Abstract
The supplementary motor area coordinates movements. Synkinesia is a rare disorder in which an involuntary movement occurs coordinated with a voluntary movement. Here, we test the hypothesis that the supplementary motor area is involved in involuntary coordination of movement. We collected functional magnetic resonance imaging (fMRI) data from two patients with ipsilateral hand-foot synkinesia and two control participants while they performed rhythmic tasks. In synkinesia patients, both the supplementary motor area and the foot motor cortex were significantly activated during the hand motor task. This pattern was not seen in controls. Our findings suggest that the supplementary motor area plays a central role in involuntary coordination observed in synkinesia, and provides insight into how the supplementary motor area orchestrates movements.
Keywords: Ipsilateral synkinesia, fMRI, Supplementary motor area, Hand-foot coordination, Motor cortex
1. Introduction
Coordination of movement is essential to the performance of complex tasks. Evidence from transcranial magnetic stimulation (TMS) [3] and brain imaging [7] demonstrates that the supplementary motor area (SMA) [5,18] is involved in voluntary coordination of movements among ipsilateral limbs. Synkinesia is a rare movement disorder in which an involuntary movement occurs coordinated with a voluntary movement in a different limb. This disorder is observed in disorders of dopamine systems such as Parkinson’s disease [15]; however, the brain networks that are involved in synkinetic involuntary movements are unclear. To test the hypothesis that the SMA also plays a role in synkinetic involuntary limb coordination, we studied two patients with ipsilateral hand-foot synkinesia using functional magnetic resonance imaging (fMRI), and compared these data with controls. To the best of our knowledge, this is the first study of the neuroanatomical basis of synkinesia, and our findings support the role of SMA in this rare coordinated movement.
2. Methods
Two patients with hand-foot synkinesia participated in this study. The first patient was a left handed 61-year-old male with Parkinson’s disease, in whom movement of either hand caused involuntary movements of the ipsilateral leg. The second patient was a right handed 64-year-old female with essential tremor and long term metoclopramide therapy for gastric hypomotility in whom movement of the right hand caused involuntary movements of the right foot. Two age-matched asymptomatic volunteers in their early sixties participated in this study as controls. All research was approved by the Human Investigation Committee of Yale University.
Participants performed two motor tasks while fMRI data was collected. For the hand motor task, they rhythmically squeezed a sponge with the hand of interest. For the foot motor task, they rhythmically flexed the foot of interest. Movements were self-paced. They performed each task for 15 s, followed by 15 s of rest. Each block consisted of 6 cycles of task performance, followed by rest (3 min); patients performed 5 blocks of each task. Visual instructions from a Macintosh computer running Psyscope software were used to instruct patients. All participants underwent a practice session prior to scanning. Supine, head-immobilized participants were imaged using a 1.5T Siemens Sonata system. Anterior and posterior commissures were identified via a sagittal localizer, and anatomic images were identified using T1 weighted, spin-echo, axial-oblique slices (TE = 11 ms, TR = 500 ms, 256 × 192 matrix, FOV = 22 cm, slice thickness 6 mm, 0 mm gap, 18 slices). Functional imaging was performed using gradient echo EPI sequence (FA = 80 degrees, TE = 45, TR = 1500 ms, 64 × 64 matrix) with the same parameters as the anatomical scan. A high resolution 3D anatomical image was also collected (FA = 45 degrees, TE = 3.28 ms, TR = 20 ms, 256 × 256 × 144, slice thickness 1 mm, 1 average, bandwidth 130 Hz).
Functional images were motion-corrected offline with Statistical Parametric Mapping 99 (www.fil.ion.ucl.ac.uk/spm/software/spm99) for three translational directions (x, y or z) and three possible rotations (pitch, yaw or roll). Images were spatially smoothed with a Gaussian kernel of FWHM 6.25 mm [6]. Trials with motion of 2 mm of translation or 3° of rotation were rejected. Individual subject data were analyzed using a General Linear Model on each voxel in the entire brain volume with regressors specific for each task. For the hand and foot motor task, regressors were defined for the motor task relative to fixation. Functional images for each task were spatially smoothed with an 8.08 mm Gaussian kernel. The output maps were beta-maps, and voxels with a T-statistic greater than 1.7 were interpreted as significant at p < 0.05.
The Yale BioImage Suite software was used to transform these data into a standard common reference space [6]. Registrations were calculated between the functional image and 2D anatomical images, the 2D anatomical image and the participant’s 3D anatomical image, and non-linearly between the individual 3D anatomical image and a reference 3D image [12]. All three registrations were applied sequentially to the individual beta-maps to bring all data into the common reference space [17]. Regions of interest were defined anatomically based on Brodmann areas (BA).
