Abstract
Aims
Whether patients with hypertensive preclinical cardiovascular disease (CVD) are at higher risk of incident diabetes has never been studied.
Methods and results
We assessed incident diabetes in 4176 hypertensive non-diabetic patients (age 58.7 ± 8.9 years, 58% male) with ≥1 year follow-up (median: 3.57 years; inter-quartile range: 2.04–7.25). Left ventricular (LV) hypertrophy (LVH) was defined as LV mass index (LVMi) ≥51 g/m2.7. Carotid atherosclerosis (CA) was defined as intima-media thickness >1.5 mm. During follow-up, diabetes developed in 393 patients (9.4%), more frequently in those with than without initial LVH or CA (odds ratio = 1.97 and 1.67, respectively; both P < 0.0001). In the Cox regression, the presence of either initial LVH or CA was associated with higher hazard of diabetes [hazards ratio (HR) = 1.30 and 1.38, respectively; both P = 0.03], independently of the type and number of anti-hypertensive medications, initial systolic blood pressure (P < 0.001), body mass index, fasting glucose, family history of diabetes (all P < 0.0001), and therapy with β-blockers. The presence of one of the, or both, markers of preclinical CVD increased the chance of incident diabetes by 63 or 64%, respectively (both P < 0.002), independently of significant confounders, a result that was confirmed (HR = 1.70 or 1.93, respectively; both P < 0.0001) using ATPIII metabolic syndrome (HR = 2.73; P < 0.0001) in the Cox model.
Conclusion
Initial LVH and CA are significant predictors of new onset diabetes in a large population of treated hypertensive patients, independently of initial metabolic profile, anti-hypertensive therapy, and other significant covariates. This sequence may be attributable to risk factors common to preclinical CVD and diabetes, but a vascular origin of diabetes cannot be excluded.
Keywords: Diabetes, Hypertension, Target organ damage, Metabolic syndrome
Introduction
A large body of epidemiological and pathological data indicates that type 2 diabetes mellitus (diabetes) is an independent risk factor for preclinical cardiovascular (CV) disease (CVD),1 including carotid atherosclerosis (CA) and left ventricular (LV) hypertrophy (LVH),2,3 as well as for overt CVD in both men and women.4,5 Diabetes also worsens survival in patients who develop clinical CVD.6
We have previously demonstrated that uncontrolled blood pressure (BP) is associated with a two-fold increased risk of incident diabetes in treated hypertensive subjects,7 extending previous observations indicating that incident diabetes is more frequent in hypertensive than in normotensive subjects,8 and that combinations of hypertension, obesity, and abnormal lipid profile, which often coexist with diabetes, are associated with preclinical cardiovascular abnormalities.1,9,10 Interestingly, clusters of metabolic abnormalities and the presence of LVH are also reported to increase the probability of uncontrolled hypertension.11
These findings raise questions regarding the temporal sequence of these abnormalities and development of both hypertension and diabetes.
It is unclear whether the presence of hypertensive preclinical CVD (target organ damage), such as LVH and/or CA, is associated with increased risk of incident diabetes, independent of metabolic risk factors and arterial hypertension.8 Accordingly, the present study was designed to test whether or not prevalent hypertensive preclinical CVD increases the risk of incident diabetes in the hypertensive participants of the Campania Salute Network Registry.
Methods
Participants
Hypertensive patients without diabetes and known CVD were studied, with at least 1-year follow-up, within the Campania Salute Network Registry. The Campania Salute is an open registry collecting information from a network of general practitioners and community hospitals networked with the Hypertension Center of the Federico II University Hospital. The database generation of the Campania Salute Network was approved by the Federico II University Hospital Ethic Committee. Signed informed consent was obtained from all the participants to use data for scientific purposes. Detailed characteristics of this population have been previously reported.7,12
Exclusion criteria for the present analysis were: pre-existing CVD, diabetes, secondary hypertension, chronic kidney disease more than grade 3 (GFR by simplified MDRD <30 mL/min/1.73 m2), aortic and/or mitral regurgitation more than mild, any degree of aortic stenosis, and follow-up <1 year. Pre-existing CVD was defined as the history of previous myocardial infarction, angina, coronary revascularization, stroke, transitory ischaemic attack, and congestive heart failure at the time of the admission visit in our outpatient clinic. Thus, from the initial population of 10 254 hypertensive patients, 3157 patients were excluded due to the presence of pre-existing CVD or diabetes, 2392 for insufficient follow-up period or poor quality echocardiographic windows, and 529 because of the lack of same-day baseline echocardiogram and carotid ultrasound. Thus, the present analysis included 4176 hypertensive non-diabetic participants free of pre-existing CVD and renal failure, with at least 1-year follow-up.
During initial and follow-up visits, BP, heart rate, body mass index (BMI), fasting glucose, and lipid profile were measured for each patient by standard methods. All hypertensive patients of the network underwent baseline echocardiogram and carotid ultrasound.
Measurements and definitions
Impaired fasting glucose and diabetes were defined according to 1997 ADA criteria (fasting plasma glucose >110 mg/dL, or >125 mg/dL or anti-diabetic treatment, respectively). Family history of diabetes (in >1 family member) was recorded at the time of the first visit.
Obesity was defined as a BMI ≥30 kg/m2. Metabolic syndrome (MetS) was defined according to modified ATPIII criteria,13 changing waist circumference with BMI ≥30 kg/m2, as previously done when waist girth was not available.14,15
Fasting glucose and lipid profile were measured by standard methods. Systolic and diastolic BP were measured by standard aneroid sphygmomanometer after 5 min rest in the supine position, according to current guidelines.16 Three BP measurements were obtained in the sitting position at 2 min intervals. The averages of these measurements were used for the analysis. Incident diabetes was adjudicated based only on detection of abnormal fasting glucose.
