Abstract
Background:
Pharmacovigilance is a useful to assure the safety of medicines and protect consumers from their harmful effects. Healthcare professionals should consider Adverse Drug Reaction (ADR) reporting as part of their professional obligation and participate in the existent pharmacovigilance programs in their countries. In India, the National PV Program was re-launched in July 2010.
Objectives:
This survey was conducted in order to assess the knowledge, attitude and practice of Indian pharmacists with the aim of exploring the pharmacists’ participation in ADR reporting system, identifying the reasons of under reporting and determining the steps that could be adopted to increase reporting rates.
Materials and Methods:
A cross-sectional survey was carried out among the pharmacists in India using a pretested questionnaire with 33 questions (10 questions on knowledge, 6 on attitude, 7 on practice, 7 on future of ADR reporting in India and 3 on benefits of reporting ADRs.). The study was conducted, over a period of 3 months from May 2012 to July 2012.
Results:
Out of the 600 participants to whom the survey was administered, a total of 400 were filled. The response rate of the survey was 67%. 95% responders were knowledgeable about ADRs. 90% participants had a positive attitude towards making ADRs reporting mandatory for practicing pharmacists. 87.5% participants were interested in participating in the National Pharmacovigilance program, in India. 47.5% respondents had observed ADRs in their practice, and 37% had reported it to the national pharmacovigilance center. 92% pharmacists believed reporting ADRs immensely helped in providing quality care to patients.
Conclusion:
The Indian pharmacists have poor knowledge, attitude, and practice (KAP) towards ADR reporting and pharmacovigilance. Pharmacists with higher qualifications such as the pharmacists with a PharmD have better KAP. With additional training on Pharmacovigilance, the Indian Pharmacists working in different sectors can become part of ADR reporting system.
Keywords: Adverse drug reaction, adverse drug reaction reporting, knowledge, attitude, and practice, national pharmacovigilance program, pharmacists
INTRODUCTION
Adverse drug reactions (ADRs) are a significant cause of morbidity and mortality worldwide.[1] According to the definition provided by the World Health Organization, “an ADR is any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy.”[2] The socio-economic and health consequences of ADRs have been highlighted in several studies.[1,3,4] While a majority of the studies cited above show prevalence of this problem in developed countries there is a paucity of accurate data from many developing countries like India. A study carried out in South India by Ramesh et al., observed 0.7% admissions due to ADRs and a total of 3.7% of the hospitalized patients experienced an ADR, of which 1.3% were fatal.[5] Another study conducted by Arulmani et al. showed that ADR was responsible for 3.4% of total hospital admissions and 3.7% ADRs developed during hospital stay.[6] Ahmad et al., reported that the incidence of ADRs in rural South India was 8%[7] and in total serious ADRs occurred in 6.7%.[8]
Spontaneous (yellow card) reporting of ADRs remains the most widely used and cost effective surveillance system and is the cornerstone of safety monitoring of drugs in clinical practice. It detects previously unrecognized adverse reactions and identifies risk factors that pre-dispose to drug toxicity and investigates causality. In addition to identifying drug safety problems, it helps to facilitate risk-benefit judgments and comparisons within therapeutic categories.[9,10] Intrinsic factors such as knowledge, attitude and practice can help in understanding the relationship of pharmacists with patients and other healthcare professionals and formulating strategies to encourage pharmacists to report ADRs.
A few studies carried out in India have shown poor knowledge, attitude, and deficient practices involving ADR reporting among prescribers and healthcare professionals, mainly physicians.[11,12,13] However, very few studies delve into the reasons that impact the knowledge, attitude and practice of pharmacists with regard to ADR reporting. Hence, this study was conducted to analyze the knowledge, attitude, and practice (KAP) related to ADR reporting among pharmacists in India. Our study also explores the views of pharmacists about the future of ADR reporting in India.
Pharmacovigilance in India-the need
According to the 2011 census, India has the 2nd highest population in the world with over 1.21 billion[14] people. Some of the ADRs are avoidable. Spontaneous reporting by healthcare professionals is a crucial step for preventing or reducing ADRs.[7] The ADR reporting rate in India is below 1% compared to the worldwide rate[14] of 5%. ADR management can cost the institution or the patient as much as US $15-150 in India.[5,15,16] Given the lower rate in India, one of the reasons might be attributed to the awareness about pharmacovigilance and ADR monitoring among the Indian healthcare providers.
