Abstract
The treatment options in clinical stage I nonseminomatous germ cell tumor (NSGCT) of testis are either surveillance, chemotherapy or retroperitoneal lymph node dissection (RPLND). While open RPLND still serves as the gold standard, laparoscopic and robot assisted laparoscopic approaches are gaining popularity. In this report, we share our experience and technique of robot assisted laparoscopic RPLND in a patient with clinical stage Ib NSGCT of testis.
Keywords: Retroperitoneal lymph node dissection, robotic, testicular tumor
INTRODUCTION
In clinical stage I nonseminomatous germ cell tumor (NSGCT) of the testis, histopathology of the testicular tumor has an important bearing on the surgeon aiding the patient to decide between surveillance, chemotherapy or retroperitoneal lymph node dissection (RPLND) as a management option. RPLND has the distinct advantage of being a staging as well as a therapeutic modality, where the open approach is considered the gold standard. The disadvantages of open RPLND are mainly a large abdominal incision and high perioperative morbidity. In the era of minimally invasive surgery, laparoscopic RPLND (LRPLND) has gained popularity among experienced laparoscopic onco-surgeons. However, LRPLND has a steep learning curve and requires advanced laparoscopic skills in handling major vessels in retroperitoneum as well as considerable experience in open retroperitoneal surgery. With the increasing experience in robotic radical prostatectomy and cystectomy, the da Vinci surgical system is now being used in various uro-oncological procedures. The 3D visualisation and 7° of tremor free movement in robotics has made it possible for transfer of open surgical skills to the laparoscopic approach so that complex procedures such as LRPLND are easily performed. In this report, we share our experience of robot assisted laparoscopic RPLND in a patient with clinical stage Ib NSGCT of the testis.
CASE REPORT
A 23 year old male presented to us with right testicular swelling for 4 months. There was no significant past history or family history of testicular tumors. On examination, the right testis was enlarged and hard while the rest of the clinical examination was unremarkable. Tumor markers were found to be marginally raised. The levels of α-foetoprotein and β-HCG were 370 ng/ml and 650 U/L respectively. A computed tomography (CT) scan abdomen, however, revealed only subcentrimetric lymhnodes (clinically insignificant) in the retroperitoneum [Figure 1]. A right high inguinal orchidectomy was performed and the histopathology showed mixed NSGCT with predominnent embryonal component with vascular and lymphatic invasion. The tumor markers after 6 weeks were normalized. The patient was staged as clinical stage Ib American Joint Committee on -T2N0M0. He was given the option of RPLND or chemotherapy, and after explaining about the implications of both forms of treatment, he opted for RPLND. The patient was subsequently taken up for right modified template robot assisted laparoscopic transperitoneal RPLND.
Figure 1.
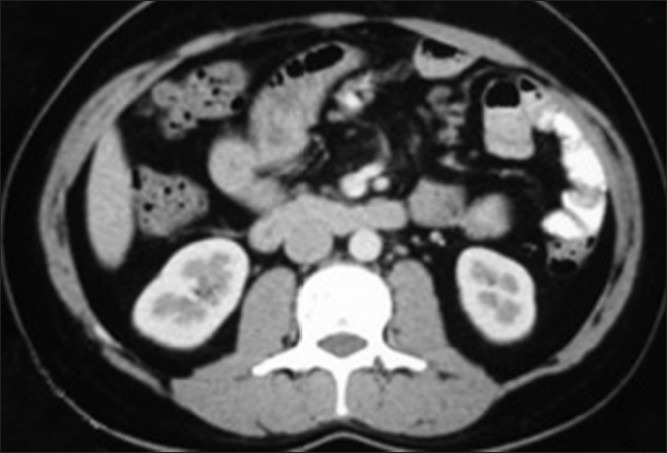
CT scan abdomen/pelvis-Retroperitoneal lymph nodes not enlarged
Technique
The patient was placed in right lateral oblique position (60°) and pneumoperitoneum was created using Verres’ needle. A total of 6 ports were used [Figure 2]. The camera port (12 mm) was placed just lateral to umbilicus on the left. Two 8 mm robotic metallic ports were placed just lateral to umbilicus on the right at a distance of 8 cm from the camera port. A third robotic port was placed for the 4th robotic arm just medial to the anterior superior iliac spine. One 5 mm port was placed for liver retraction at the subxiphoid area. An assistant port (12 mm) for retraction, suture transfer and specimen retrieval was placed below the camera port towards the pelvis. The robot was docked with the cart being kept behind the back of the patient. On the right robotic arm, a monopolar curved scissors and needle holder were used. On the left, a bipolar Maryland forceps, needle holder and prograsp forceps were used. The prograsp forceps was very useful in holding and retracting tissues. However, the third robotic arm was not helpful because of crossing over and collision of the robotic instruments.
Figure 2.