Echocardiography
Echocardiograms were recorded in videotapes, using commercial machines and a standardized protocol, were digitally mastered and read offline by one expert reader under the supervision of a senior faculty member, using dedicated work-stations (MediMatic). Measurements were made according to the American Society of Echocardiography/European Association of Echocardiography recommendations.17
Left ventricular mass was calculated from a necropsy-validated formula18 and normalized for height in metres to the power of 2.7 (LVMi).11,19
Left ventricular hypertrophy (LVH) was defined as LVMi ≥51 g/m2.7.11
Carotid ultrasound
Carotid ultrasound was carried out in the supine position with the neck extended in mild rotation. The scanning protocol was performed with an ultrasound device (SONOS 2500/5500, HP, Philips) equipped with a 7.5 MHz high-resolution transducer with an axial resolution of 0.1 mm. Examinations were recorded on S-VHS videotapes and analysed as previously described.20 The maximal arterial intima-media thickness (IMT) was estimated offline in up to 12 arterial walls, including the right and the left, near and far distal common carotid (1 cm), bifurcation, and proximal internal carotid artery, and using an image-processing dedicated workstation (MediMatic). Evidence of IMT value higher than 1.5 mm was considered as ‘plaque’.16 The presence of carotid plaque was considered a marker of CA.
Statistical analysis
Data were analysed using SPSS (version 20.0; SPSS, Chicago, IL, USA) and expressed as mean ± 1SD. Variables not normally distributed were log-transformed. Descriptive comparison between patients with or without initial LVH and/or evidence of CA was performed using t-test. Unadjusted prevalence of specific conditions in population subgroups was compared using the χ2 distribution, and Monte Carlo simulation to generate exact P-values.
To account for therapy, single classes of medications, including anti-renin–angiotensin system drugs (anti-RAS, including ACE inhibitors and/or AT1 receptor antagonists), calcium channel blockers (CCBs), β-blockers, and thiazide diuretics, were dichotomized according to their overall use during the individual follow-up, based on the frequency of prescriptions during the control visits, considering as the end of follow-up time of diabetes diagnosis for patients with incident diabetes. Thus, all medications used for more than 50% of control visits were considered as covariates in proportional hazards analysis, a method that has been previously reported.7
Incident diabetes in relation to the presence of either initial LVH or CA was assessed using two models of the Cox regression analysis (one for each marker), controlling for demographic, haemodynamic, and metabolic variables participating to the phenotypes of MetS (age, sex, reported duration of hypertension, initial BP, heart rate, BMI, fasting glucose, HDL-cholesterol, triglycerides) and number and type of anti-hypertensive medications that were significantly different in exploratory statistics. In alternative Cox models, we assessed the effect of the generic presence of either one of the two markers of preclinical CVD (LVH or CA) or both, adjusting for the same covariates. Finally, the latter Cox model was also run by substituting individual risk factors (i.e. glucose, HDL-cholesterol, BP, BMI, and triglycerides) with MetS, in the whole, as well as in subsets of, study population.
A two-tailed α-value of <0.05 was used to reject the null hypothesis.
Results
Among the 4176 non-diabetic hypertensive outpatients included in this analysis (mean age 58.7 ± 8.9 years, 58% male), 992 (24%) were obese and 1158 met the criteria for the diagnosis of MetS (28%). Initial LVH was found in 1136 patients (27%). Initial CA was found in 1548 patients (37%).
Table 1 shows that patients with baseline LVH were older (P < 0.0001) and more often male (P < 0.003). They also exhibited longer history of hypertension, higher baseline BMI, systolic and diastolic BP, and lower heart rate than those without LVH (all P < 0.0001). Baseline fasting glucose and triglycerides were also higher, HDL-cholesterol was lower, and MetS was more prevalent in the presence than in the absence of LVH (all P < 0.0001, Table 1). No difference was found in the proportion of patients with a family history of diabetes among the two groups.
Table 1.
Baseline demographic and clinical characteristics of participants with or without initial LV hypertrophy (LVH)
| Non-LVH (n = 3040) | LVH (n = 1136) | P≤ | |
|---|---|---|---|
| Age (years) | 57.61 ± 8.9 | 61.69 ± 8.3 | 0.0001 |
| Sex (M/F %) | 56.6/43.4 | 61.6/38.4 | 0.003 |
| BMI (kg/m2) | 26.84 ± 3.6 | 29.77 ± 4.4 | 0.0001 |
| Hypertension duration (years) | 5.13 ± 6.0 | 7.39 ± 7.3 | 0.0001 |
| Systolic BP (mmHg) | 140.14 ± 15.8 | 146.54 ± 19.1 | 0.0001 |
| Diastolic BP (mmHg) | 89.37 ± 9.9 | 91.48 ± 11.2 | 0.0001 |
| Heart rate (b.p.m.) | 74.97 ± 11.4 | 73.09 ± 11.4 | 0.0001 |
| Fasting plasma glucose (mg/dL) | 93.36 ± 12.0 | 95.90 ± 12.0 | 0.0001 |
| HDL Cholesterol (mg/dL) | 50.83 ± 12.7 | 48.99 ± 12.4 | 0.0001 |
| Triglycerides (mg/dL) | 130.9 ± 71.9 | 141.5 ± 79.2 | 0.0001 |
| Metabolic syndrome (%) | 28.2 | 46.9 | 0.0001 |
| Family history of diabetes (%) | 30.2 | 29.8 | 0.809 |
| CCB (%) | 18.8 | 32.2 | 0.0001 |
| β-Blockers (%) | 26.5 | 27.3 | 0.614 |
BMI, body mass index; BP, blood pressure; HDL, high density lipoprotein; CCB, calcium channel blockers.
Similarly, patients with baseline CA were more often men and older (both P < 0.004), had longer history of hypertension, higher initial systolic but lower diastolic BP, higher fasting glucose and triglycerides, and lower baseline and heart rate than those without CA (0.004 < P < 0.0001, Table 2), without statistical differences for HDL-cholesterol prevalence of MetS and family history of diabetes. Calcium channel blockers were more frequently used in patients with than in those without LVH or CA, whereas no difference was found for β-blockers and other classes of meds (Tables 1 and 2).