In about 3-6% patients of varying ages, ADRs lead to hospital admissions whereas this number can go as high as 24% in elders. About 5.9-22.3% of all emergency cases can be attributed to ADRs.[17,18,19,20] ADRs rank among the 4-6th highest cause of mortality in the US, leading to as many as 106,000 deaths on a yearly basis.[1,21] In Southern India, Ramesh et al. found that in a small tertiary care hospital, 0.7% cases were admitted as a result of ADRs and as many as 18 among 1000 patients died because of the same.[5] In US, hospital admissions and mortality rates in patients with ADRs were 8.25% and 19.18% higher respectively.[22]
National pharmacovigilance program (NPP) of India
The National pharmacovigilance program (NPP) was launched by the Ministry of Health and Family Welfare in July 2010, primarily overseen by CDSCO, New Delhi. ADR reports collected from the affiliated medical colleges will be dispatched to the national coordinating center. The coordinating center will conduct causality assessment and upload the reports into the pharmacovigilance software. Lastly, the integrated ADR data will be transmitted through vigiflow software interface into the Uppsala Monitoring Center's ADR database where signal processing will be carried out.[14,23]
Aim of the study
This survey was conducted to assess the knowledge, attitude and practice of pharmacists in India with the aim of identifying reasons for under-reporting of ADRs and determining the steps that could be adopted to increase reporting rates.
MATERIALS AND METHODS
A questionnaire was prepared to investigate knowledge, attitude and practices of Indian pharmacists about ADR reporting. The questionnaire consisted of questions included in previous studies that examined the knowledge and attitude of healthcare professionals,[3,4,14,24] about ADR reporting. Questions were framed taking into account not only the phamacovigilance system in place in the individual healthcare institutions where pharmacists were working at but also its relation to the working of the pharmacovigilance system at the national level. The questionnaire comprised of 33 questions. The questions were distributed as follows: 10 questions were related to knowledge, 6 questions were related to attitude and skills, 7 questions were related to practice and the remaining 10 questions were related to the future of ADR reporting and benefits of ADR reporting. Four questions were included at the beginning of the survey to collect demographic data like age, gender, highest qualification achieved and profession (community pharmacist, hospital pharmacist, academician pharmacist, student pharmacist and others). The pretested questionnaire was made available to the pharmacists (n = 600) at their work place by E-mail and via social networking sites like Facebook (including pharmacy professional groups like Indian academy of pharmacists, pharma trend setter, pharma times etc.), Linkindin and Orkut. The study was conducted over a period of 3 months from May 2012 to July 2012. The responses to the questionnaire were analyzed by performing descriptive statistics. Data were analyzed using the Statistical Package for Social Sciences (SPSS for MS Windows version 9).
RESULTS
Demographics
Out of the 600 questionnaires sent by E-mail and by social sites, 400 questionnaires were returned, giving a response rate of 67% [Table 1]. In our study, 77.5% respondents were males and 22.5% were female pharmacists. The average age of all the responding pharmacists was 29.5 years. Of all the pharmacists answering the questionnaire, 31% were M.Pharm, 28% were PharmD, 20% had B.Pharm, 11% were PhD and remaining 10% were D.Pharm. 37.5% were students, 10% were academicians, 10% were community pharmacists, 6.25% were hospital pharmacists and the remaining 36.25% were working professionals (manufacturing, marketing, regulatory affairs and clinical research industry, and pharmacovigilance, etc.).
Table 1.
Demographic profile of the sample (n=400)
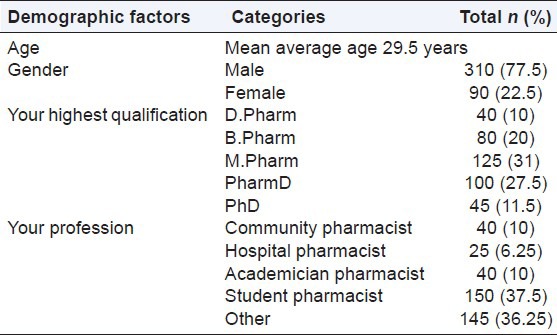
There were 9 questions assessing knowledge of the pharmacists about ADR reporting and pharmcovigilance. Among the 400 respondents, 95% responders were aware of the term ADRs. Only 57.5% (n = 230) were aware of the starting year of the pharmacovigilance activities in India. Similarly, <1/3 (n = 120; 30%) of the respondents knew about the location of the nearest pharmacovigilance center. A small number (n = 20; 5%) of responders believed that all drugs available in the market were safe. 16% pharmacists believed that herbal drugs had no ADRs or that they were safe, and only 6% pharmacists believed that ADRs associated with herbal products should not be reported. In our study, only 59% responders knew which organization was responsible for collecting and monitoring ADRs in India (CDSCO). 75% responders knew when ADRs should be reported, and only 50% pharmacists knew which type of ADRs were reported. Further details are shown in Table 2.
