, the archetype of a group of pathogens that adhere to host enterocytes via the formation of attaching and effacing (A/E) lesions and cause extensive host cell cytoskeletal rearrangements (Dean and Kenny, 2009). When secreted, EspA undergoes self-polymerization, thereby forming a long extracellular filamentous extension that coats the needle and connects it to the translocation pore in the eukaryotic plasma membrane and likely acts as a molecular conduit for TTS protein translocation (Knutton et al., 1998). EspA has a high tendency to self-oligomerize and thus is retained in a monomeric, soluble state in the cytoplasm by forming a complex with the CesAB chaperone (Creasey et al., 2003; Yip et al., 2005).
Here we show that the homodimeric CesAB chaperone exists in a partially unfolded state and does not interact with the EscN ATPase. In contrast, formation of the CesAB–EspA chaperone–substrate complex results in strong affinity for EscN. Structural analysis demonstrated that EspA binding to CesAB results in extensive folding of many regions in the chaperone. The induced structure in one of these regions is specifically recognized by EscN and mediates the formation of the ternary EscN–CesAB–EspA complex. Interestingly, a homodimeric CesAB variant designed to adopt a folded structure, similar to the one induced by EspA binding, is capable of interacting with EscN. Amino acid substitutions in the EscN-interacting CesAB region abrogate targeting of CesAB–EspA to EscN resulting in severe secretion and infection defects.
RESULTS
The Substrate-Free CesAB Chaperone does not Interact with the EscN ATPase
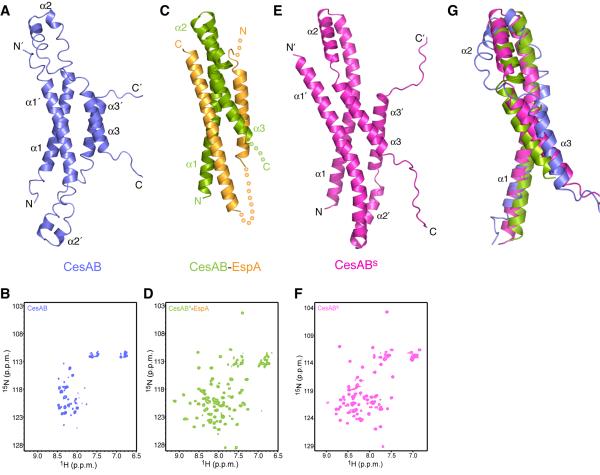
In the absence of its substrate EspA, CesAB exists as a loosely packed, conformationally dynamic homodimer in solution (Chen et al., 2011) (Figure 1A and 1B). CesAB adopts a four-helix bundle structure with each of the subunits in an all-helical conformation consisting of three helices of variable stability (Chen et al., 2011) (Figure 1A). We used NMR spectroscopy, which is a very sensitive reporter of even transient binding interactions (Takeuchi and Wagner, 2006), to test whether CesAB interacts with the ATPase EscN (Gauthier and Finlay, 2003; Zarivach et al., 2007). For this reason, we prepared full-length EscN, which we show here that it forms a stable hexamer in solution with stimulated ATPase activity (Figure S2A and S2C). The NMR data show that none of the CesAB resonances is affected by the addition of EscN (Figure S2D), thereby clearly demonstrating that there is no interaction between CesAB and EscN. Thus, CesAB appears not to be engaged by the injectisome ATPase in its substrate-free form.
Figure 1. Structures of CesAB, CesAB–EspA and CesABs.
(A) Solution structure of the homodimeric CesAB, which adopts a molten-globule-like structure in solution (Chen et al., 2011).
(B) 1H-15N HSQC spectrum of CesAB.
(C) Crystal structure of the heterodimeric CesAB–EspA (Yip et al., 2005). Regions of the proteins that were not crystallographically resolved are represented as dotted lines.
(D) 1H-15N HSQC spectrum of CesAB–EspA. The CesAB subunit is 15N labeled whereas the EspA subunit is unlabeled.
(E) Solution structure of the CesABs variant (D14L/R18D/E20L), determined in this work.