Table 2.
Baseline demographic and clinical characteristics of participants with or without initial carotid atherosclerosis (CA)
| Non-CA (n = 2628) | CA (n = 1548) | P≤ | |
|---|---|---|---|
| Age (years) | 53.57 ± 8.9 | 62.37 ± 7.7 | 0.0001 |
| Sex (M/F %) | 56.2/43.8 | 60.9/39.1 | 0.004 |
| BMI (kg/m2) | 27.64 ± 4.1 | 27.62 ± 3.9 | 0.891 |
| Hypertension duration (years) | 5.03 ± 5.9 | 6.95 ± 7.2 | 0.0001 |
| Systolic BP (mmHg) | 140.92 ± 16.3 | 143.50 ± 18.0 | 0.0001 |
| Diastolic BP (mmHg) | 90.38 ± 10.0 | 89.21 ± 10.6 | 0.0001 |
| Heart rate (b.p.m.) | 74.87 ± 11.5 | 73.76 ± 11.5 | 0.002 |
| Fasting plasma glucose (mg/dL) | 93.27 ± 11.8 | 95.38 ± 12.4 | 0.0001 |
| HDL Cholesterol (mg/dL) | 50.51 ± 12.8 | 50.03 ± 12.3 | 0.233 |
| Triglycerides (mg/dL) | 131.0 ± 74.0 | 138.5 ± 74.2 | 0.001 |
| Metabolic syndrome (%) | 33.1 | 33.5 | 0.780 |
| Family history of diabetes (%) | 30.0 | 30.1 | 0.954 |
| CCB (%) | 20.4 | 26.0 | 0.0001 |
| β-Blockers (%) | 26.9 | 26.4 | 0.680 |
BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; CCB, calcium channel blockers.
Incident diabetes
During follow-up (median: 3.57 years; inter-quartile range: 2.04–7.25), incident diabetes was adjudicated in 393 patients (9.4%). Diabetes developed near two-fold more frequently in patients with than in those without initial LVH [14.1 and 7.7%, respectively; odds ratio (OR) = 1.97, 95% confidence interval (CI): 1.59–2.45, P < 0.0001] and similarly, the incidence of diabetes was significantly more frequent among patients with baseline evidence of carotid plaque (12.3 vs. 7.7%; OR = 1.67, 95% CI: 1.36–2.06, P < 0.0001). Hypertensive patients developing diabetes during follow-up were given more often β-blockers and CCB than patients without incident diabetes (32.6 vs. 26.1%; 30.0 vs. 21.7%, respectively; both P < 0.006), whereas no difference was found for the other classes of antihypertensive meds. Patients with incident diabetes also took a greater number of antihypertensive meds (1.8 ± 0.98) than those free of incident diabetes (1.5 ± 0.95, P < 0.0001). No difference was found in the number of visit per year in patients with or without incident diabetes (1.29 ± 1.02 vs. 1.39 ± 1.03, respectively; P = 0.620).
In the Cox regression, the presence of initial LVH remained associated with 30% higher risk of incident diabetes [hazards ratio (HR) = 1.30; (95% CI 1.02–1.64); P = 0.03], independently of the type and number of anti-hypertensive medications, initial higher systolic BP (P = 0.001), BMI, fasting glucose, and family history of diabetes (all P < 0.0001). Similarly, the presence of CA was associated with nearly 40% higher risk of incident diabetes [HR = 1.38; (95% CI 1.11–1.70); P = 0.003], independently of the type and number of anti-hypertensive medications, initial higher systolic BP, BMI, fasting glucose, and family history of diabetes (all P < 0.0001).
The presence of either of the two markers of preclinical CVD (n = 1582) increased the chance of incident diabetes by more than 60% [HR = 1.63; (95% CI 1.27–2.08); P < 0.0001], a risk that remained similar in the presence of both markers [n = 551; HR = 1.64; (95% CI 1.19–2.23); P = 0.002], and was independent of initial higher systolic BP [HR = 1.05/5 mmHg; (95% CI 1.02–1.11); P = 0.001], greater BMI [HR = 1.05/kg/m; (95% CI 1.02–1.08)], higher fasting glucose [HR = 1.08/mg/dL; (95% CI 1.07–1.09)], family history of diabetes [HR = 1.59; (95% CI 1.29–1.96); all P < 0.0001], and type and number of anti-hypertensive medications (not significant).
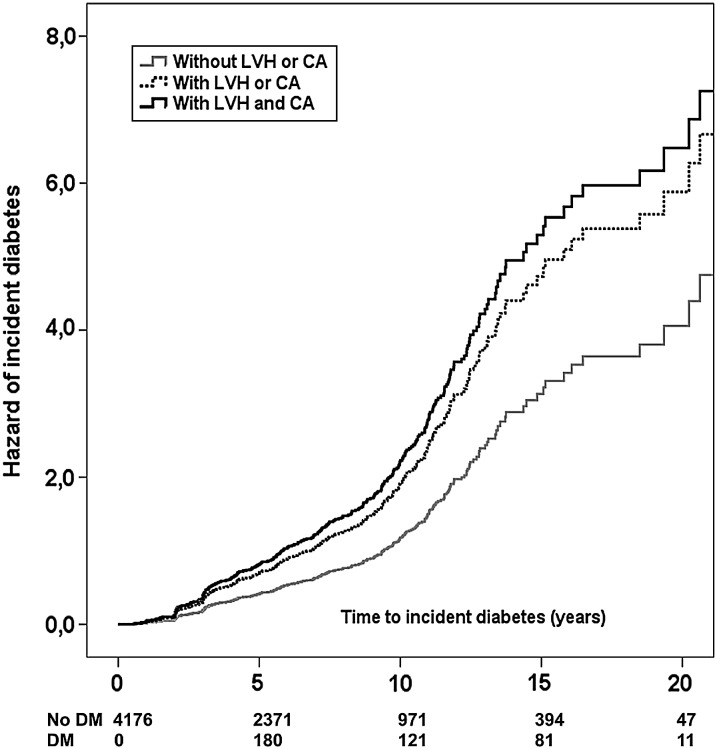
The last model of the Cox regression was repeated clustering all the components of MetS in a single variable as initial presence or absence of MetS. In this analysis, incident diabetes was independently predicted by initial presence of one or both markers of preclinical CVD, with additional significant effects for MetS, older age, and family history of diabetes, with no detectable effect for the other covariates (Table 3, Figure 1).