Table 2.
Knowledge of ADR reporting and monitoring by pharmacists
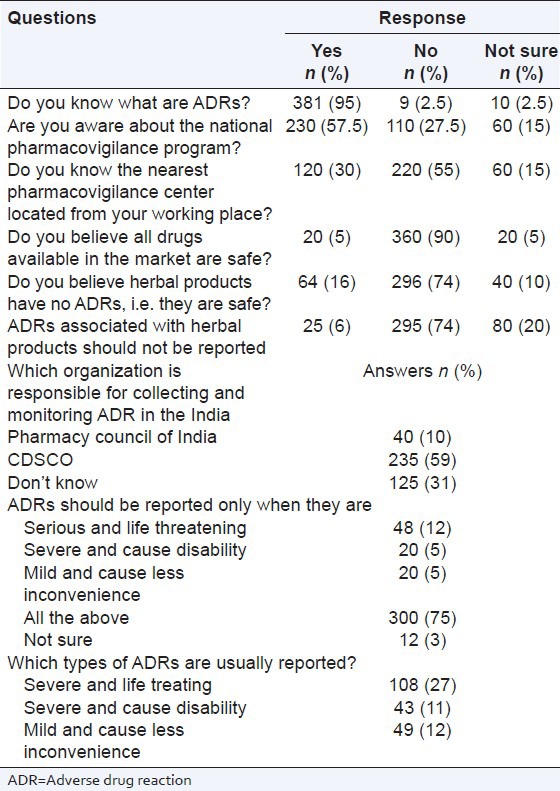
There were 6 questions related to the attitudes of the pharmacists towards ADR reporting and pharmacovigilance. In general, the respondents had a good attitude towards ADR reporting and pharmacovigilance. Nearly all pharmacists (n = 325; 81%) felt that ADR reporting was their duty. 340 (85%) participants thought that serious ADRs encouraged pharmacists to report to the relevant authority. 360 (90%) participants are believed ADR reporting should be made mandatory for practicing pharmacists. 320 (80%) participants believed that ADR reporting system is not widely promoted by relevant authorities and 350 (87.5%) participants were interested in participating in the National Pharmacovigilance Programme of India. The details regarding the responses of pharmacists about their attitudes towards ADR reporting and pharmacovigilance are listed in Table 3.
Table 3.
Responses of professionals to the attitude related questions
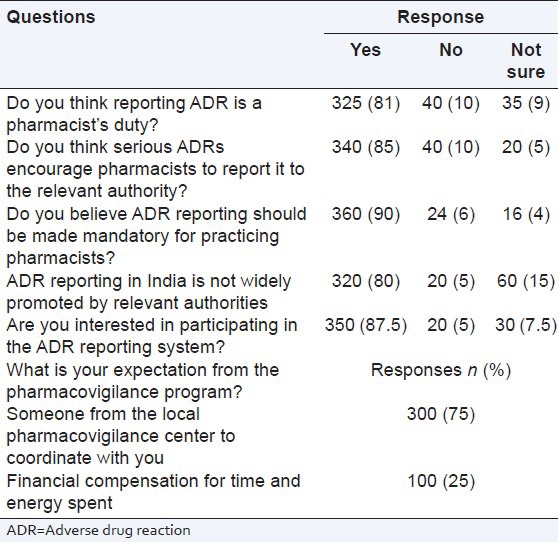
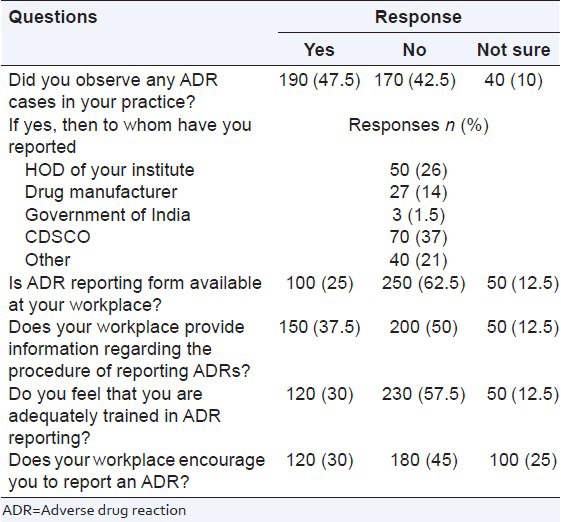
There were 6 pharmacovigilance practice related questions. It was found that nearly half the responders (n = 190; 47.5%) had experienced of ADRs in their practice and submitted (n = 70; 37%) an ADR report to the national pharmacovigilance center. A sizable number (n = 250; 62.5%) said that the ADR reporting form were not available in their work place, and only 30% (n = 120) participants reported that they were trained for ADR reporting. The details about the responses of the healthcare professionals are listed in Table 4.
