Abstract
Objective
To evaluate the effectiveness of maternal combination antiretroviral prophylaxis for prevention of mother-to-child transmission of HIV (PMTCT) in a program setting
Design
Prospective cohort study
Setting
Nine primary care clinics in rural Zambia
Participants
284 HIV-infected pregnant women at ≥28 weeks gestation initiating PMTCT services between April 2009 and January 2011 and their newborn infants
Intervention
In four “intervention” sites, PMTCT comprised universal combination antiretroviral prophylaxis (i.e., irrespective of CD4 count) from pregnancy until the cessation of breastfeeding. In five “control” sites, women received antenatal zidovudine and peripartum nevirapine, the standard of care at the time. Prophylaxis during breastfeeding was not available in control sites.
Main outcome measure
Cumulative infant HIV infection and death at 12 months postpartum
Results
At 12 month postpartum, 1 of 104 (1.0%) infants born to mothers at the intervention sites were HIV-infected, compared to 14 of 116 (12.1%) receiving care in the control sites (relative risk [RR]: 12.6, 95%CI: 2.2-73.1; P=0.005). When we considered the composite outcome of HIV infection or death, similar trends were observed in the overall study population (RR: 3.4, 95%CI: 1.6-7.6; P=0.002) and in a sub-analysis of women with CD4 >350 cells/μL (RR: 3.2; 95%CI: 1.1-9.6; P=0.04).
Conclusions
When compared to PMTCT services based on antenatal zidovudine and peripartum nevirapine, the provision of maternal combination prophylaxis imparted measurable health benefits to HIV-exposed infants. Implementation research is needed to further tailor and optimize these strategies for similar field settings.
Keywords: HIV, prevention of mother to child transmission, antiretroviral therapy, breastfeeding, Zambia
INTRODUCTION
Antiretroviral therapy (ART) during pregnancy improves maternal survival and reduces infant HIV infection [1]. For those who do not yet require long-term treatment, combination antiretroviral prophylaxis (ART prophylaxis) can also effectively reduce mother-to-child HIV transmission in the antenatal, intrapartum, and postpartum periods [2-9]. In the 2010 World Health Organization (WHO) guidelines, maternal ART prophylaxis was included as one of two recommended options for the prevention of mother-to-child HIV transmission (PMTCT), as the so-called “Option B” approach [10]. By 2012, however, the WHO clearly favored maternal three-drug antiretroviral combinations for a number of reasons: simplification of regimens, integration with comprehensive HIV services, reductions in maternal morbidity and mortality, and potential for preventing horizontal HIV transmission to serodiscordant partners [11].
The WHO’s Option B strategy for PMTCT calls for initiation of maternal combination antiretroviral regimens early in pregnancy (as early as 14 weeks), with continuation until breastfeeding stops. All infants receive six weeks of either zidovudine (ZDV) or nevirapine (NVP). Women who require ART for maternal health continue lifelong treatment following breastfeeding cessation. Despite its introduction in 2010, there are few published reports about the field implementation of Option B. In a “real world” context, several factors could affect its public health impact. For example, the successful uptake of the intervention is critical; yet, studies of the “PMTCT cascade” demonstrate attrition at numerous time points, even for relatively simple regimens such as single-dose nevirapine [12]. For those who successfully initiate prophylaxis or treatment, questions around program retention and long-term drug adherence persist [13-16]. Studies focusing on the field effectiveness of Option B are urgently needed, particularly as this strategy is scaled up across sub-Saharan Africa. Our objective was to evaluate the effectiveness of combination ART prophylaxis for PMTCT in a public health setting in rural Zambia.
METHODS
Setting
The Kafue District is a rural area approximately 40 kilometers south of the Zambia’s capital city of Lusaka. The district covers 9,400 square-kilometers and, in 2010, had a population of 242,754 [17]. Over the past three years, the antenatal HIV prevalence in the Kafue District reported by the Ministry of Health was approximately 17% (personal communication, M Mzumara). The public health sector in the district comprises a network of 14 primary care clinics – all with outpatient antenatal services – with more complicated patients referred to a single district hospital.
From 2007 to 2010, PMTCT in the Kafue District followed the Zambian Ministry of Health guidelines [18]. Opt-out HIV counseling and testing was provided within antenatal clinics and diagnosis was established using a rapid antibody test algorithm. HIV-infected women were referred to 1 of 4 HIV care and treatment units in the district where they underwent clinical and immunologic screening for ART eligibility. Women who met eligibility criteria for HIV treatment — CD4 cell count ≤350 cells/μL or WHO clinical stage 3 or 4 — were prescribed ART. Those who did not meet these criteria were prescribed twice-daily ZDV from 28 weeks of gestation onward. At the time of labor, these women were instructed to ingest a single dose of NVP, typically dispensed at an earlier visit, followed by a 1-week zidovudine-lamivudine (ZDV-3TC) “tail.” HIV-exposed newborns received a single-dose of NVP within the first 72 hours, followed by seven days of ZDV prophylaxis. Mothers were encouraged to exclusively breastfeed for 6 months and then provide complementary feeds with continued breastfeeding until 24 months if they remained HIV negative. In accordance with national guidelines, additional maternal or infant antiretroviral prophylaxis was not provided during breastfeeding. For our primary comparisons (see below), we describe this PMTCT protocol as the “standard of care” and individuals receiving this care as the “control group.”