Table 3.
Baseline predictors of incident diabetes including either one of or both markers of preclinical CV disease
| HR | CI 95.0% HR |
P≤ | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age (years) | 1.02 | 1.00 | 1.03 | 0.028 |
| MetS (n/y) | 2.73 | 2.21 | 3.37 | 0.0001 |
| Family history of diabetes (n/y) | 1.85 | 1.51 | 2.27 | 0.0001 |
| LVH or CA (n/y) | 1.70 | 1.33 | 2.17 | 0.0001 |
| LVH and CA (n/y) | 1.93 | 1.42 | 2.63 | 0.0001 |
| Heart rate (b.p.m.) | 0.99 | 0.99 | 1.01 | 0.762 |
| Duration of hypertension (years) | 1.01 | 0.99 | 1.02 | 0.270 |
| Number of antihypertensive drugs | 1.09 | 0.95 | 1.24 | 0.213 |
| CCB (n/y) | 0.99 | 0.78 | 1.27 | 0.920 |
| β-Blockers (n/y) | 1.04 | 0.82 | 1.32 | 0.749 |
MetS, metabolic syndrome; LVH, left ventricular hypertrophy; CA, carotid atherosclerosis; CCB, calcium channel blockers; β-blockers, beta-blockers.
Figure 1.
Hazard of incident diabetes according to the initial presence of one (dotted line) or both markers (continuous black line) of preclinical cardiovascular disease (left ventricular hypertrophy, carotid atherosclerosis), compared with the absence of preclinical cardiovascular disease (continuous grey line).
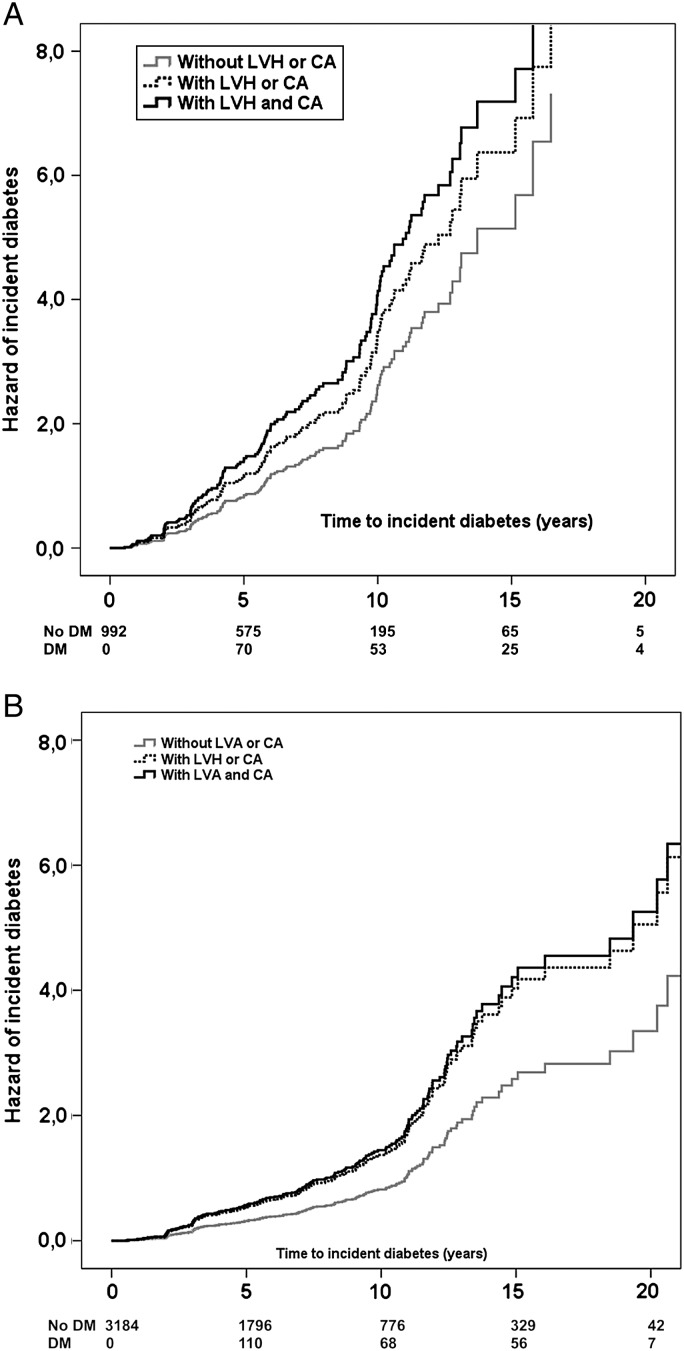
The Cox regression, adjusting for age, family history of diabetes, heart rate, duration of hypertension, number of anti-hypertensive meds, and use of CCB or β-blockers, was also run in specific subpopulations, based on the presence or absence of obesity or impaired fasting glucose. Patients with BMI ≥30 were younger, had higher baseline glucose, triglycerides, systolic and diastolic BP, heart rate and duration of hypertension, lower HDL cholesterol, and more frequent MetS and family history of diabetes (all P < 0.04) than those without obesity. In the obese sub-population (n = 992), incident diabetes was independently predicted by the combined presence of both markers of preclinical CVD (Table 4; Figure 2A). In the non-obese sub-population (n = 3184), incident diabetes was independently predicted also by the initial presence of one of the markers of preclinical CVD (Table 4; Figure 2B). Similarly, incident diabetes was independently predicted by the initial presence of one of the markers of preclinical CVD either in the presence (n = 1389) or in the absence of MetS (n = 2787, all P < 0.02, Table 4).