(F) 1H-15N HSQC spectrum of CesABs
(G) Superposition of the CesAB subunit of CesAB, CesAB–EspA and CesABs.
The CesAB–EspA Heterodimer Interacts Specifically with the EscN ATPase
The CesAB dimer undergoes subunit exchange to interact with its cognate substrate EspA, resulting in the formation of a 1:1 heterodimeric complex with a molecular mass of ~33 kDa (Figure 1C) (Yip et al., 2005; Chen et al., 2011). The complex forms a four-helix bundle, with each protein contributing two α helices. Interestingly, CesAB, which is poorly folded in the homodimer, acquires a well-folded structure upon binding to EspA (Chen et al., 2011) (Figure 1C and 1D). We used NMR to test the interaction between CesAB–EspA and hexameric EscN. The NMR data show that addition of EscN causes a very significant effect to a large number of CesAB–EspA resonances (Figure 2A and S2E), indicating formation of the ternary CesAB–EspA–EscN complex. The dissociation constant (Kd) of the ternary complex, measured by NMR line shape analysis, is ~3 μM. Thus, the heterodimeric chaperone–substrate complex binds with a significant affinity to the active hexameric state of the injectisome ATPase. Interestingly, no binding was observed between CesAB–EspA and a N-terminal-truncated EscN variant lacking the first 98 residues (EscNΔN) that exists in a monomeric state and lacks enzymatic activity (Figures S2B, S2C and S2F). Thus, EscN has to be in a functional oligomeric state in order to engage the chaperone–substrate complex.
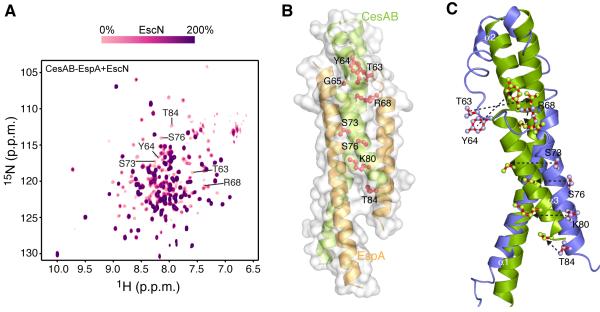
Figure 2. Interaction of CesAB–EspA with EscN.
(A) Overlaid 1H-15N HSQC spectra of the titration of U-2H-15N labeled CesAB–EspA with unlabeled hexameric EscN. Stepwise addition of EscN results in gradual resonance broadening of the interacting residues in CesAB–EspA. Spectra recorded at 10 different titration points are overlaid. The CesAB residues most affected by EscN binding are shown. The resonances not affected by EscN binding even at saturating concentrations of EscN are located in flexible regions of EspA that were crystallographically unresolved.
(B) CesAB–EspA residues (shown in red sticks) identified by NMR to be most affected upon binding to EscN. All these residues are located in helices α2 and α3 in CesAB.
(C) Superposition of the CesAB subunit of the homodimeric CesAB (blue) and the heterodimeric CesAB–EspA complex (green). The residues identified to mediate the binding to CesAB–EspA to EscN are shown.
The CesAB–EspA Interaction with EscN is Mediated by CesAB
To determine the specific residues that mediate the interaction between CesAB–EspA and EscN, we used NMR differential line broadening analysis (Matsuo et al., 1999; Panchal et al., 2003; Zamoon et al., 2005; Takeuchi and Wagner, 2006) (Figure 2A). Because EscN has a large molecular mass (~350 kDa), complex formation with labeled CesAB–EspA will result in severe line broadening of the resonances. The broadening effect is related to the chemical shift difference of the CesAB–EspA resonances between the EscN-free and EscN-bound form. Thus, using this method the residues in CesAB–EspA that are most affected by the formation of the ternary complex with EscN can be identified.
The results indicated that the residues most affected by EscN binding belong to CesAB. Specifically, Thr63, Tyr64, Arg68, Ser73, Ser76, Lys80 and Thr84 of CesAB experience the strongest effect (Figure 2B). These residues are located in helices α2 and α3 of CesAB in the heterodimer and form a contiguous solvent-exposed surface. The NMR data strongly suggest that this region forms the EscN-binding surface in CesAB–EspA.