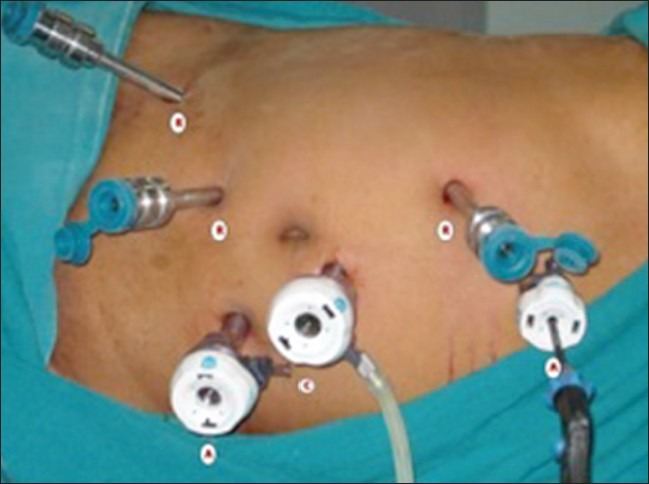
Right oblique 60° position with port position
The dissection was started with mobilisation of hepatic flexure, ascending colon and caecum followed by kocherisation of the duodenum. The right gonadal vessels were identified and dissected from the inferior vena cava (IVC) till the right deep inguinal ring. The silk suture, which was used to ligate the vessels and cord during orchidectomy was identified and the right gonadal vessels along with a part of spermatic cord were excised. The right ureter, which was the right lateral limit of dissection, was identified, and carefully dissected from the paracaval tissue. Paracaval lymphatic tissue was dissected from the right renal hilum (proximal limit) to the right common iliac artery bifurcation [distal limit, Figure 3]. Excised tissues were retrieved through the 12 mm assistant port using home made plastic bag at the end of the procedure. The retrocaval tissue behind the IVC and the interaortocaval tissue between aorta and IVC were carefully dissected, and divided between clips (liga, weck). We didn’t use any hemostatic agent. During this dissection, the aorta and IVC were gently retracted by the assistant and the lumbar vessels were identified and preserved. With the help of 3D view and magnification, postganglionic sympathetic fibres and sympathetic ganglia were also identified and preserved. Pre-aortic dissection was done from the left renal vein (proximal limit) to the inferior mesenteric artery (distal limit). While dissecting, the wall of the aorta was injured, which was easily repaired with the 5-0 prolene suture. At the end of the dissection, we could see the skeletonised IVC, exposed aorta, right ureter and right and left renal hilum [Figure 4]. Hemostasis was ensured at the end of the procedure and the mobilised colon was repositioned.
Figure 3.
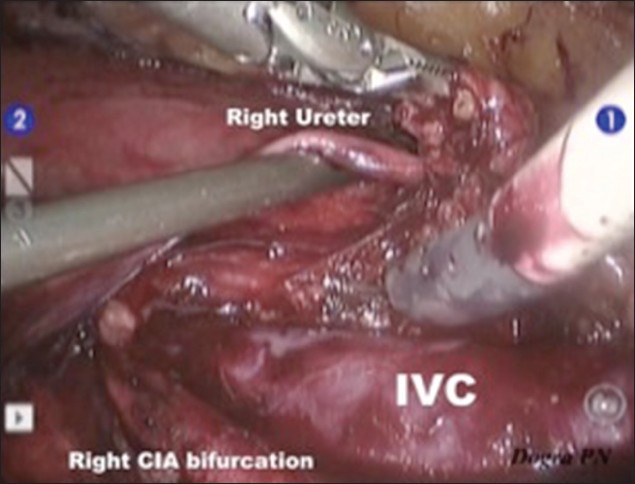
Right Paracaval Dissection: Distal limit-Right CIA (common iliac artery) bifurcation, lateral limit-Right Ureter
Figure 4.
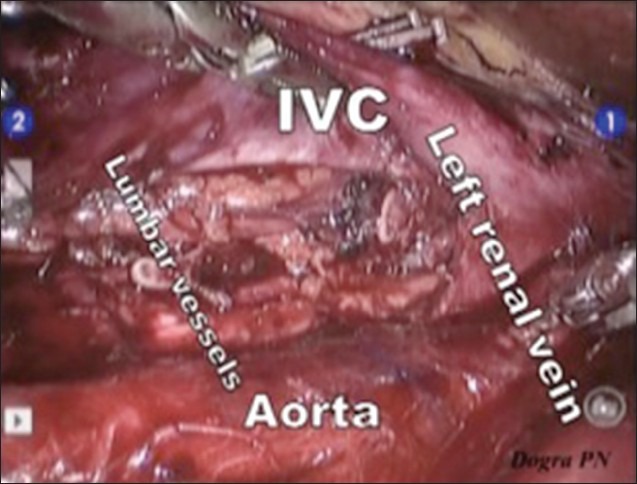
At the end of surgery-IVC: Inferior vena cava, Aorta, Left renal vessels and Lumbar vessels clearly seen
RESULTS
The total operative time, console time and estimated blood loss were 210 min, 170 min and 450 ml respectively. The patient didn’t require any blood transfusion. The histopathology showed a total 13 lymph nodes: 7 paracaval, 3 preaortic and 3 interaortocaval and all of which were negative for tumor deposits [Figure 5]. Postoperative pain management was done with 8th hourly injectable diclofenac sodium (75 mg) for 24 h which was changed to oral diclofenac on post operative day 1. Epidural analgesia was not used. The patient was allowed orally on the first postoperative day and discharged on postoperative day 3. At the time of writing this report, the patient has completed 6 months of follow up. His tumor markers, CT scan are normal and has normal ejaculations.