Table 4.
ADR reporting in your workplace (practice)
There were 7 questions assessing the problems faced by pharmacists while reporting ADRs at their work place. Out of the total 400 responders, 63% (n = 252) mentioned that they lacked information due to failure on the part of patients to provide information related to ADRs. Only 18% pharmacists reported lack of time to report ADRs, whereas 65% pharmacists stated that ADR reporting was not widely promoted by the relevant authority, and they were unaware about the existent national ADR reporting system, need to report ADRs and feared facing legal problems due to reporting ADRs [Table 5].
Table 5.
Which of the problems do you face while reporting ADRs in your work place?

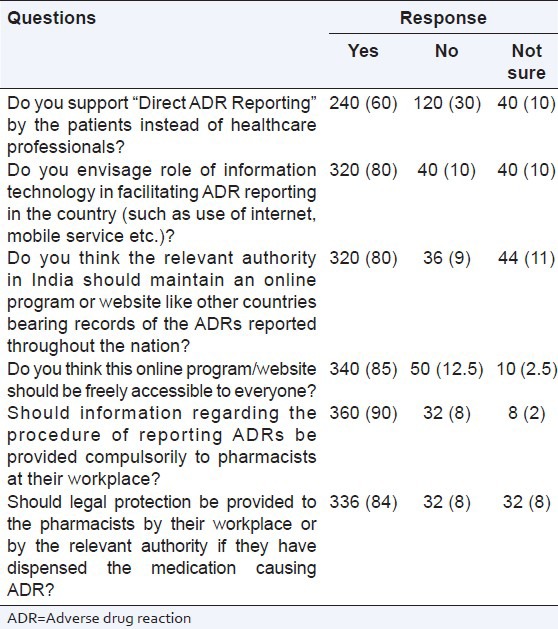
There were 6 questions related to the future of ADR reporting in India. 60% pharmacists supported direct ADR reporting by patients rather than healthcare professionals and more than 80% respondents supported using information technology like internet, freely accessible online programs, information related to ADR reporting provided at their work place and provision of legal protection at the work place for the pharmacist if ADR was caused by the prescribed drug [Table 6].
Table 6.
Future of ADR reporting in India
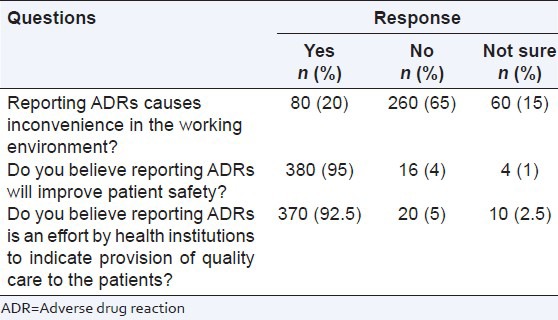
There were 3 questions related to the benefits of reporting ADRs. 20% responders mentioned that ADR reporting caused inconvenience in the working environment, 95% pharmacists believed reporting ADRs will improve patient safety and more than 92% pharmacists believed reporting ADR is an effort by healthcare institutions to provide quality care to the patients [Table 7].
Table 7.