It is of interest that CesAB, rather than EspA, appears to be responsible for mediating the binding between CesAB–EspA and EscN. This is surprising since CesAB in the homodimer does not interact with EscN (Figure S2D). Structural analysis of the CesAB homodimer and the CesAB–EspA heterodimer shows that the CesAB protomer adopts a similar overall fold; however, in the CesAB homodimer all three α helices are much shorter and largely unwound and dynamic, in contrast to the CesAB–EspA complex wherein they are well folded (Figure 1G) (Yip et al., 2005; Chen et al., 2011). Superposition of the CesAB structures of the homodimer and heterodimer shows that the EscN-binding region in CesAB is well formed in the heterodimer but extensively unfolded in the homodimer (Figure 2C).
A Well Folded CesAB Homodimer Variant Binds Specifically to EscN
The present results suggest that binding of EspA to CesAB poises CesAB for interacting with EscN by eliciting a disorder-to-order transition that stabilizes the formation of a region that is specifically recognized by EscN (Figure 2C). To further test this hypothesis, we assessed the effect of a CesAB variant that was previously shown to mimic the EspA binding effect and stabilize a well-folded structure of the CesAB homodimer (Chen et al., 2011). Specifically, the D14L/R18D/E20L triple amino acid substitution optimizes coiled-coil interactions at the CesAB helical bundle interface. The stabilized CesAB-D14L/R18D/E20L (henceforth CesABs) has NMR and CD features that are characteristic of a well folded protein (Figure S1A–D).
We have determined the solution structure of CesABs using NMR (Figures 1E, 1F and S1E and Table S1). CesABs adopts a structure that is overall very similar to the one of CesAB (Figure 1G and S1F). However, the packing at the helical bundle in CesABs is drastically improved (Figure S1E), with CesABtm burying ~3,200 Å2 in its dimeric interface, compared to only ~1,100 Å2 buried in CesAB.
Interestingly, the CesABs structure is very similar to the structure that CesAB adopts in the CesAB–EspA heterodimer (Figure 1G and S1G), with all of the three helices being well folded. Thus, the triple mutant appears to induce the same structural transition to CesAB as EspA binding does. Most importantly, the EscN-binding region is well formed in CesABs. We have used NMR to assess whether CesABs interacts with EscN. The results show clearly that there is a specific interaction between CesABs and EscN (Figure S2G), with the Kd of the ternary complex estimated at ~12 μM. The residues most affected upon complex formation are very similar in CesABs and CesAB–EspA (Figure S1H and S1I). Taken together, the results showed that the triple amino-acid substitution in CesABs induces to CesAB an overall structure that is similar to the one induced by EspA binding and, as a result, EscN specifically recognizes CesABs, in contrast to CesAB.
Disruption of CesAB–EspA Binding to EscN Gives Rise to Secretion and Functional Defects
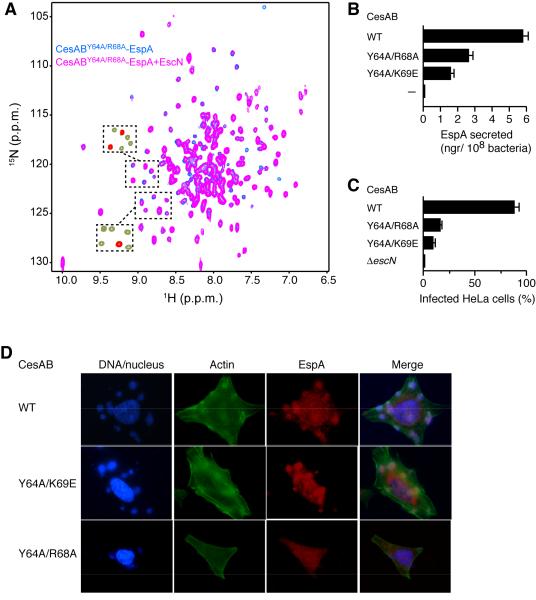
To test the binding between CesAB–EspA and EscN, we generated CesAB mutants. Amino acids in the region identified to mediate the interaction (Figure 2B) were mutated and their effect on the formation of the CesAB–EspA–EscN ternary complex was assessed by NMR and in vivo secretion assays (Figure 3). The data showed that substitutions at Glu60, Tyr64, Arg68 and Lys69 decrease substantially the affinity of CesAB–EspA for EscN. For example, Kd of CesAB–EspA-Y64A/R68A for EscN is larger than 80 μM, more than a factor of 20 than CesAB–EspA. As a result of the much weaker interaction between the mutated CesAB–EspA and EscN, secretion of EspA decreases substantially (Figure 3B). In addition, infection of HeLa cells by EPEC strains carrying cesAB mutated genes causes minimal actin polymerization and pedestal formation, indicating defective secretion of TTS effectors (Figure 3C and 3D). It should be noted that in the absence of EscN, EspA is not secreted (Figure S3). Collectively, the results provide strong evidence that efficient targeting of CesAB–EspA to EscN is required for EspA secretion.