Table 4.
Cox's regressions performed for subgroups stratified by BMI (obese vs. non-obese), presence or absence of MetS or impaired fasting glucose, adjusting for age, family history of diabetes, heart rate, duration of hypertension, number of anti-hypertensive meds, and the use of CCB or β-blockers
| HR | CI 95.0% HR | P≤ | ||
|---|---|---|---|---|
| Lower | Upper | |||
| BMI ≥30 (n = 992) | ||||
| LVH or CA | 1.40 | 0.91 | 2.17 | 0.126 |
| LVH and CA | 1.76 | 1.06 | 2.92 | 0.030 |
| BMI <30 (n = 3184) | ||||
| LVH or CA | 1.73 | 1.28 | 2.33 | 0.0001 |
| LVH and CA | 1.83 | 1.22 | 2.74 | 0.003 |
| With MetS (n = 1389) | ||||
| LVH or CA | 1.50 | 1.08 | 2.08 | 0.015 |
| LVH and CA | 1.68 | 1.12 | 2.52 | 0.012 |
| Without MetS (n = 2787) | ||||
| LVH or CA | 1.97 | 1.37 | 2.83 | 0.001 |
| LVH and CA | 2.29 | 1.41 | 3.72 | 0.001 |
| First fasting glucose ≥110 mg/dL (n = 453) | ||||
| LVH or CA | 1.63 | 1.10 | 2.41 | 0.015 |
| LVH and CA | 1.70 | 1.05 | 2.76 | 0.029 |
| First fasting glucose <110 mg/dL (n = 3723) | ||||
| LVH or CA | 1.73 | 1.27 | 2.36 | 0.001 |
| LVH and CA | 2.00 | 1.34 | 2.99 | 0.001 |
MetS was also added as a covariate in the models using BMI or impaired fasting glucose.
Figure 2.
Hazard of incident diabetes in subgroup with (A) or without obesity (B), according to the initial presence of one (dotted line) or both markers (continuous black line) of preclinical cardiovascular disease (left ventricular hypertrophy, carotid atherosclerosis), compared with the absence of preclinical cardiovascular disease (continuous grey line).
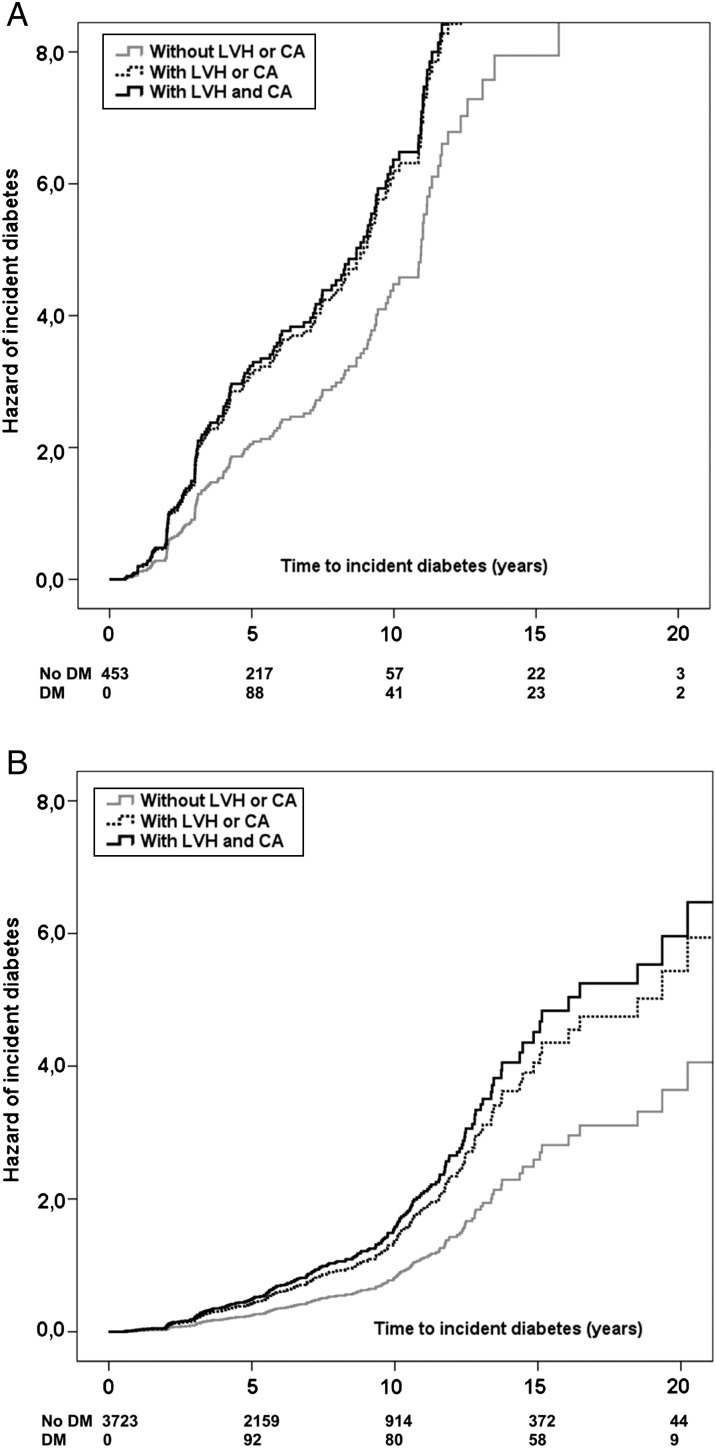
Patients with impaired fasting glucose were older and more frequently men, had higher BMI, triglycerides, longer duration of hypertension, lower HDL cholesterol, and more frequent MetS and family history of diabetes (all P < 0.01) than those with normal fasting glucose. In patients with impaired fasting glucose (n = 453), the presence of one of the markers of preclinical CVD was associated with more than 1.6-fold greater risk of incident diabetes and the presence of both markers increased the risk to 1.7 folds (both P < 0.001, Table 4; Figure 3A). In patients with normal fasting glucose, incident diabetes was associated with more than 1.7-fold greater risk of incident diabetes in patients with either LVH or CA and more than two-fold in patients with both LVH and CA (both P < 0.001, Table 4; Figure 3B). No interaction was found between the presence of one or two markers of preclinical CV and BMI, MetS, or fasting plasma glucose at the first available visit, after adjusting for age, family history of diabetes, heart rate, duration of hypertension, number of anti-hypertensive meds, and use of CCB or β-blockers.