Benefits of reporting ADRs
DISCUSSION
The present study was a questionnaire based study which included pharmacist from all over India. This is the first study in India that evaluated the KAP of pharmacists regarding ADR reporting and the functioning of the NPP. Overall, the (KAP) scores of the pharmacists were low. Pharmacovigilance deals with detection, assessment, understanding and prevention of adverse effects or any other drug related problems. The ultimate aim of pharmacovigilance is to ensure patient safety and rational use of medicines once a new medicine is released for general use in the society. The most notable outcome of pharmacovigilance is the prevention of patients being affected unnecessarily due to the negative consequences of pharmacotherapy.[25] Pharmacovigilance programs have played a crucial role in detection of ADRs and banning of several drugs from the market. However, under-reporting of ADRs is one of the main problems associated with pharmacovigilance programs.[26] It is known that spontaneous reporting programs (one of the most widely used methods of pharmacovigilance) are associated with relatively low levels of reporting. Spontaneous reporting programs operate on the basis that all ADRs should be reported despite uncertainty about a causal relationship. Even in countries like the United Kingdom (UK), where pharmacovigilance programs are well-established, a high level of under-reporting is documented.[27]
Previous studies have shown that while the right attitude for ADR reporting exists among most of the physicians, the actual practice of ADR reporting is lacking. Studies in Mumbai,[13] Mysore,[27] Muzzafarnagar[28] and Ahmedabad[11] have shown that prescribers have high knowledge and attitude with regards to ADR reporting but practice it poorly. Our study also found similar results. The response rate (67%) was similar to other studies carried out in Ahmedabad (India),[11] and UK[29] but lower compared to Netherlands.[27]
This study shows that post graduate pharmacists (M. Pharm, PharmD, PhD) (70%) responded significantly more than pharmacists with other qualifications such as B.Pharm (20%) and D.Pharm (10%). This may be because online resources like E-mail and professional networking sites and groups are more accessible to pharmacists who are post graduates compared to pharmacists who are graduates and diploma holders. Pharmacists with a Bachelor’ degree, i.e. B.Pharm mostly works in manufacturing units, and pharmacists who are diploma holders work in the community and hospital pharmacy. They either have no access to internet facilities or have no time to access it. Also, their curriculum does not cover the use of health related information technology.
Inman[30] has stated some of the reasons for under reporting of ADRs. These reasons include lack of financial incentives; fear that the reporter might face legal proceedings, complacency, i.e. holding the impression that the drug was introduced in the market accompanied by disclosure of all ADRs, diffidence, i.e. holding the belief that reporting should be backed by an assurance that an ADR is associated with that particular drug, showing indifference towards reporting assuming that a single ADR is not serious enough to be reported, being ignorant about the seriousness of ADR reporting and coming up with excuses for not reporting due to lethargy and laziness. Some of these reasons have also been observed in studies conducted in Ahmedabad,[11] Mysore,[21] Mumbai[13] and Muzaffar Nagar.[11] in order to address some of the determinants of under-reporting found in this study, ADR reporting guidelines should be made available in the form of booklets and posters at conspicuous locations in health care facilities as a constant reminder. This should be done in addition to regular sensitization of all health care workers on the importance of pharmacovigilance in the quest to decrease morbidity and mortality among the populace.
In our study, 25% responders wanted money for spending time and energy for reporting ADRs, and 75% pharmacists wanted some entity to coordinate the pharmacovigilance activities in their workplace with the local pharmacovigilance center. Apart from the fact that the use of incentives has not been widely accepted and practiced, it raises the possibility of over-reporting by some health care workers in a bid to obtain financial rewards. This also gives rise to another debate if ADR reporting should be a fundamental responsibility of health care workers or not.
Improvement in ADR reporting in future, apart from reducing the incidence of ADRs in clinical practice, will also lead to a reduction in health care costs. Another way to increase the reporting of ADRs is through the promotion of patient self-reporting. The benefits of this idea have been confirmed in different studies.[31,32] Patient self-reporting has a complimentary role to play in increasing the level of ADR reporting in a developing country such as India. In our study 60% pharmacists supported direct ADR reporting by the patients instead of healthcare professionals. 80% pharmacists supported online programs or websites for ADR reporting via internet, mobile service etc., and free access to nationwide ADR related information to everyone similar to western countries. More than 85% pharmacists wanted compulsory provision of information regarding the procedure of reporting ADRs at their work place and legal protection provided by the relevant authority. The suggestion given by the respondents to improve ADR reporting corresponded with those observed in other studies. In a study carried out in Nigeria[33] impacting continuous medical education; training and encouragement feedback from patients, prescribers and dispensers, publicity of a reporting scheme in local journals and appointing an ADR specialist in every hospital helped in improving reporting. It was also opined that reporting of serious ADRs should be prioritized considering the workload of the prescribers. Reporting should be made easy and convenient by E-mail/website, telephone, fax etc., This can improve the quantum and quality of the reports. In our study, when asked about the benefits of reporting ADRs, pharmacists believed that ADR reporting improves patient safety and ADR reporting is an effort by health institutions to provide quality care to patients. These were similar to the results of some other studies showing that ADR reporting improved patient safety and medical care.