Pilot PMTCT program in Kafue District
In April 2009, the Zambia Ministry of Health implemented a four-facility demonstration project in Kafue District offering ART prophylaxis to all HIV-infected pregnant women irrespective of clinical stage or CD4 cell count. Our group at the Centre for Infectious Disease Research in Zambia (CIDRZ) provided technical assistance and support in its design and oversight. We developed an integrated clinic model where pregnant women diagnosed with HIV were provided CD4 screening and WHO clinical staging within the antenatal clinic setting. Those who met criteria for HIV treatment were immediately started on ART and advised to continue it for life. Women who did not meet criteria for HIV treatment were offered ART prophylaxis starting at 28 weeks of gestation, with a plan to stop the regimen at the cessation of breastfeeding. A nucleoside reverse transcriptase inhibitor backbone of ZDV and 3TC was preferentially prescribed. NVP was prescribed as the “third drug” when the woman’s CD4 was ≤250 cells/μL; when the CD4 count was above this threshold, we dispensed efavirenz (EFV) or lopinavir-ritonavir (LPV/r) [19,20]. In cases of anemia, ZDV was substituted with tenofovir (TDF), abacavir (ABC), or stavudine (D4T) [21]. Infant feeding counseling corresponded with the standard of care: exclusive breast-feeding until 6 months with the addition of complementary feeding until 24 months. For our primary comparisons, we describe the patients receiving these services as the “intervention group.”
Study activities
The pilot program presented a unique opportunity to evaluate the field effectiveness of combination ART prophylaxis for PMTCT in a public health setting. Using a quasi-experimental design, we enrolled a cohort of pregnant women seeking care across the 4 pilot sites into the intervention group. To provide a contemporaneous control group, we also enrolled pregnant women from 5 other government clinics providing PMTCT according to the prior standard of care. (The fifth control site was added due to slow enrollments at one rural health facilities.) Across all 9 sites, HIV-infected pregnant women who had been screened for ART eligibility were approached from 28 weeks gestation onward to participate. Gestational age was estimated based on reported last menstrual period. Ultrasound confirmation was not available due to the limited resources in these rural areas. The study was described and written informed consent obtained. Specific reasons for non-participation were not collected as part of routine clinical practice or by study staff; therefore, we were unable to include them in our final analyses.
All participants in both groups of this prospective cohort study received intensive clinical and laboratory monitoring. Following enrollment, they were scheduled for 2 week, 4 week, and 8 week visits. Clinical assessments were conducted immediately after delivery and at 6 weeks, 3 months, 6 months, and 12 months postpartum. We tested infants for HIV infection at birth, 6 weeks, 6 months, and 12 months of life with the Roche Amplicor version 1.5 DNA PCR assay with manual extraction (Roche Diagnostics, Branchburg, NJ, USA). We required a second positive specimen from a separate blood draw to confirm infant infection. We evaluated medication adherence in the intervention group using the medication possession ratio, a measure of the cumulative number of days late for pharmacy refills divided by total days on therapy [22]. Participants with missed visits were followed-up at home by community health workers and reminded about study visits. We sought to enroll 320 HIV-infected pregnant women: 160 from intervention sites and 160 from control sites. Because of recognized differences in patient volume at participating facilities, enrollment at any single site was capped at 40 participants. This sample size was based on an expected decrease in HIV transmission from 18% to 4% [23,24], assuming 80% power and a 0.05 two-sided significance level. We used the formula published by Hayes and Bennett [25] with an estimated coefficient of variation of 0.6.
Statistical analysis
Our primary outcomes were (1) infant HIV infection and (2) infant HIV infection or death, measured at 6 weeks, 6 months, and 12 months postpartum [26]. The primary study population included all live born infants. Infants categorized as lost to follow-up were censored at the time of last visit and thus did not contribute to later point estimates for HIV infection or death.
We compared baseline demographic and clinical characteristics between groups using Fisher’s exact test for categorical variables and non-parametric Wilcoxon rank sum tests for continuous variables. To measure differences in breastfeeding and follow-up losses, important potential confounders to our analysis, we conducted Kaplan-Meier analyses and adjusted Cox proportional regression. Age, CD4 count, WHO clinical stage, renal function, liver function, parity, time on ART prophylaxis during pregnancy, and gestational age were included in the multivariate Cox models. For our primary analyses, we used a log-linked binomial regression model to estimate the relative risk (RR) of our primary outcomes between the intervention and control groups using generalized estimating equations with an exchangeable correlation structure to account for clustering at the site level. We calculated 95% confidence intervals using exact binomial methods. Because of differences in our comparison groups due to early ART referrals in the control sites, we conducted a secondary analysis restricted to infants whose mothers had a CD4 cell count of >350 cells/μL. We performed all analyses with SAS version 9.1.3 (SAS Institute, Cary, NC). The study was approved by the Biomedical Research Ethics Committee at the University of Zambia (Lusaka, Zambia) and the Institutional Review Boards at the University of North Carolina at Chapel Hill (Chapel Hill, North Carolina, USA) and the University of Alabama at Birmingham (Birmingham, Alabama, USA).