Figure 3.
Hazard of incident diabetes in subgroup with (A) or without impaired fasting glucose (B) according to the initial presence of one (dotted line) or both markers (continuous black line) of preclinical cardiovascular disease (left ventricular hypertrophy, carotid atherosclerosis), compared with the absence of preclinical cardiovascular disease (continuous grey line).
Finally, we compared the risk of incident diabetes in patients with different follow-up period (1, 2, and 5 years). The odds of diabetes referred to initial preclinical CVD increased with longer period of follow-up, but was already evident at a 2-year follow-up (Table 5).
Table 5.
Risk of incident diabetes in patients with different follow-up period (1, 2, and 5 years) with the initial presence of one or both markers of preclinical cardiovascular disease
| P≤ | OR | CI 95.0% OR |
||||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| No LVH (n = 3040) | LVH (n = 1136) | |||||
| Incident diabetes after 1 year follow-up | 8 (0.3%) | 7 (0.6%) | 0.14 | 1.00 | 1.00 | 1.01 |
| Incident diabetes after 2 years follow-up | 23 (0.8%) | 21 (1.8%) | 0.003 | 1.01 | 1.00 | 1.02 |
| Incident diabetes after 5 years follow-up | 106 (3.6%) | 74 (6.5%) | 0.0001 | 1.03 | 1.01 | 1.05 |
| No CA (n = 2628) | CA (n = 1548) | |||||
| Incident diabetes after 1 year follow-up | 6 (0.2%) | 9 (0.6%) | 0.104 | 1.00 | 1.00 | 1.01 |
| Incident diabetes after 2 years follow-up | 21 (0.8%) | 23 (1.5%) | 0.041 | 1.01 | 1.00 | 1.01 |
| Incident diabetes after 5 years follow-up | 92 (3.5%) | 88 (5.7%) | 0.001 | 1.02 | 1.01 | 1.04 |
| No LVH+CA (n = 3625) | LVH+CA (n = 551) | |||||
| Incident diabetes after 1 year follow-up | 10 (0.3%) | 5 (0.9%) | 0.038 | 1.01 | 1.00 | 1.01 |
| Incident diabetes after 2 years follow-up | 33 (0.9%) | 11 (2.0%) | 0.039 | 1.01 | 1.00 | 1.02 |
| Incident diabetes after 5 years follow-up | 144 (4.0%) | 36 (6.5%) | 0.009 | 1.03 | 1.00 | 1.05 |
Discussion
This study indicates for the first time that the presence of hypertensive target organ damage (preclinical CVD), specifically LVH or CA or the combination of both, significantly increases the risk of future development of diabetes, independent of metabolic profile, BP, antihypertensive treatment and MetS, in a large outpatient-based cohort of clinically healthy, treated, non-diabetic hypertensive patients. This finding could be confirmed in the subgroups of non-obese patients, whereas in obese subjects, only the combination of both markers could add to the risk of diabetes. The explanation of the difference between non-obese and obese participants relies in the higher absolute risk of diabetes in the presence of obesity, which also tracks significant preclinical CVD, making reasonable that only the combination of both markers adds to the risk of incident diabetes, already high in the condition of obesity. In the non-obese subpopulation, the exposure is substantially lower and, therefore, the condition of preclinical CVD can better modulate risk, according to severity.
Compared with the hazard of incident diabetes in the Cox model including single components of MetS, the risk related to preclinical CVD increased when MetS was accounted for in the Cox regression, suggesting that hypertensive target organ damage is particularly discriminating when MetS is present. This finding is consistent with the evidence that MetS significantly increases CV risk, independently of the effect of its single components.15
Similar to what has been already suggested regarding the temporal relation between hypertension and LV hypertrophy,21,22 our results indicate that the traditional assumption that diabetes precedes preclinical CVD might be revised.2,3
In particular, diabetes is reported to be associated with greater LV mass, more concentric LV geometry, and lower myocardial function.2 These associations are also independent of potential confounders including age, sex, MetS, initial fasting glucose, and hypertension.23 The common vision about the temporal relations between these abnormalities and diabetes was that diabetes is a cause or a concurrent cause of them. Similarly, both impaired fasting glucose and diabetes have been demonstrated to be independently associated with increased carotid IMT, a subclinical measure of atherosclerosis, independently of several potentially important metabolic and coagulation factors.24 Similar to the temporal relation found with LVH, also CA could be demonstrated to precede diabetes, a finding contributing to overlook to scenarios different from the traditional cause–effect relation. The association between preclinical CVD and incident diabetes was not inflated by the presence in the study population of patients with impaired fasting glucose, as it could also be confirmed in the subpopulation without impaired fasting glucose, using the most conservative approach proposed by the ATPIII in the 2002.25
Our findings may be conciliated with the evidence that endothelial dysfunction precedes and predicts incident diabetes, to raise the hypothesis that vascular disease precedes the β-cell failure, which signs the shift from a condition of insulin resistance to diabetes.26 Endothelial dysfunction and impaired nitric oxide-mediated vasodilatation have also been suggested to directly lead to reduced insulin delivery to skeletal muscles, resulting in peripheral insulin resistance and hyperglycaemia.27
The association of insulin resistance with arterial hypertension28 further suggests that a vascular impairment might precede development of diabetes. The ability of arterial hypertension to predict incident diabetes8 also confirms that the vascular changes associated with diabetes cannot be simply considered as a consequence of the disease. These findings are eventually reinforced by the evidence that poor therapeutic control of BP further increases the risk of incident diabetes in hypertensive subjects.7 Considering this evidence, it is not surprising that we found that conditions of preclinical CVD, such as LVH and CA, precede and predict incident diabetes.