3. Results
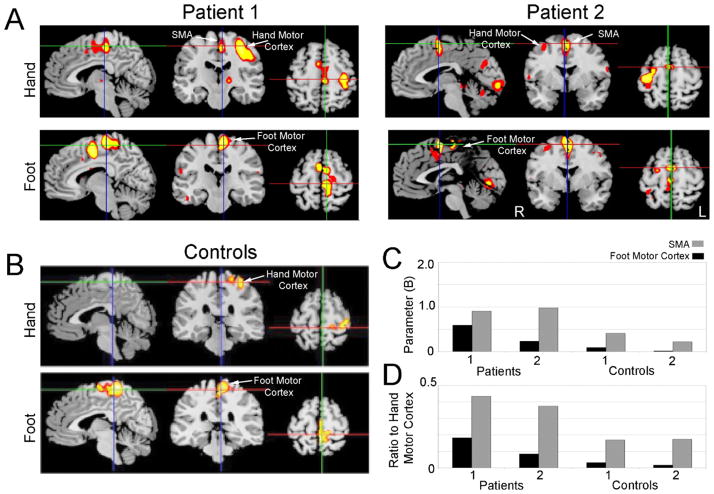
In this study, fMRI data was collected from patients with hand-foot synkinesia in whom voluntary hand movement resulted in ipsilateral involuntary movement of their foot. Synkinetic movements were observed during the collection of fMRI data. In these patients, performance of the hand motor task resulted in significant activation of the contralateral hand motor cortex (Fig. 1A; BA 4) of the precentral gyrus. In addition, activation was seen in mesial frontal regions in the contralateral foot motor cortex (mesial BA 4) and the SMA (BA 6). In patient 2, small areas of activation were seen in ipsilateral hand motor cortex, cerebellum, and occipital cortex. In comparison, the controls showed no comparable activation in mesial frontal cortex during the hand motor task (Fig. 1B). During the foot motor task, both patients and controls demonstrated activation primarily in the foot motor cortex. For each individual subject, foot motor cortex and SMA were selected as anatomical regions of interest. The mean beta values of the foot task and the hand task were computed across these areas. During the hand motor task, the activation of the SMA and the foot motor cortex was higher in both synkinesia patients compared to controls (Fig. 1C). These data demonstrate that in patients with synkinesia, SMA and foot motor cortex are activated during hand movements in patients with hand-foot synkinesia.
Fig. 1.
(A) fMRI activation maps of patients performing the hand motor task (top row) and the foot motor task (bottom row). Synkinesia patients had prominent activity in the supplementary motor area and foot motor cortex during the hand motor task. Patient 1 was right handed, and patient 2 was left handed; images are displayed in radiological convention. (B) Average fMRI activation maps of two controls performing the hand motor task (top row) and the foot motor task (bottom row). (C) Activation of mesial cortical regions of interest from the supplementary area (SMA) and the foot motor cortex during performance of the hand motor task. Patients with synkinesia both had greater activation in these areas compared to controls. (D) The ratio SMA or foot motor cortex activation to hand motor cortex activation during performance of the hand motor task. Patients with synkinesia had higher ratios of SMA and foot motor cortex activity than controls, suggesting that increases in activation could not be explained by increased cortical excitability.
Medications modulating dopaminergic transmission can affect cortical excitability. To ensure these results were distinct from a general increase in cortical activity, we calculated ratios of activation within SMA and foot motor cortex (BA 6) to activation within hand motor cortex (Fig. 1D). These data suggest that increased activity in SMA and foot motor cortex cannot be accounted for by increased cortical excitability.
4. Discussion
The present study investigated whether SMA is involved in the coordination of involuntary movements. To test this idea, we collected fMRI data of patients with hand-foot synkinesia. During hand movements, we find activity in the SMA and in foot motor cortex in patients with synkinesia. Our findings provide novel evidence that the SMA is involved in the involuntary coordination of movement, and provide the first glimpse of the neuroanatomical basis of synkinesia. The SMA can be involved generally in voluntary movements [2]. In this study, we find some activation in the SMA in our controls; however, SMA activity is stronger during involuntarily coordinated movements in synkinesia patients relative to controls. Taken together, these data shed light on how the SMA coordinates both voluntary and involuntary movements.