RESULTS
We recruited participants from April 2009 to January 2011. In the four intervention sites, 2,469 pregnant women agreed to HIV testing and 451 (18.3%) were diagnosed with HIV. Among those identified as HIV-infected, 218 (48.3%) initiated ART prophylaxis; of these, 143 (65.6%) enrolled in this study. In the five control sites, 3,158 pregnant women agreed to HIV testing, of which 513 (16.2%) were diagnosed with HIV. All women were offered PMTCT according to the standard of care and 369 (71.9%) received at least one dispensation of antenatal ZDV. Of these 141 (38.2%) enrolled in the study.
Of the 284 total participants, 4 (1.4%) withdrew from the study and 10 (3.5%) were lost to follow-up prior to delivery (Figure 1). At delivery, 7 (2.5%; 95% CI 1.0-5.3%) stillbirths were documented. Our analysis cohort comprised 263 live born infants for the primary analysis: 129 in the intervention group and 134 in the control group. At 12 months, 104 mother-infant pairs in the intervention group and 116 mother-infant pairs in the control group remained active in follow-up including those who had achieved a study outcome. There was 1 (0.7%) maternal death in the control group. A few differences were noted between study groups at enrollment (Table 1). These were consistent when this analysis was limited to mother-infant pairs remaining active in follow-up at 12 months. CD4 at baseline was lower in the intervention group compared to those in the control (339 vs. 440 cells/μl, P<0.001). The median time on antiretroviral drugs during pregnancy was shorter in the intervention group (7.6 vs. 9.1 weeks; P<0.01). Among the 143 women starting ART for treatment or prophylaxis in the intervention group, the initial prescribed regimen was ZDV+3TC+EFV (n=74, 52.1%), ZDV+3TC+NVP (n=35, 24.7%), ZDV+3TC+LPV/r (n=14, 9.9%), D4T+3TC+EFV (n=9, 6.3%), D4T+3TC+NVP (n=9, 6.3%), and ABC+3TC+NVP (n=1, 0.7%). Of 104 women who remained active at 12 months in the intervention group, 100 (96.2%) had documented medication pick-up throughout this period. Among these women, 70 (70.0%) remained on the ART prophylaxis regimen they initiated at enrollment, 20 (20.0%) had undergone a single-drug substitution, and 10 (10.0%) had 2 drugs replaced with a fixed-dose combination.
Figure 1.
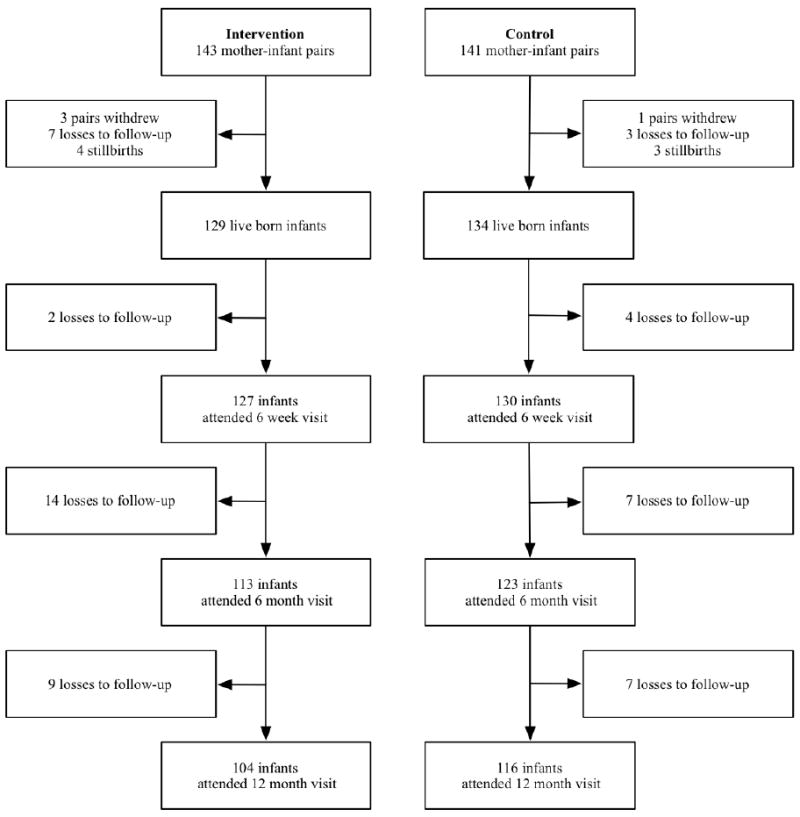
Cohort profile for enrolled mother-infant pairs across participating study sites in Kafue District, Zambia, April 2009 – January 2012. Intervention denotes women who received ART prophylaxis. Control indicates women who received ZDV monotherapy with peripartum nevirapine and ZDV-3TC tail.