Suggestions for improving ADR reporting
Each hospital should establish a local “Pharmacovigilance Unit” for reporting ADRs and collecting related data.
Pharmacovigilance workshops should be conducted to provide guidance to physicians, pharmacists and nurses for recognizing and reporting ADRs.
ADR reporting by patients should also be encouraged along with reporting by healthcare professionals.
Representatives from NPP should co-ordinate with healthcare professionals at their work place.
A separate column should be provided for ADR reporting in patient medication chart.
Incentives should be provided to pharmacists reporting ADRs not associated with human errors.
Periodic meetings of experts from NPP with pharmacists should be arranged to boost reporting.
The NPP should periodically collect ADR forms from hospitals by sending representatives.
ADR drop boxes should be introduced at strategic sites in hospitals.
ADR reporting should be facilitated by E-mail, fax and phone.
Pharmacovigilance studies should be incorporated in the pharmacy syllabus.
Assurance of non-involvement in legal matters, if they arise.
ADR reporting should be made mandatory for all manufacturing companies and healthcare professionals.
Each hospital should have a data-base on ADRs, which should be possible to be accessed by pharmacists.
Periodic meetings between pharmacists, physicians and nurses for effective co-ordination are necessary.
Positively changing the mindset, so that ADR reporting becomes an accepted and understood routine should be the overall objective of a healthcare system.
Limitations of the study
The main limitation of our study was the relatively small number of respondents (pharmacists). In addition, since our study was a self-report, it might have biases such as recall bias and social desirability bias. The opinion of the non-responders in general and participants who did not respond to certain questions could have also affected the interpretation.
CONCLUSION
Indian pharmacists have a relatively better attitude towards ADR reporting. However, they have a limited knowledge and practice with regard to ADR reporting and pharmacovigilance. Even though, pharmacists felt ADR monitoring to be essential and were willing to report, they are unaware about the NPP. They lacked knowledge about the location of the nearest ADR reporting centers. Lack of adequate number of ADR reporting centers was also a significant finding. The findings of our study suggest that there is scope for improving the ongoing pharmacovigilance activities in India. There is a need for continuing educational initiatives for pharmacists and other healthcare professionals.
ACKNOWLEDGMENTS
Authors acknowledge all the pharmacists who participated in this study by filling up the questionnaires.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 2.Geneva, Switzerland: World Health Organization; 2002. [Last cited on 2012 Jun 29]. World Health Organization. Safety of Medicines. A Guide to Detecting and Reporting Adverse Drug Reactions. Available from: http://www.WHO/EDM/QSM/2002.2 . [Google Scholar]
- 3.Moore N, Lecointre D, Noblet C, Mabille M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol. 1998;45:301–8. doi: 10.1046/j.1365-2125.1998.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White TJ, Arakelian A, Rho JP. Counting the costs of drug-related adverse events. Pharmacoeconomics. 1999;15:445–58. doi: 10.2165/00019053-199915050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital – Their severity and cost involved. Pharmacoepidemiol Drug Saf. 2003;12:687–92. doi: 10.1002/pds.871. [DOI] [PubMed] [Google Scholar]
- 6.Arulmani R, Rajendran SD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol. 2008;65:210–6. doi: 10.1111/j.1365-2125.2007.02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad A, Parimalakrishnan S, Mohanta GP, Manna PK, Manavalan R. Incidence of adverse drug reactions with commonly prescribed drugs in tertiary care teaching hospital in India. Int J Pharm Sci. 2011;3:79–83. [Google Scholar]
- 8.Importance of ADR reporting in India. [Last cited on 2012 Jul 21]. Available from: http://www.pharmacovigilance.co.in/whyadrreporting.html .