This scenario is also consistent with the evidence that most vascular damage associated with diabetes seems to be related to the coexisting MetS, more than to diabetes itself.29,30 Taken together with our findings, this evidence also suggests that the potent atherogenic effect of insulin resistance might be part of the biological mechanism yielding β-cell failure. Under this scenario, diabetes might be at least in part a consequence more than a cause of vascular impairment.
Microvascular disease has been hypothesized as a possible pathogenic factor in development of diabetes.31 This hypothesis is largely based on observations of microvascular abnormalities, such as arteriolar narrowing and impaired microvascular blood flow in the skin and skeletal muscles of persons with, or at high risk of, diabetes (e.g. those with impaired glucose tolerance and a family history of diabetes).32 Retinal arteriolar narrowing and hypertension have been associated with incident diabetes, independent of known risk factors. Parallel pancreatic microvascular disease might also precede clinical manifestation of diabetes.33 The hypothesis that diabetes might be the consequence of pancreatic microvascular dysfunction and ischaemia has been raised,34 but never proven. However, this hypothesis is supported by the experimental evidence of massive necrosis (but not apoptosis) associated with development of diabetes in diabetes-prone rats.35
Limitations
We need to highlight some limitations of this study. First of all, this analysis has been performed in an observational registry, with the potential limitation related to this type of data repository.36 As a consequence, the frequency of follow-up visits was not standardized. However, we show that the number of visit/year was not different between patients developing or not developing diabetes. In addition, follow-up time was very variable, ranging from 1 to more than 20 years. However, sub-analyses performed at fixed duration of follow-up (1, 2, 5 years) could exclude a substantial bias related to the wide range of follow-up (Table 5), being the difference in incidence already significant after 2 years. Although the number of patients lost to follow-up is very low in the Campania Salute network (<9%), the possibility of a bias related to patients lost to follow-up cannot be excluded with certainty. β-Blockers were more used in patients developing diabetes than in those without incident diabetes. However, the proportion of patients taking β-blockers was not statistically different in the presence or absence of markers of preclinical CVD, making unlikely a confounding effect, which was in fact excluded in the Cox models.
Finally, direct assessments of endothelial function, β-cell function, or sympathetic activity could have helped substantially the understanding of our findings and should track future research.
Conclusions
We provide first evidence that initial LVH and CA are significant predictors of new onset diabetes in a large population of treated, relatively healthy hypertensive patients, independently of initial metabolic profile, anti-hypertensive therapy, and other significant covariates. This temporal sequence is likely to be attributable to risk factors both affecting CV system and yielding to diabetes, for which LVH and CA represent a more severe and advanced stage, but a vascular origin of diabetes cannot be excluded.
There are important implications of these findings for primary CV prevention, because they help identifying hypertensive patients at high risk of incident diabetes, a condition that would enormously increase CV risk in the setting of arterial hypertension. The possibility of refining identification of hypertensive patients at high risk to develop diabetes, by pooling ultrasound with clinical information, increases the chance to target individuals who might benefit from more aggressive pharmacological management, even independently of BP control, to reduce their vascular burden. Further studies are warranted to evaluate mechanisms explaining the reverse causation suggested by our findings.
Authors’ contributions
R.I., G.d.S., and N.D.L. conceived the study, made the analysis, and wrote the manuscript. V.T. and R.G. gave substantial help in data management, contributed to the analysis, and edited the manuscript. E.G., B.T., and O.V. gave substantial conceptual help in writing, editing, and finalizing the manuscript. R.I. and G.d.S. edited the final version of the manuscript. B.T. and G.d.S. gave final approval for submission. The guarantors of the study are G.d.S., N.D.L., and B.T.
Funding
This work has been supported in part by grant AIFA (Italian Agency for Drugs): AIFA/FARM5STRH9.
Conflict of interest: none declared.