Table 1.
Comparison by study arm of mother and infant characteristics for all participants and among those active at 12 months, Kafue District, Zambia, April 2009 – January 2012.
| Enrollment cohort | Mother-infant pairs active at 12 months | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intervention (n = 143) | Control (n = 141) | P | Intervention (n = 104) | Control (n = 116) | P | |
| Age, median years (IQR) | 27.1 (23.9, 32.5) | 27.1 (23.8, 31.5) | 0.49 | 29.2 (24.5, 33.6) | 27.5 (24.2, 31.9) | 0.18 |
| Education, n (%) | ||||||
| None | 26 (18.6%) | 40 (28.6%) | 0.13 | 20 (19.2%) | 29 (25.05%) | 0.59 |
| Primary | 46 (32.9%) | 44 (31.4%) | 34 (32.7%) | 36 (31.0%) | ||
| Secondary/Tertiary | 68 (48.6%) | 56 (40.0%) | 50 (48.1%) | 51 (44.0%) | ||
| Gravidity, median (IQR) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.41 | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.80 |
| Estimated gestational age at initiation, median weeks (IQR) | 31.4 (29.7, 33.3) | 30.3 (28.4, 32.1) | 0.01 | 31.4 (29.3, 33.3) | 30.1 (28.4, 32.0) | 0.01 |
| CD4 count, median cells/uL (IQR) | 339 (218, 464) | 440 (334, 585) | <0.001 | 345 (215, 469) | 433 (333, 560) | <0.001 |
| WHO stage, n (%) | ||||||
| 1 | 100 (81.3%) | 118 (91.5%) | 0.05 | 75 (79.8%) | 98 (91.6%) | 0.05 |
| 2 | 19 (15.4%) | 8 (6.2%) | 16 (17.0%) | 7 (6.5%) | ||
| 3 / 4 | 4 (3.3%) | 3 (2.3%) | 3 (3.2%) | 2 (1.9%) | ||
| BMI, median kg/m2 (IQR) | 24.2 (21.9, 26.4) | 24.0 (22.3, 26.1) | 0.92 | 24.2 (21.8, 26.3) | 23.8 (22.2, 25.8) | 0.81 |
| Hemoglobin, median g/dL (IQR) | 10.6 (9.7, 11.4) | 10.7 (9.7, 11.6) | 0.63 | 10.5 (9.7, 11.3) | 11.0 (10.0, 14.0) | 0.53 |
| ALT, median IU/L (IQR) | 12 (10, 15) | 11.0 (9, 14) | 0.25 | 12.0 (10.0, 15.5) | 11.0 (10.0, 14.0) | 0.20 |
| Creatinine, median umol/L (IQR) | 64 (58, 69) | 60 (57, 67) | 0.05 | 64 (59, 69) | 60 (58, 68) | 0.06 |
| Time on ART prophylaxis in pregnancy, median weeks (IQR) | 7.6 (5.0, 9.6) | 9.1 (6.3, 11.1) | 0.002 | 7.6 (5.1, 9.6) | 9.2 (6.5, 11.3) | 0.001 |
| Gestational age, median weeks (IQR) | 38.6 (36.6, 40.7) | 39.4 (37.2, 41.1) | 0.18 | 38.8 (36.3, 40.9) | 39.4 (37.1, 41.0) | 0.30 |
| Gestational age, n weeks (%) | ||||||
| < 34 | 9 (7.3%) | 13 (10.2%) | 0.13 | 9 (9.0%) | 10 (9.0%) | 0.45 |
| 34 to 37 | 29 (23.6%) | 18 (14.1%) | 22 (22.0%) | 17 (15.3%) | ||
| > 37 | 85 (69.1%) | 97 (75.8%) | 69 (69.0%) | 84 (75.7%) | ||
| Delivery location, n (%) | ||||||
| Health facility | 110 (82.7%) | 109 (79.6%) | 0.54 | 87 (83.7%) | 95 (81.9%) | 0.62 |
| Home | 23 (17.3%) | 28 (20.4%) | 17 (16.3%) | 21 (18.1%) | ||
| Stillbirth, n (%) | ||||||
| Yes | 4 (3.0%) | 3 (2.2%) | 0.72 | - | - | - |
| No | 129 (97.0%) | 134 (97.8%) | - | - | ||
| Infant ARV Received, n (%) | ||||||
| AZT/NVP | 107 (78.7%) | 119 (86.9%) | 0.20 | 93 (89.4%) | 107 (92.2%) | 0.49 |