- 9.Rawlins MD. Spontaneous reporting of adverse drug reactions. I: The data. Br J Clin Pharmacol. 1988;26:1–5. doi: 10.1111/j.1365-2125.1988.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dukes MN. The importance of adverse reactions in drug regulation. Drug Saf. 1990;5:3–6. doi: 10.2165/00002018-199005010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Desai CK, Iyer G, Panchal J, Shah S, Dikshit RK. An evaluation of knowledge, attitude, and practice of adverse drug reaction reporting among prescribers at a tertiary care hospital. Perspect Clin Res. 2011;2:129–36. doi: 10.4103/2229-3485.86883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehan HS, Vasudev K, Tripathi CD. Adverse drug reaction monitoring: Knowledge, attitude and practices of medical students and prescribers. Natl Med J India. 2002;15:24–6. [PubMed] [Google Scholar]
- 13.Gupta P, Udupa A. Adverse drug reaction reporting and pharmacovigilance: knowledge, attitude and perception among resident doctors. J Pharm Sci. 2011;3:1064–9. [Google Scholar]
- 14.Amrita P, Kharbanda B. Knowledge, attitude and skills of nurses of Delhi towards adverse drug reaction reporting. Indian J Pharm Pract. 2012;5:45–51. [Google Scholar]
- 15.Malhotra S, Karan RS, Pandhi P, Jain S. Drug related medical emergencies in the elderly: Role of adverse drug reactions and non-compliance. Postgrad Med J. 2001;77:703–7. doi: 10.1136/pmj.77.913.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel KJ, Kedia MS, Bajpai D, Mehta SS, Kshirsagar NA, Gogtay NJ. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin Pharmacol. 2007;7:8. doi: 10.1186/1472-6904-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm World Sci. 2002;24:46–54. doi: 10.1023/a:1015570104121. [DOI] [PubMed] [Google Scholar]
- 18.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18 820 patients. BMJ. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, et al. Adverse drug reactions as cause of hospital admissions: Results from the Italian group of pharmacoepidemiology in the Elderly (GIFA) J Am Geriatr Soc. 2002;50:1962–8. doi: 10.1046/j.1532-5415.2002.50607.x. [DOI] [PubMed] [Google Scholar]
- 20.Nelson KM, Talbert RL. Drug-related hospital admissions. Pharmacotherapy. 1996;16:701–7. [PubMed] [Google Scholar]
- 21.Bates DW. Drugs and adverse drug reactions: How worried should we be? JAMA. 1998;279:1216–7. doi: 10.1001/jama.279.15.1216. [DOI] [PubMed] [Google Scholar]
- 22.Bond CA, Raehl CL. Adverse drug reactions in United States hospitals. Pharmacotherapy. 2006;26:601–8. doi: 10.1592/phco.26.5.601. [DOI] [PubMed] [Google Scholar]
- 23.Pharmacovigilance Programme of India for assuring drug safety. [Last cited on 2012 Jun 29]. Available from: http://www.cdsco.nic.in/pharmacovigilance.htm .
- 24.Green CF, Mottram DR, Rowe PH, Pirmohamed M. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2001;51:81–6. doi: 10.1046/j.1365-2125.2001.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsson S. The need for pharmacovigilance. In: Gupta SK, editor. Pharmacology and Therapeutics in the New Millennium. New Delhi: Narosa Publishing House; 2001. pp. 502–8. [Google Scholar]
- 26.Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernández-Díaz S, Lasheras B. Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf. 2007;30:1073–82. doi: 10.2165/00002018-200730110-00006. [DOI] [PubMed] [Google Scholar]
- 27.Belton KJ, Lewis SC, Payne S, Rawlins MD, Wood SM. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39:223–6. doi: 10.1111/j.1365-2125.1995.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramesh M, Parthasarathi G. Adverse drug reactions reporting: attitudes and perceptions of medical practitioners. Asian J Pharm Clin Res. 2009;2:10–4. [Google Scholar]
- 29.Eland IA, Belton KJ, van Grootheest AC, Meiners AP, Rawlins MD, Stricker BH. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol. 1999;48:623–7. doi: 10.1046/j.1365-2125.1999.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inman WH. Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol. 1996;41:434–5. [PubMed] [Google Scholar]
- 31.Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: A review of published literature and international experience. Br J Clin Pharmacol. 2007;63:148–56. doi: 10.1111/j.1365-2125.2006.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hunsel F, Passier A, van Grootheest K. Comparing patients’ and healthcare professionals’ ADR reports after media attention: The broadcast of a Dutch television programme about the benefits and risks of statins as an example. Br J Clin Pharmacol. 2009;67:558–64. doi: 10.1111/j.1365-2125.2009.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