Figure 3. Disruption of CesAB–EspA Binding to EscN Gives Rise to Secretion and Functional Defects.
(A) Effect of the Y64A/R68A substitution on the interaction of CesAB–EspA with EscN. Overlaid 1H-15N HSQC spectra of CesABY64A/R68A–EspA in the absence (blue) and presence (magenta) of EscN. Compared to wild-type CesAB–EspA binding to EscN, the NMR data indicate a significant decrease in the affinity of the ternary complex (Kd is larger than 80 μM). The boxed areas show the corresponding regions of the spectra of CesAB–EspA (green) superimposed on the spectra of its complex with EscN (red). Whereas the majority of the peaks are broadened beyond detection in the CesAB–EspA–EscN complex, they are still present at substantial intensity in the CesABY64A/R68A–EspA–EscN complex.
(B) In vivo secretion of EspA from EPECΔcesAB strains complemented with pASK-IBA7 plasmids expressing wild-type or mutated CesAB. The graph reports the total amount of EspA secreted in 90 min following CesAB expression.
(C) In vivo infection of HeLa cells from EPECΔcesAB or EPECΔescN strains complemented with pASK-IBA7 plasmids expressing wild-type or mutated CesAB. The graph reports the percentage of HeLa cells infected after 90-min inoculation with the bacteria.
(D) Infection of HeLa cells by bacterial EPECΔcesAB strains complemented with plasmids expressing wild-type or mutant CesAB. The results show very little actin pedestal formation, indicating uninfected HeLa cells, after 90-min inoculation with bacteria.
Targeting and Secretion Signals are Distinct
It was previously shown that the first ~20 N-terminal residues of EspA bear a secretion signal for EspA since deletion of this region resulted in EspA secretion defects (Munera et al., 2010). NMR analysis of the interaction of CesAB–EspA with EscN (Figure 2A) shows that the N-terminal region of EspA does not participate in the binding interaction. To further corroborate this we characterized by NMR the interaction of the isolated N-terminal region of EspA (EspA1–34) with EscN. The data showed that indeed this region does not interact with EscN (Figure S2J). Additional evidence that the region of EspA carrying the secretion signal does not participate in the binding to EscN is provided by NMR analysis of CesAB–EspAΔ29, a variant comprising an N-truncated EspA lacking the first 29 residues. CesAB–EspAΔ29, retains the same fold and structure as the full-length CesAB–EspA (Figure S2K), but it is fully engaged by EscN (Figure S2L). Thus, although EspA is targeted to the ATPase by means of CesAB, there are regions in EspA that control its secretion, most likely during a downstream process following targeting of EspA at the injectisome. The results argue that the targeting and secretion signals are distinct and the two processes are likely regulated by different mechanisms.