| None | 22 (16.2%) | 15 (10.9%) | 11 (10.6%) | 9 (7.8%) | ||
| Unknown | 7 (5.1%) | 3 (2.2%) | 0 (0.0%) | 0 (0.0%) | ||
| Ever breastfed, n (%) | ||||||
| Yes | 129 (100.0%) | 133 (99.3%) | 1.00 | 104 (100.0%) | 115 (99.1%) | 0.49 |
| No | 0 (0.0%) | 1 (0.7%) | 0 (0.0%) | 1 (0.9%) | ||
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Known infant HIV status at delivery, n (%) | ||||||
| Yes | 116 (89.9%) | 119 (88.8%) | 0.84 | 97 (93.3%) | 106 (91.4%) | 1.00 |
| No | 13 (10.1%) | 15 (11.2%) | 7 (6.7%) | 10 (8.6%) | ||
All 129 women with live births in the intervention group and 133 of 134 (99.2%) women in the control group reported ever breastfeeding (P=1.00). Women in the control group had a higher risk of breastfeeding cessation prior to 12 months compared to women in the intervention group (AHR: 2.31; 95%CI: 1.50-3.54) (Figure 2A). In Kaplan-Meier analysis, 80.1% of women in the intervention group remained active in the study at 12 months, compared to 85.5% in the control group (P=0.13) (Figure 2B). Women in the control group may have been more likely to be retained in care (AHR: 1.56; 95%CI: 0.63–3.87), but this finding was not statistically significant. Data on adherence was captured for 103 (99.0%) of the intervention participants still active in the study at 12 months. Of these, 21 (20.4%) were found to have poor adherence to the ART prophylaxis (MPR <80%); 46 (44.7%) demonstrated sub-optimal adherence (80-94%); and 36 (34.9%) had optimal adherence (≥95%).
Figure 2.
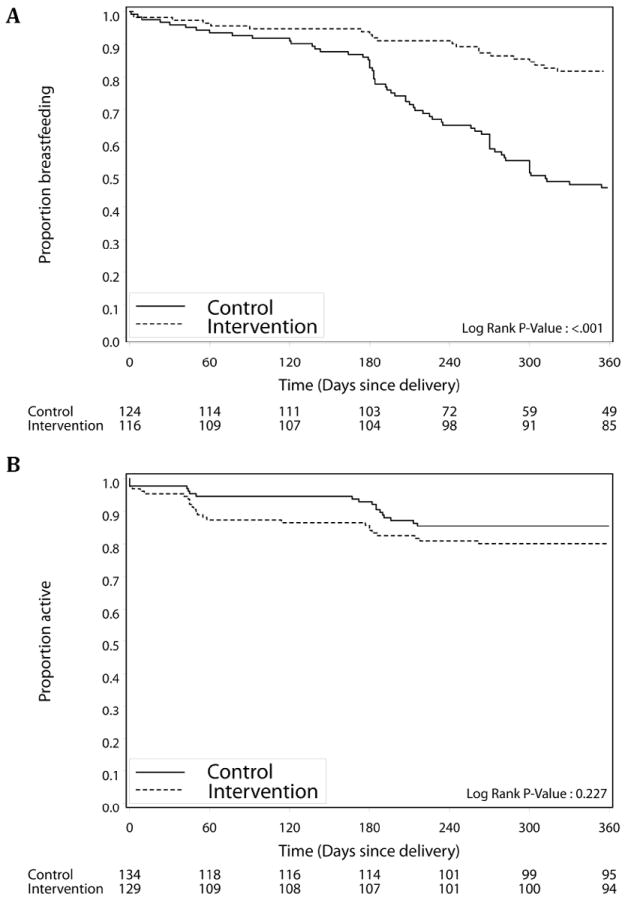
Kaplan-Meier estimates of time to (A) breastfeeding cessation and (B) loss to follow-up among live born infants of mothers in intervention sites (dashed line) and control sites (solid line) from birth to 12 months.
HIV infection was documented in 15 infants during longitudinal follow-up: 1 in the intervention group and 14 in the control group. The timing of HIV infection in the control group was as follows: at birth (n=1), <4 weeks (n=1), 4 weeks to <3 months (n=4), and 3 months to 12 months postpartum (n=8). The single case of infant HIV infection in the intervention group occurred at 2 weeks postpartum. Of the 15 HIV-positive infants, 13 (86.7%) had the diagnosis confirmed by a second PCR test and were referred for HIV treatment. One infant (6.7%) was lost to follow-up and one infant (6.7%) died before a confirmatory test could be performed. Cumulative risk for HIV infection was consistently higher in the control group when measured at 6 weeks, 6 months, and 12 months (Figure 3A). The risk of HIV infection at 12 months was 1.0% (1/104) in the intervention group and 12.1% (14/116) in the control group (RR: 12.6, 95%CI: 2.2-73.1; P=0.005). Similar trends were observed when the composite outcome of infant HIV infection or death was considered (RR: 3.4, 95%CI: 1.6-7.6; P=0.002; Figure 3B).
Figure 3.