Innate synchronies of limb movements are essential to the maintenance of balance and the execution of gait. During development, such synchronies of movement occasionally manifest as synkinesia, where involuntary movements in one muscle group follow voluntary movements of another muscle group. Most developmental synkinesias disappear by adolescence but can re-emerge in neurological disease states [1,14]. Individualized limb movements are made possible by the maturation of intracortical inhibitory fibers, and motor programs that utilize these connections when coordinating multiple limbs. Thus synkinesia provides a unique opportunity for the study of the control mechanisms underlying synchronous movements.
The SMA coordinates movements of multiple limbs. Whereas there are no known direct connections between hand and foot motor cortex, the loci representing the upper and the lower limbs in the SMA are overlapping [10]. A recent TMS study demonstrated functional connections between the SMA and motor cortex, suggesting that SMA facilitates the linkage of movements in distinct limbs [3]. Brain imaging studies have demonstrated that the SMA is a key node in a distributed network that coordinates voluntary movements [4]. Consonant with this idea, we find activation in several other areas during synkinesia, suggesting that even involuntary coordination of movement invokes a distributed network orchestrated by the SMA.
Neurological syndromes that involve dopamine, such as Parkinson’s disease, schizophrenia, Tourette’s syndrome, and Huntington’s disease, are associated with synkinesia. In these disorders, synkinesia is often heterologous, or ‘mirror movements’, involving the same limb on the opposite side of the body [8,9,11,13,19]. Although the brain network involved in these mirror movements is unclear, mirror movements could invoke dense subcortical connections as well as the SMA [16]. Future brain imaging or TMS studies will explore this issue (Tables 1 and 2).
Table 1.
The clinical and demographic particulars of the two patients studied.
| Age/Sex | Handedness | Diagnosis | Medication | Synkinesia | |
|---|---|---|---|---|---|
| Patient 1 | 64/F | Right | Essential tremor since age 20 (hands) | Trazodone 300 mg daily Zoloft 200 mg daily Reglan 40 mg daily |
Voluntary movement of the right hand caused involuntary movement of the right foot. Voluntary movements of the feet and the left hand did not elicit synkinesia. Mental rehearsal of right hand movement did not produce concurrent right foot movement. |
| Patient 2 | 61/M | Left | Parkinson’s disease for 7 years | Sinemet 25/100 mg tid | Voluntary movements of either hand produced involuntary movements in the ipsilateral foot. This effect was most pronounced on the left side. Voluntary movements of the feet did not elicit feet-hand synkinesia. Mental rehearsal of the left hand movement did not produce hand-foot synkinesia |
Table 2.
Talairach coordinates of regions of activation for individual patients and controls during the hand motor task and foot motor task.
| Anatomical region/Broadmann area | Right hand task
|
Left hand task
|
Right foot task
|
Left foot task
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | x | y | z | x | y | z | ||
| Patient 1 | Primary motor cortex BA4 | −36 | −22 | 50 | 40 | −16 | 50 | −12 | −36 | 60 | 11 | −31 | 65 |
| Supplementory motor cortex (SMA) BA6 | −4 | −24 | 50 | 7 | −18 | 50 | −7 | −24 | 60 | 5 | −18 | 60 | |
| Patient 2 | Primary motor cortex BA4 | −34 | −24 | 50 | 35 | −19 | 50 | −4 | −35 | 60 | 4 | −35 | 60 |
| Supplementory motor cortex (SMA) BA6 | −4 | −10 | 50 | 4 | −4 | 50 | −4 | −8 | 60 | 5 | −20 | 60 | |
| Control 1 | Primary motor cortex BA4 | −36 | −24 | 50 | 36 | −22 | 50 | −11 | −36 | 60 | 11 | −36 | 60 |
| Supplementory motor cortex (SMA) BA6 | – | – | −4 | −20 | 60 | 5 | −20 | 60 | |||||
| Control 2 | Primary motor cortex BA4 | −35 | −24 | 50 | 35 | −20 | 50 | −11 | −36 | 60 | 11 | −36 | 60 |
| Supplementory motor cortex (SMA) BA6 | – | – | −4 | −20 | 60 | 4 | −20 | 60 | |||||
Our study has a number of limitations. The relative rarity of ipsilateral synkinesia has made the size of the study necessarily small. In addition, we did not compare our patients on and off medications. However, our patients could only adequately perform the motor tasks in this study on medication. Furthermore, we cannot distinguish if the SMA activation that we observe is from feedback or direct motor control. Techniques such as TMS may resolve this issue. One additional possibility is that increased SMA activation during involuntary synkinetic movements may be related to additive BOLD signal of both hand and leg movements. Despite these limitations, our data describing SMA involvement in involuntary movement could provide an opportunity to treat synkinesia patients based on selective manipulations of this cortical network.