Footnotes
See page 3395 for the editorial comment on this article (doi:10.1093/eurheartj/eht365)
References
- 1.Devereux RB, Alderman MH. Role of preclinical cardiovascular disease in the evolution from risk factor exposure to development of morbid events. Circulation. 1993;88(4 Pt 1):1444–1455. doi: 10.1161/01.cir.88.4.1444. [DOI] [PubMed] [Google Scholar]
- 2.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 3.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68:85–89. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 4.Danaei G, Lawes CM, Vander Hoorn S, Murray CJ, Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–1659. doi: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 5.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-year cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 6.Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T, BraunWald E, Jaffe AS The MILIS Study Group. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol. 1989;14:49–57. doi: 10.1016/0735-1097(89)90053-3. [DOI] [PubMed] [Google Scholar]
- 7.Izzo R, de Simone G, Chinali M, Iaccarino G, Trimarco V, Rozza F, Giudice R, Trimarco B, De Luca N. Insufficient control of blood pressure and incident diabetes. Diabetes Care. 2009;32:845–850. doi: 10.2337/dc08-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Atherosclerosis Risk in Communities Study. N Engl J Med. 2000;342:905–912. doi: 10.1056/NEJM200003303421301. [DOI] [PubMed] [Google Scholar]
- 9.de Simone G, Devereux RB, Roman MJ, Alderman MH, Laragh JH. Relation of obesity and gender to left ventricular hypertrophy in normotensive and hypertensive adults. Hypertension. 1994;23:600–606. doi: 10.1161/01.hyp.23.5.600. [DOI] [PubMed] [Google Scholar]
- 10.de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens. 2002;20:323–331. doi: 10.1097/00004872-200202000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Izzo R, de Simone G, Devereux RB, Giudice R, De Marco M, Cimmino CS, Vasta A, De Luca N, Trimarco B. Initial left-ventricular mass predicts probability of uncontrolled blood pressure in arterial hypertension. J Hypertens. 2011;29:803–808. doi: 10.1097/HJH.0b013e328343ce32. [DOI] [PubMed] [Google Scholar]
- 12.De Luca N, Izzo R, Iaccarino G, Malini PL, Morisco C, Rozza F, Iovino GL, Rao MAE, Bodenizza C, Lanni F, Guerrera L, Arcucci O, Trimarco B. The use of a telematic connection for the follow-up of hypertensive patients improves the cardiovascular prognosis. J Hypertens. 2005;23:1417–1423. doi: 10.1097/01.hjh.0000173526.65555.55. [DOI] [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Schillaci G, Pirro M, Vaudo G, Gemelli F, Marchesi S, Porcellati C, Mannarino E. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol. 2004;43:1817–1822. doi: 10.1016/j.jacc.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 15.de Simone G, Olsen MH, Wachtell K, Hille DA, Dahlof B, Ibsen H, Kjeldsen SE, Lyle PA, Devereux RB. Clusters of metabolic risk factors predict cardiovascular events in hypertension with target-organ damage: the LIFE study. J Hum Hypertens. 2007;21:625–632. doi: 10.1038/sj.jhh.1002203. [DOI] [PubMed] [Google Scholar]
- 16.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 19.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 20.Lembo G, De Luca N, Battagli C, Iovino G, Aretini A, Musicco M, Frati G, Pompeo F, Vecchione C, Trimarco B. A common variant of endothelial nitric oxide synthase (Glu298Asp) is an independent risk factor for carotid atherosclerosis. Stroke. 2001;32:735–740. doi: 10.1161/01.str.32.3.735. [DOI] [PubMed] [Google Scholar]
- 21.de Simone G, Devereux RB, Roman MJ, Schlussel Y, Alderman MH, Laragh JH. Echocardiographic left ventricular mass and electrolyte intake predict arterial hypertension. Ann Intern Med. 1991;114:202–209. doi: 10.7326/0003-4819-114-3-202. [DOI] [PubMed] [Google Scholar]
- 22.de Simone G, Devereux RB, Chinali M, Roman MJ, Welty TK, Lee ET, Howard BV. Left ventricular mass and incident hypertension in individuals with initial optimal blood pressure: the Strong Heart Study. J Hypertens. 2008;26:1868–1874. doi: 10.1097/HJH.0b013e3283050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman E, Shemesh J, Shamiss A, Thaler M, Carroll J, Rosenthal T. Left ventricular mass in diabetes-hypertension. Arch Intern Med. 1992;152:1001–1004. [PubMed] [Google Scholar]
- 24.Wagenknecht LE, D'Agostino R, Jr, Savage PJ, O'Leary DH, Saad MF, Haffner SM. Duration of diabetes and carotid wall thickness. The Insulin Resistance Atherosclerosis Study (IRAS) Stroke. 1997;28:999–1005. doi: 10.1161/01.str.28.5.999. [DOI] [PubMed] [Google Scholar]
- 25.Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 26.Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev. 1998;19:477–490. doi: 10.1210/edrv.19.4.0336. [DOI] [PubMed] [Google Scholar]
- 27.Balletshofer BM, Rittig K, Enderle MD, Volk A, Maerker E, Jacob S, Matthaei S, Rett K, Haring HU. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101:1780–1784. doi: 10.1161/01.cir.101.15.1780. [DOI] [PubMed] [Google Scholar]
- 28.Ferrannini E, Natali A. Insulin resistance and hypertension: connections with sodium metabolism. Am J Kidney Dis. 1993;21(5 Suppl 2):37–42. doi: 10.1016/s0272-6386(12)70253-6. [DOI] [PubMed] [Google Scholar]
- 29.Alexander CM, Landsman PB, Teutsch SM, Haffner SM. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 30.Howard BV, Best LG, Galloway JM, Howard WJ, Jones K, Lee ET, Ratner RE, Resnick HE, Devereux RB. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. 2006;29:391–397. doi: 10.2337/diacare.29.02.06.dc05-1299. [DOI] [PubMed] [Google Scholar]
- 31.Tooke JE. Microvascular function in human diabetes. A physiological perspective. Diabetes. 1995;44:721–726. doi: 10.2337/diab.44.7.721. [DOI] [PubMed] [Google Scholar]
- 32.Jaap AJ, Shore AC, Tooke JE. Relationship of insulin resistance to microvascular dysfunction in subjects with fasting hyperglycaemia. Diabetologia. 1997;40:238–243. doi: 10.1007/s001250050669. [DOI] [PubMed] [Google Scholar]
- 33.Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165:1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- 34.Tal MG. Type 2 diabetes: microvascular ischemia of pancreatic islets? Med Hypotheses. 2009;73:357–358. doi: 10.1016/j.mehy.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Fehsel K, Kolb-Bachofen V, Kroncke KD. Necrosis is the predominant type of islet cell death during development of insulin-dependent diabetes mellitus in BB rats. Lab Invest. 2003;83:549–559. doi: 10.1097/01.lab.0000063927.68605.ff. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell PM. External validity of randomised controlled trials: ‘to whom do the results of this trial apply? Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]