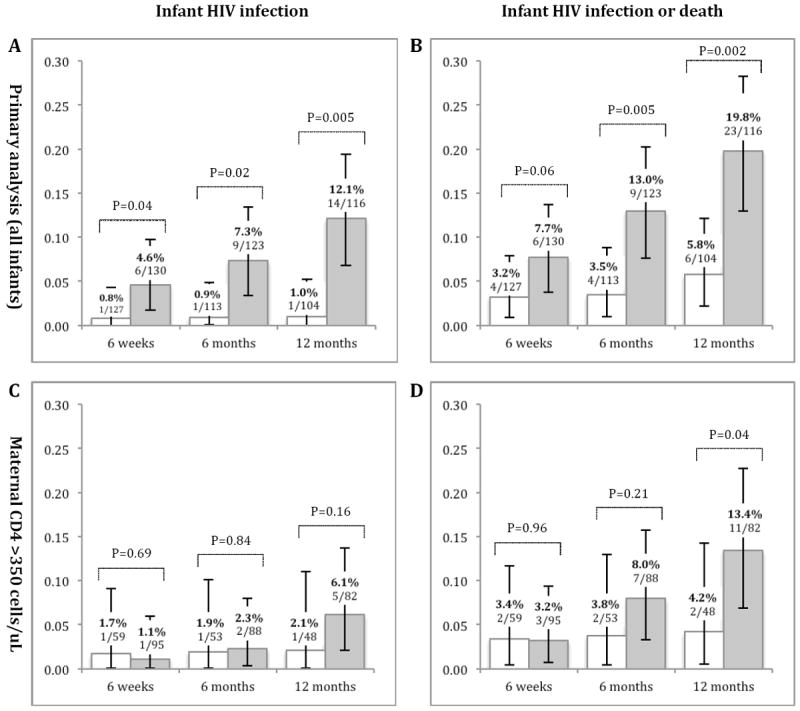
Risk of (A) infant HIV infection and (B) infant HIV infection or death among participants in the intervention group (white) and in the control group (gray), in the primary analysis at 6 weeks, 6 months, and 12 months. Analysis was based on infants active in study at given time point. Among infants born to women with CD4 count > 350 cells/μL at time of regimen initiation (C) infant HIV infection and (D) infant HIV infection or death at 6 weeks, 6 months, and 12 months. P-values denoted above bracket at each time point were calculated based on log-linked binomial regression model adjusted for clustering using generalized estimating equations. 95% CI were calculated using exact binomial methods.
Of the 263 live-born infants included in our primary analysis, 154 (58.6%) were born to mothers with CD4 count > 350 cells/μL at time of regimen initiation. Of these, 95 (61.7%) were in the control group and 59 (38.3%) in the intervention group. In a secondary analysis restricted to this sub-population, we were unable to detect differences in HIV infection by 12 months (RR: 2.9, 95%CI: 0.7–13.0; p=0.16; Figure 3C). However, we observed greater risk of HIV infection or death among individuals in the control group at 12 months (RR: 3.2; 95%CI: 1.1–9.6; P=0.04; Figure 3D).
DISCUSSION
Use of combination ART prophylaxis during pregnancy and breastfeeding was associated with a lower rate of infant HIV infection and death when compared to short-course antenatal ZDV and peripartum NVP. Similar trends were observed among infants whose mothers had CD4 cell counts >350 cells/μL. These measureable improvements in infant outcomes support the expansion of ART prophylaxis to all women irrespective of CD4 count, as is recommended in the WHO’s Option B PMTCT policy.
When our study was first designed and implemented in 2009, the standard of care for PMTCT in Zambia focused on the timely triage of HIV-infected pregnant women for HIV treatment. The goal was to urgently identify and initiate treatment for women eligible for ART. In accordance with WHO recommendations at the time, those who did not meet eligibility criteria were initiated on short-course zidovudine and peripartum NVP from 28 weeks gestation onward. Antiretroviral prophylaxis during breastfeeding – to either mother or infant – was not offered. In the ensuing years, however, the science of PMTCT evolved rapidly. Services similar to those provided in our four Kafue pilot sites have been introduced as policy across much of Africa [11,27]. In this report, we share our early programmatic experiences and demonstrate the clear benefits to infant health when services are “upgraded” from the previous standard of care (i.e., PMTCT as provided in our control sites) to the current standard of care (i.e., services provided at our intervention sites).
Several clinical trials have demonstrated the efficacy of ART prophylaxis during pregnancy and through breastfeeding [3-9]. Universally, these regimens have been shown to reduce mother-to-child HIV transmission; however, study design and primary endpoints have differed from trial to trial. Perhaps most similar to our study is the Kesho Bora study, a randomized controlled trial conducted in Kenya, Burkina Faso, and South Africa. Participants in Kesho Bora were randomized to receive either ART prophylaxis from 28 to 34 weeks gestation through breastfeeding or short-course antenatal ZDV and peripartum NVP. At 12 months postpartum, the risk of HIV infection or death was lower in the intervention group when compared to the control group (5.4% vs. 9.5%; P=0.029), findings that approximate those of our study.