Figure 5.
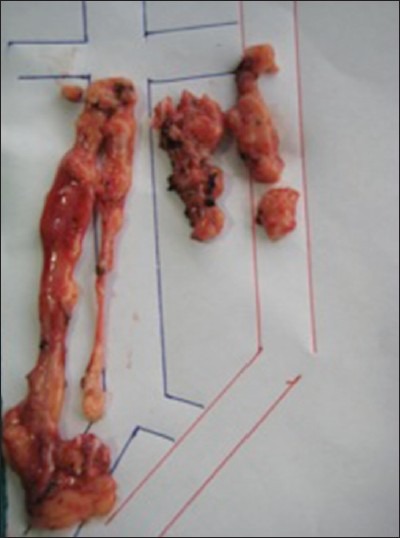
Tissue retrieved –Paracaval lymphatic tissue: 7 lymph nodes, Interaortocaval lymphatic tissue: 3 lymph nodes, Preaortic lymphatic tissue: 3 lymph nodes
DISCUSSION
LRPLND was earlier considered a diagnostic procedure with minimal therapeutic impact. A recent meta analysis of LRPLND by Rassweiler et al.[1] has changed this myth. He analysed over >800 patients in 34 publications and showed that the mean operative time of LRPLND was 204 min (range, 138-261 min) and the mean conversion rate was 3.8% with a mean hospital stay of 3.3 days. These results were significantly better than the early laparoscopic series and were attributed to the increasing experience of surgeons in laparoscopic techniques. LRPLND patients were shown to have less postoperative analgesic requirement and decreased hospital stay as compared to open RPLND.[2] The initial fear of decreased therapeutic potential of minimally invasive RPLND has since been abolished after Rassweiler et al.[1] demonstrated similar staging accuracy and long-term outcomes as well as decreased complications in LRPLND as compared to open RPLND.
The technical difficulty with laparoscopy needs experience and skill. With the introduction of the robotics in urology, the surgeon has 7° of hand movements, a 3D view and tremorless instrument movements. The precise movement of robotic arms help the surgeon to dissect major vessels and also helps in quick suturing, if required during the surgery. Planning ahead for port placement and positioning is very important as improperly placed port may be more of a nuisance than help, as we found in this case while using the 3rd robotic port. For a successful robot assisted LRPLND the surgeon should be well versed with retroperitoneal anatomy. In a classical RPLND, the position of the patient has to be changed after completing one side. The assistant port is helpful for retraction of major vessels, suctioning, suture exchange and clip application.
There are few reports of robot assisted laparoscopic RPLND in literature.[3,4] Their mean operative time in these reports was 182.45 min which was slightly less as compared to ours (210 min).
CONCLUSION
Robot assisted laparoscopic RPLND is a feasible procedure and may initially be considered in primary RPLND for clinical stage I patients. As application to post chemotherapy patients requires more skill and expertise, larger patient series and long term follow up are required to substantiate the role of Robot assisted laparoscopic RPLND in the management of patients with testicular tumors. There is a need to study this use of robot assisted laparoscopic RPLND rigorously and that it must be done with curative and therapeutic intent and not as a staging procedure.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Rassweiler JJ, Scheitlin W, Heidenreich A, Laguna MP, Janetschek G. Laparoscopic retroperitoneal lymph node dissection: Does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol. 2008;54:1004–15. doi: 10.1016/j.eururo.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MJ, Kavoussi LR. Controversial technology: The Chunnel and the laparoscopic retroperitoneal lymph node dissection (RPLND) BJU Int. 2010;106:950–9. doi: 10.1111/j.1464-410X.2010.09659.x. [DOI] [PubMed] [Google Scholar]
- 3.Davol P, Sumfest J, Rukstalis D. Robotic-assisted laparoscopic retroperitoneal lymph node dissection. Urology. 2006;67:199. doi: 10.1016/j.urology.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 4.Williams SB, Lau CS, Josephson DY. Initial series of robot-assisted laparoscopic retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell testicular cancer. Eur Urol. 2011;60:1299–302. doi: 10.1016/j.eururo.2011.03.009. [DOI] [PubMed] [Google Scholar]