DISCUSSION
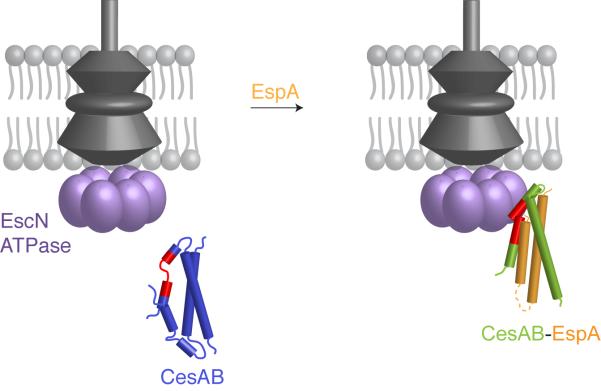
Targeting of TTS secreted proteins at the injectisome is the first crucial step in a process that ultimately results in the secretion of needle and translocator proteins or translocation of effectors into the eukaryotic cell. The cytosolic basal structure of the injectisome is capable of discerning the TTS substrates from all the other bacterial proteins; yet, the molecular basis for this process remains poorly understood. Here we have identified the targeting signal in the EPEC CesAB–EspA chaperone–substrate system. We show that the signal is encoded in a conformational switch in the chaperone that is induced only upon binding of the physiological substrate (Figure 4). Our results clearly demonstrate that chaperones contain targeting signals and thus act to usher bound substrates to the injectisome.
Figure 4. Targeting of CesAB–EspA to the ATPase.
Although CesAB carries the targeting signal (colored in red), this is presented to EscN only when EspA is bound to CesAB by means of an induced conformational switch on CesAB. As a result, EscN recognizes the re-structured CesAB region and engages the CesAB–EspA complex.
Several studies have focused on identifying the so-called secretion signal, that is, the region of the TTS substrate that controls its secretion (Mota et al., 2005). The results have suggested in certain cases that specific regions in the N-terminal region of effectors and translocators constitute the secretion signal (Sory et al., 1995; Lloyd et al., 2001; Amer et al., 2011), whereas in other cases it has been suggested that the secretion signal is encoded in the mRNA sequence (Anderson and Schneewind, 1997; Ramamurthi and Schneewind, 2005). However, secretion is a complex process and targeting is just the first step of it. Indeed, here we demonstrate that targeting and secretion of the CesAB–EspA chaperone–substrate system are controlled by different signals: the targeting signal is encoded in the chaperone (CesAB), whereas the secretion signal is located in the N-terminal region of EspA (Munera et al., 2010). This region of EspA is not required for binding to the ATPase, but is required for its ultimate secretion, a process that occurs after the chaperone–substrate complex has been engaged by the injectisome.
The TTS ATPase is conserved in all TTS systems and is thought to play important roles in both engaging TTS substrates and in the secretion process (Galán, 2008). Our results show that in the EPEC system the EscN ATPase functions as a docking platform for chaperone–substrate complexes. The interactions are functional since abrogation of the binding causes severe secretion and infection defects. However, it is likely that additional proteins of the cytosolic basal body of the injectisome may also function as docking points for TTS substrates (Diepold et al., 2012).
EXPERIMENTAL PROCEDURES
Protein Preparation
The cesAB and espA genes encoding the wild type CesAB (B7UMC4_ECO27) and the wild type EspA (B7UM94_ECO27) were isolated and cloned as described previously (Chen et al., 2011). The ATPase escN encoding EscN (B7UMA6_ECO27) was isolated by PCR from cosmid pCVD462 (a gift from Dr. J.B. Kaper) derived from the locus of enterocyte effacement (LEE) of E2348/69 cloned into pCVD551 (McDaniel and Kaper, 1997) and were finally cloned into pET16b. The mutants were constructed by site-directed mutagenesis using PfuUltraTM High Fidelity DNA polymerase (Quick-Change; Stratagene). The constructs were transformed in BL21(DE3) cells and grown at 37 °C, and protein synthesis was induced by addition of 0.5 mM of IPTG at A600 ~0.4. Isotopically labeled samples for NMR studies were prepared as described (Gelis et al., 2007; Popovych et al., 2009). All protein samples were purified over a nickel-chelating Sepharose column (GE Healthcare), followed by a Superdex-75 size exclusion column (GE Healthcare).