A strength of our study was its focus on field effectiveness, which considered program attrition over time. However, this “real world” perspective also led to analytical challenges as well. For example, women who met immunologic criteria for HIV treatment in the control facilities were immediately referred to the nearest antiretroviral therapy clinic – typically the Kafue District Hospital – prior to 28 weeks gestation. Women in the intervention facilities were not referred in the same manner; in our integrated service model, they could access antiretroviral therapy on site. Because we did not include CD4 eligibility criteria for the study, these differences in patient management led to imbalances between the comparison populations. We attempted to account for these differences in our secondary analysis, which restricted the study population to infants whose mothers had CD4 counts >350 cells/μL. We were reassured to find that results in this sub-analysis were consistent with those of the larger study cohort.
We observed a greater than two-fold hazard for stopping breastfeeding before 12 months in the control group, even after adjusting for baseline participant characteristics. Although we used a core group of study nurses, many factors — including differences in counseling — could have contributed to this incongruity. Breastfeeding cessation may also have been influenced by the perception that ART prophylaxis during breastfeeding imparts additional protection against mother-to-child HIV transmission. If confirmed, this change in maternal infant feeding behavior could present an important collateral benefit of ART prophylaxis.
As part of our analysis, we sought to determine the extent of follow-up losses in our programmatic cohort. We found that at 12 months of age nearly one-fifth of infants had no study outcome and could not be traced. This high percentage is concerning, but in line with other reports. Myer and colleagues showed that 19% of women pregnant at initiation of ART were lost to follow-up after one year [16]. Similarly, Kaplan and colleagues showed 32% of pregnant women who initiated ART were lost to follow-up after three years [14]. Among women in the intervention group whose infants were retained in care, only 35% demonstrated optimal adherence to their three-drug regimen as measured by medication possession ratio. This is significantly lower than previous findings in non-pregnant adults on ART, but consistent with studies of pregnant women [15,22]. Evidence-based strategies are clearly needed to improve retention and adherence among pregnant and postpartum populations, particularly as Ministries of Health move to Option B or Option “B+” (i.e., initiation of lifelong antiretroviral therapy in pregnancy irrespective of CD4 cell count for PMTCT) [27]. Since most women in this population will be asymptomatic [10], there may be less motivation to adhere to long-term treatment once the risk of HIV transmission to the infant has passed.
We note several limitations to the current analysis. First, we were unable gather routine data about reasons behind non-participation in our cohort study. This may have led to unmeasured selection biases between the groups. Second, since neither the participants nor the facilities providing the intervention were randomized, our study data may contain systematic biases. There were several differences between our intervention and comparison groups. Because of the small number of events, particularly in the intervention group, we were unable to perform multivariable analyses. However, the most important potential confounder – maternal CD4 cell count – was examined in greater detail in our secondary analysis through stratification. Third, owing to the swiftly advancing field of PMTCT, the standard of care provided at our control sites is no longer the recommended WHO standard. The lack of antiretroviral coverage during the breastfeeding period, where the majority of infant HIV infections occurred, is most worrisome. Our study was designed to demonstrate the incremental health gains made possible by the implementation of the WHO’s Option B for PMTCT in a field setting.
In summary, our findings demonstrate a significant benefit of maternal ART prophylaxis to HIV-exposed infants. However, patient attrition and poor adherence among those receiving combination regimens in the postpartum period could diminish regimen effectiveness and carry long-term consequences for women on treatment. As country programs seek to implement antiretroviral regimens of longer duration, particularly after delivery, investments are urgently needed to improve timely access to care and retention.
Acknowledgments
We thank Eleanor Turnbull for her contributions to study implementation and oversight. We acknowledge the Zambian Ministry of Health for its ongoing support for implementation research in the field of PMTCT. We also thank participants in Kafue District and the study team of district nurses, data associates, and clinical officers.
Funding source: The work reported herein was funded by Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061). Additional trainee support was provided by the National Institutes of Health through the International Clinical Research Scholars Program at Vanderbilt University (R24 TW007988). Funding agencies played no role in the study design, data collection, data analysis, or manuscript writing.
Footnotes
Author Contributions: Dr. Chi and Mr. Gartland had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
BHC, JSAS, EMS, MB, MKL, and NTC conceived and developed the study design. MGG, SNM, and MKL were responsible for data collection and management. Data analysis and interpretation was performed by all authors. Statistical analysis was undertaken by MSL, PM, BHC, and MGG. Drafting or writing of this manuscript was performed by MGG, BHC, MSL, and PM. All authors contributed to the critical revision of this manuscript. Study supervision was performed by MGG, BHC, SNM, MB, and MKL.
The authors have no conflicts of interest to declare.
References
- 1.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to- child HIV transmission. AIDS. 2010;24:1374–1377. [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. [September 8, 2012];Global Plan Towards The Elimination of New HIV Infections Among Children by 2015 And Keeping Their Mothers Alive. 2011 :1–48. at http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110609_JC2137_Global-Plan-Elimination-HIV-Children_en.pdf.