HIGHLIGHTS.
We explore the cortical basis of synkinesia with functional brain imaging.
Rhythmic movements in synkinesia patients recruit the supplementary motor cortex.
Rhythmic movements in controls did not recruit the supplementary motor cortex.
These data provide insight into the involuntary coordination of movement.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Baldissera F, Cavallari P, Civaschi P. Preferential coupling between voluntary movements of ipsilateral limbs. Neuroscience Letters. 1982;34:95–100. doi: 10.1016/0304-3940(82)90098-2. [DOI] [PubMed] [Google Scholar]
- 2.Boecker H, Kleinschmidt A, Requardt M, Hänicke W, Merboldt KD, Frahm J. Functional cooperativity of human cortical motor areas during self-paced simple finger movements. A high-resolution MRI study. Brain. 1994;117(Pt 6):1231–1239. doi: 10.1093/brain/117.6.1231. [DOI] [PubMed] [Google Scholar]
- 3.Byblow WD, Coxon JP, Stinear CM, Fleming MK, Williams G, Müller JFM, et al. Functional connectivity between secondary and primary motor areas underlying hand-foot coordination. Journal of Neurophysiology. 2007;98:414–422. doi: 10.1152/jn.00325.2007. [DOI] [PubMed] [Google Scholar]
- 4.Debaere F, Swinnen SP, Béatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. Neuroimage. 2001;14:947–958. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- 5.Donchin O, de Oliveira SC, Vaadia E. Who tells one hand what the other is doing: the neurophysiology of bimanual movements. Neuron. 1999;23:15–18. doi: 10.1016/s0896-6273(00)80748-5. [DOI] [PubMed] [Google Scholar]
- 6.Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH. Geometric strategies for neuroanatomic analysis from MRI. Neuroimage. 2004;23(Suppl 1):S34–S45. doi: 10.1016/j.neuroimage.2004.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrsson HH, Naito E, Geyer S, Amunts K, Zilles K, Forssberg H, et al. Simultaneous movements of upper and lower limbs are coordinated by motor representations that are shared by both limbs: a PET study. European Journal of Neuroscience. 2000;12:3385–3398. doi: 10.1046/j.1460-9568.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 8.Espay AJ, Li JY, Johnston L, Chen R, Lang AE. Mirror movements in parkinsonism: evaluation of a new clinical sign, Journal of Neurology. Neurosurgery and Psychiatry. 2005;76:1355–1358. doi: 10.1136/jnnp.2005.062950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer SF. Mirror movements in neurology, Journal of Neurology. Neurosurgery and Psychiatry. 2005;76:1330. doi: 10.1136/jnnp.2005.069625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. Journal of Neurophysiology. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- 11.Georgiou-Karistianis N, Hoy KE, Bradshaw JL, Farrow M, Chiu E, Churchyard A, et al. Motor overflow in Huntington’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:904–906. doi: 10.1136/jnnp.2003.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 13.Johnson KA, Cunnington R, Bradshaw JL, Phillips JG, Iansek R, Rogers MA. Bimanual co-ordination in Parkinson’s disease. Brain. 1998;121(Pt 4):743–753. doi: 10.1093/brain/121.4.743. [DOI] [PubMed] [Google Scholar]
- 14.Largo RH, Caflisch JA, Hug F, Muggli K, Molnar AA, Molinari L. Neuromotor development from 5 to 18 years. Part 2: associated movements. Developmental Medicine and Child Neurology. 2001;43:444–453. doi: 10.1017/s0012162201000822. [DOI] [PubMed] [Google Scholar]
- 15.Salazar G, Casas E, Oliveras D, Rando A, Sergio P. Ocular-jaw synkinesia in normal, Parkinson’s disease, and multiple system atrophy subjects: clinical and electrophysiological findings. Clinical Neurophysiology. 2010;121:94–97. doi: 10.1016/j.clinph.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nature Reviews Neuroscience. 2002;3:348–359. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- 17.Talaraich J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Thieme; New York, NY: 1998. [Google Scholar]
- 18.Tanji J. The supplementary motor area in the cerebral cortex. Neuroscience Research. 1994;19:251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 19.Verheul MHG, Geuze RH. Inter-limb coupling in bimanual rhythmic coordination in Parkinson’s disease. Human Movement Science. 2004;23:503–525. doi: 10.1016/j.humov.2004.08.021. [DOI] [PubMed] [Google Scholar]