Differential Line Broadening NMR Experiments
Because EscN has a large molecular mass (~350 kDa), complex formation with labeled CesAB–EspA resulted in severe line broadening of the resonances. The broadening effect depends on the chemical shift difference of the CesAB–EspA resonances between the EscN-free and EscN-bound form (Matsuo et al., 1999). Thus, using this method the residues in CesAB–EspA that are most affected by the formation of the ternary complex with EscN can be identified. The experiments were performed with a concentration of 15N-labeled CesAB–EspA complex fixed at 0.2 mM while increasing amount of unlabeled EscN was titrated. The concentration ratio of CesAB–EspA complex to the ATPase EscN varied from 0.2 to 2. The intensity of CesAB–EspA resonances as a function of EscN concentration was measured in 2D 1H-15N HSQC spectra.
ATPase Activity Measured via Isothermal Titration Calorimetry
ATPase activity assays were performed via isothermal titration calorimetry on a VP-ITC (GE Healthcare, Inc.) employing a multi-injection analysis protocol (Todd and Gomez, 2001; Bianconi, 2007). This methodology is based on the observed proportionality between the rate of reaction (v) and thermal power (dq/dt) generated upon titration of discrete substrate aliquots into an enzyme solution. The rate of product formation is determined according to the following relation:
where V represents the sample cell volume and ΔHapp corresponds to the molar reaction enthalpy. ATPase activity was monitored calorimetrically at 25 ⍛C in a buffer comprised of 50 mM HEPES, 100 mM NaCl, 5 mM MgCl2, and 5.0 mM β-mercaptoethanol adjusted to pH 7.5. In a typical calorimetric assay, the reaction rate is monitored by titrating successive microliter aliquots of ATP substrate into the sample cell containing EscN. The thermal power is monitored for 60 seconds upon each substrate injection under a constant stirring rate of 250 rpm. Assuming that the reaction proceeds via a steady state mode in which [substrate] >> [enzyme], the differential power upon each addition of substrate represents the maximal enzyme reaction rate and is characterized by an exothermic heat that remains nearly constant until the next injection. The thermal power data monitoring ATP hydrolysis is converted to the turnover rate (mM sec−1) and plotted as a function of substrate concentration. The reaction profiles depicted in Supplementary Fig. 2c employ initial substrate (ATP) and enzyme (EscN) concentrations of 2.5 and 0.006 mM, respectively.
In Vivo Secretion from EPEC Strains and Infection of HeLa Cells
The assays were performed as described in previously. Details can be found in Extended Experimental Procedures.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grant AI094623 (C.G.K.), the Operational Program “Competitiveness and Entrepreneurship” (OPCE II - EPAN II) - National Strategic Reference Framework (NSRF 2007–2013) 09SYN-11-902 (A.E.) and an Excellence grant from the Greek General Secretariat of Research 1473-Nonaco (A.E.).
Footnotes
ACCESSION NUMBERS
The Protein Data Bank accession number for the atomic coordinates for CesABs is 1M1N.