- 3.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, et al. Triple-Antiretroviral Prophylaxis to Prevent Mother-To-Child HIV Transmission through Breastfeeding—The Kisumu Breastfeeding Study, Kenya: A Clinical Trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or Infant Antiretroviral Drugs to Reduce HIV-1 Transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peltier CA, Ndayisaba GF, Lepage P, van Griensven J, Leroy V, Pharm CO, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS. 2009;23:2415–2423. doi: 10.1097/QAD.0b013e32832ec20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 8.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(Suppl 4):S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 9.Kesho Bora Study Group. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. The Lancet Infectious Diseases. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Guidelines on care, treatment and support for women living with HIV/AIDS and their children in resource-constrained settings. Geneva: World Health Organization; 2010. [10 January 2013]. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [Google Scholar]
- 11.World Health Organization. [September 8, 2012];Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infections in Infants: Executive Summary. 2012 :1–7. at: http://www.who.int/hiv/PMTCT_update.pdf. [PubMed]
- 12.Stringer EM, Ekouevi DK, Coetzee D, Tih PM, Creek TL, Stinson K, et al. Coverage of nevirapine-based services to prevent mother-to-child HIV transmission in 4 African countries. JAMA. 2010;304:293–302. doi: 10.1001/jama.2010.990. [DOI] [PubMed] [Google Scholar]
- 13.Clouse K, Maskew M, Fox MP, Bassett J, Larson B. Delayed diagnosis of HIV and high rates of loss to follow-up among pregnant women receiving antenatal services at a primary healthcare clinic in Johannesburg, South Africa. Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections 2012; Seattle, WA. 2012. pp. 1–1. Abstract 1004. [Google Scholar]
- 14.Kaplan R, Orrell C, Zwane E, Bekker L-G, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22:1679–1681. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-, middle and high income countries. AIDS. 2012;26:2039–2052. doi: 10.1097/QAD.0b013e328359590f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myer L, Cornell M, Fox MP, Garone D, Wood R, Prozesky HW, et al. Loss to Follow-up and Mortality among Pregnant and Non-pregnant Women Initiating ART: South Africa. Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. pp. 1–1. Paper 22. [Google Scholar]
- 17.Republic of Zambia Central Statistical Office. [10 January 2013];Zambia 2010 Census of Population and Housing: Preliminary Population Figures. 2011 :1–71. http://unstats.un.org/unsd/demographic/sources/census/2010_phc/Zambia/PreliminaryReport.pdf.
- 18.Ministry of Health Zambia. [10 January 2013];National Protocol Guidelines Integrated Prevention of Mother-to-child Transmission of HIV/AIDS. 2007 :1–51. http://www.aidstar-one.com/sites/default/files/treatment_documents/hiv_treatment_guidelines_zambia_pmtct_2007.pdf.
- 19.Hitti J, Frenkel LM, Stek AM, Nachman SA, Baker D, Gonzalez-Garcia A, et al. Maternal toxicity with continuous nevirapine in pregnancy: results from PACTG 1022. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2004;36:772–776. doi: 10.1097/00126334-200407010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Jamisse L, Balkus J, Hitti J, Gloyd S, Manuel R, Osman N, et al. Antiretroviral-associated toxicity among HIV-1-seropositive pregnant women in Mozambique receiving nevirapine-based regimens. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;44:371–376. doi: 10.1097/QAI.0b013e318032bbee. [DOI] [PubMed] [Google Scholar]
- 21.Ssali F, Stöhr W, Munderi P, Reid A, Walker AS, Gibb DM, et al. Prevalence, incidence and predictors of severe anaemia with zidovudine-containing regimens in African adults with HIV infection within the DART trial. Antivir Ther (Lond) 2006;11:741–749. doi: 10.1177/135965350601100612. [DOI] [PubMed] [Google Scholar]
- 22.Chi BH, Cantrell RA, Zulu I, Mulenga LB, Levy JW, Tambatamba BC, et al. Adherence to first-line antiretroviral therapy affects non-virologic outcomes among patients on treatment for more than 12 months in Lusaka, Zambia. International Journal of Epidemiology. 2009;38:746–756. doi: 10.1093/ije/dyp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamisse L, Balkus J, Farquhar C, Osman N, Djedje M, Hitti J. Perinatal HIV Transmission with Highly Active Antiretroviral Therapy During Late Pregnancy and Postpartum. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. pp. 1–1. [Google Scholar]
- 24.Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 25.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. International Journal of Epidemiology. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 26.Alioum A, Cortina-Borja M, Dabis F, Dequae-Merchadou L, Haverkamp G, Hughes J, et al. Estimating the efficacy of interventions to prevent mother-to-child transmission of human immunodeficiency virus in breastfeeding populations: comparing statistical methods. Am J Epidemiol. 2003;158:596–605. doi: 10.1093/aje/kwg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–284. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]