REFERENCES
- Akeda Y, Galán JE. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. doi: 10.1038/nature03992. [DOI] [PubMed] [Google Scholar]
- Amer AAA, Ahlund MK, Bröms JE, Forsberg A, Francis MS. Impact of the N-Terminal Secretor Domain on YopD Translocator Function in Yersinia pseudotuberculosis Type III Secretion. J Bacteriol. 2011;193:6683–6700. doi: 10.1128/JB.00210-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- Bianconi ML. Calorimetry of enzyme-catalyzed reactions. Biophys Chem. 2007;126:59–64. doi: 10.1016/j.bpc.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Birtalan SC, Phillips RM, Ghosh P. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol Cell. 2002;9:971–980. doi: 10.1016/s1097-2765(02)00529-4. [DOI] [PubMed] [Google Scholar]
- Boonyom R, Karavolos MH, Bulmer DM, Khan CMA. Salmonella pathogenicity island 1 (SPI-1) type III secretion of SopD involves N- and C-terminal signals and direct binding to the InvC ATPase. Microbiology. 2010;156:1805–1814. doi: 10.1099/mic.0.038117-0. [DOI] [PubMed] [Google Scholar]
- Chen L, Balabanidou V, Remeta DP, Minetti CASA, Portaliou AG, Economou A, Kalodimos CG. Structural Instability Tuning as a Regulatory Mechanism in Protein-Protein Interactions. Mol Cell. 2011;44:734–744. doi: 10.1016/j.molcel.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CA, Zhang K, Andres SN, Fang Y, Kaniuk NA, Hannemann M, Brumell JH, Foster LJ, Junop MS, Coombes BK. Structural and biochemical characterization of SrcA, a multi-cargo type III secretion chaperone in Salmonella required for pathogenic association with a host. PLoS Pathog. 2010;6:e1000751. doi: 10.1371/journal.ppat.1000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G. The type III secretion injectisome. Nat Rev Micro. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Creasey EA, Friedberg D, Shaw RK, Umanski T, Knutton S, Rosenshine I, Frankel G. CesAB is an enteropathogenic Escherichia coli chaperone for the type-III translocator proteins EspA and EspB. Microbiology. 2003;149:3639–3647. doi: 10.1099/mic.0.26735-0. [DOI] [PubMed] [Google Scholar]
- Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol. 2009;12:101–109. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diepold A, Wiesand U, Amstutz M, Cornelis GR. Assembly of the Yersinia injectisome: the missing pieces. Mol Microbiol. 2012;85:878–892. doi: 10.1111/j.1365-2958.2012.08146.x. [DOI] [PubMed] [Google Scholar]
- Feldman M, Cornelis G. The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol Lett. 2003;219:151–158. doi: 10.1016/S0378-1097(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Francis MS. Handbook of Molecular Chaperones: Roles, Structures and Mechanisms. 2010. Type III Secretion Chaperones:A Molecular Toolkit for All Occasions; pp. 79–147. [Google Scholar]
- Galán JE. Energizing type III secretion machines: what is the fuel? Nat Struct Mol Biol. 2008;15:127–128. doi: 10.1038/nsmb0208-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- Gauthier A, Finlay BB. Translocated intimin receptor and its chaperone interact with ATPase of the type III secretion apparatus of enteropathogenic Escherichia coli. J Bacteriol. 2003;185:6747–6755. doi: 10.1128/JB.185.23.6747-6755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelis I, Bonvin AMJJ, Keramisanou D, Koukaki M, Gouridis G, Karamanou S, Economou A, Kalodimos CG. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell. 2007;131:756–769. doi: 10.1016/j.cell.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada K, Minamino T, Tahara A, Namba K. Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits. Proc Natl Acad Sci USA. 2007;104:485–490. doi: 10.1073/pnas.0608090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izoré T, Job V, Dessen A. Biogenesis, regulation, and targeting of the type III secretion system. Structure. 2011;19:603–612. doi: 10.1016/j.str.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Knutton S, Rosenshine I, Pallen M, Nisan I, Neves B, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. Embo J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Tejero M, Kato J, Wagner S, Liu X, Galán JE. A sorting platform determines the order of protein secretion in bacterial type III systems. Science. 2011;331:1188–1191. doi: 10.1126/science.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilic M, Vujanac M, Stebbins CE. A common structural motif in the binding of virulence factors to bacterial secretion chaperones. Mol Cell. 2006;21:653–664. doi: 10.1016/j.molcel.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Lloyd SA, Forsberg A, Wolf-Watz H, Francis MS. Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol. 2001;9:367–371. doi: 10.1016/s0966-842x(01)02100-x. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Walters K, Teruya K, Tanaka T, Gassner G, Lippard S, Kyogoku Y, Wagner G. Identification by NMR spectroscopy of residues at contact surfaces in large, slowly exchanging macromolecular complexes. J Am Chem Soc. 1999;121:9903–9904. [Google Scholar]
- McDaniel T, Kaper J. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- Mota LJ, Sorg I, Cornelis GR. Type III secretion: the bacteria-eukaryotic cell express. FEMS Microbiol Lett. 2005;252:1–10. doi: 10.1016/j.femsle.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Munera D, Crepin VF, Marches O, Frankel G. N-terminal type III secretion signal of enteropathogenic Escherichia coli translocator proteins. J Bacteriol. 2010;192:3534–3539. doi: 10.1128/JB.00046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller SA, Pozidis C, Stone R, Meesters C, Chami M, Engel A, Economou A, Stahlberg H. Double hexameric ring assembly of the type III protein translocase ATPase HrcN. Mol Microbiol. 2006;61:119–125. doi: 10.1111/j.1365-2958.2006.05219.x. [DOI] [PubMed] [Google Scholar]
- Page A, Parsot C. Chaperones of the type III secretion pathway: jacks of all trades. Mol Microbiol. 2002;46:1–11. doi: 10.1046/j.1365-2958.2002.03138.x. [DOI] [PubMed] [Google Scholar]
- Pallen MJ, Bailey CM, Beatson SA. Evolutionary links between FliH/YscL-like proteins from bacterial type III secretion systems and second-stalk components of the FoF1 and vacuolar ATPases. Protein Sci. 2006;15:935–941. doi: 10.1110/ps.051958806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen MJ, Beatson SA, Bailey CM. Bioinformatics analysis of the locus for enterocyte effacement provides novel insights into type-III secretion. BMC Microbiol. 2005;5:9. doi: 10.1186/1471-2180-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S, Kaiser D, Torres E, Pollard T, Rosen M. A conserved amphipathic helix in WASP/Scar proteins is essential for activation of Arp2/3 complex. Nat Struct Biol. 2003;10:591–598. doi: 10.1038/nsb952. [DOI] [PubMed] [Google Scholar]
- Popovych N, Tzeng S-R, Tonelli M, Ebright RH, Kalodimos CG. Structural basis for cAMP-mediated allosteric control of the catabolite activator protein. Proc Natl Acad Sci USA. 2009;106:6927–6932. doi: 10.1073/pnas.0900595106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthi KS, Schneewind O. A synonymous mutation in Yersinia enterocolitica yopE affects the function of the YopE type III secretion signal. J Bacteriol. 2005;187:707–715. doi: 10.1128/JB.187.2.707-715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers L, Mukerjea R, Birtalan S, Friedberg D, Ghosh P. A solvent-exposed patch in chaperone-bound YopE is required for translocation by the type III secretion system. J Bacteriol. 2010;192:3114–3122. doi: 10.1128/JB.00113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory M, Boland A, Lambermont I, Cornelis G. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Wagner G. NMR studies of protein interactions. Curr Opin Struct Biol. 2006;16:109–117. doi: 10.1016/j.sbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Thomas NA, Deng W, Puente JL, Frey EA, Yip CK, Strynadka NCJ, Finlay BB. CesT is a multi-effector chaperone and recruitment factor required for the efficient type III secretion of both LEE- and non-LEE-encoded effectors of enteropathogenic Escherichia coli. Mol Microbiol. 2005;57:1762–1779. doi: 10.1111/j.1365-2958.2005.04802.x. [DOI] [PubMed] [Google Scholar]
- Todd MJ, Gomez J. Enzyme kinetics determined using calorimetry: a general assay for enzyme activity? Anal Biochem. 2001;296:179–187. doi: 10.1006/abio.2001.5218. [DOI] [PubMed] [Google Scholar]
- Woestyn S, Allaoui A, Wattiau P, Cornelis G. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip CK, Finlay BB, Strynadka NCJ. Structural characterization of a type III secretion system filament protein in complex with its chaperone. Nat Struct Mol Biol. 2005;12:75–81. doi: 10.1038/nsmb879. [DOI] [PubMed] [Google Scholar]
- Zamoon J, Nitu F, Karim C, Thomas D, Veglia G. Mapping the interaction surface of a membrane protein: unveiling the conformational switch of phospholamban in calcium pump regulation. Proc Natl Acad Sci USA. 2005;102:4747–4752. doi: 10.1073/pnas.0406039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarivach R, Vuckovic M, Deng W, Finlay BB, Strynadka NCJ. Structural analysis of a prototypical ATPase from the type III secretion system. Nat Struct Mol Biol. 2007;14:131–137. doi: 10.1038/nsmb1196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.