Abstract
Fluorophores are ubiquitous in nature. Naturally occurring fluorophores are exceptionally stable and have high quantum yield. Several natural systems have acquired fluorescent signature due to the presence of these fluorophores. Systematic attempt to harvest these fluorophores from natural systems could reap rich commercial benefit to bio-imaging industry. Silk cocoon biomaterial is one such example of natural system, which has acquired a fluorescent signature. The objective of this study is to develop simple, rapid, commercially viable technique to isolate silk cocoon membrane fluorophores and exploring the possibility of using them as fluorescent dye in bio-imaging. Here, we report an innovative water glass (Na2SiO3) based strategy to isolate the silk cocoon fluorophores. Isolated fluorophore is majorly quercetin derivatives and exhibited remarkable photo- and heat stability. Fluorescence and mass spectrometric analysis confirmed presence of a quercetin derivative. We further used this fluorophore to successfully label the silicate shell of diatom species Nitzschia palea.
Silk cocoon is among the best examples of naturally evolved biomaterials which protect and facilitate development of a new life1,2. Cocoon membrane possesses certain exquisite regulatory roles like CO2 gating, temperature control, water-proofing and photo-protection3,4,5,6,7. Cocoon fibers have the necessary mechanical strength to prevent physical damage to the dormant, developing pupae8. Recently it was reported that graphene oxide could be synthesized from silk cocoon membrane9.
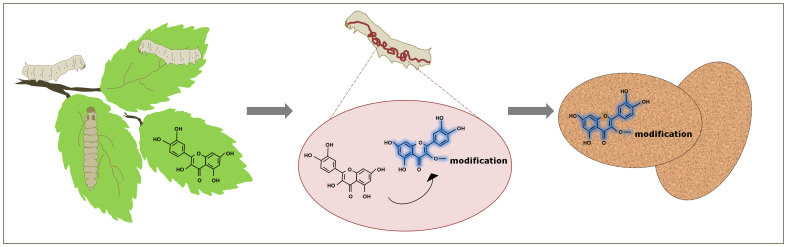
One of the least explored properties of silk cocoon is its acquired fluorescent signature. During early 19th century, it was documented that the presence of flavonoid (mostly quercetin derivatives)10,11 and minor fraction of carotenoid compounds12 in the cocoon membrane renders cocoon its acquired fluorescent nature13,14,15,16,17,18,19,20,21. It has been observed that male and female larval cocoons differ in the type and concentration of these flavonoid derivatives22,23,24,25,26. More recent studies have shown the UV shielding abilities of cocoon flavonoid derivative21. Based on the status of current studies it could be concluded that cocoon fluorophore and similar compounds on the cocoon are actually the modified versions of the precursor compounds present in plant leaves on which larva is fed (Figure 1)27,28,29,30. Similar compounds have been extracted from the gut of silk larvae during its development. No systematic efforts have been made to isolate these fluorophore molecules from silk cocoon and evaluate their potential as fluorescent dyes.
Figure 1. Genesis of cocoon fluorophore.
Plant quercetin is ingested and modified by silk larvae in its gut. This modification delivers fluorescence property to the molecule. This modified quercetin is then exported on the cocoon surface.
Here we have used a “water glass” based strategy to isolate the fluorophores from silk cocoon. Chemically, water glass or soluble glass is sodium silicate (Na2SiO3). Owing to its glassy appearance and water solubility, it is commonly called water glass or soluble glass. For over 150 years, chemical industries have been producing water glass. It has find wide range of applications in detergents, pulp and paper industries, adhesives, textile processing, soil grouting, water and waste water treatment plants, oil reclamation, mineral ore flotation and as inorganic binder31,32,33. In this study, we exploited the detergent, adhesive and binding nature of water glass to extract the fluorophore from silk cocoon. On treating the silk cocoon membrane in an aqueous solution of water glass at room temperature, we found that the fluorophore molecules gets adhered on or loosely bind to the surface of water glass molecule thereby creating a “waterglass-fluorophore ferry”. By suspending the “water glass-fluorophore complex” in methanol, the fluorophore molecules get dissociated from the water glass and get dissolved in methanol hence we could successfully extract the fluorophores. On characterizing the fluorophores, we found a large fraction of it to be a quercetin derivative. Since these fluorophores have the ability to bind to silicate, we attempted to label the natural silica frustules or cell wall of diatom, Nitzchia palea, with this fluorophore. We observed that this fluorophore indeed label the silicate frustules of diatom and thereby creating a “fluorescent diatom”.
Results
Graphic summary
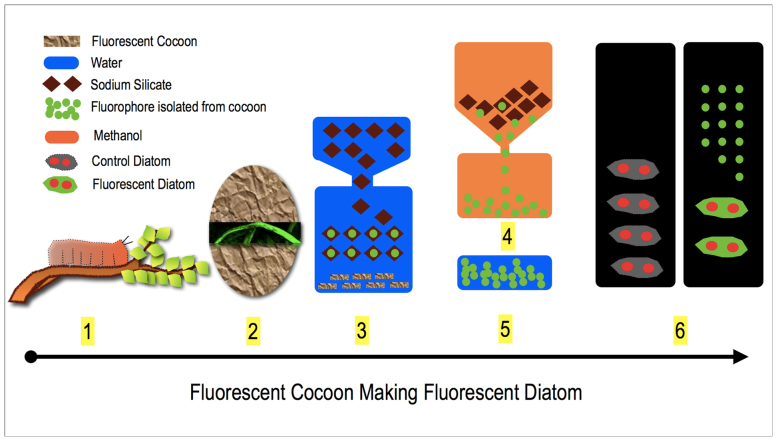
Figure 2 provides the overall graphic summary of this study. The supplementary information (Figure S1a and S1b) highlights the detailed protocol for the extraction of the fluorophores from the cocoon membrane.
Figure 2. Outline for the extraction of fluorophores from silk cocoon.
1. Insect larva is feeding on plant leaves containing different flavonoids and carotenoids. After feeding, the larva starts secreting silk fiber. 2. Silk fibers get woven around the larva and form a protective membrane called cocoon. Cocoons contain various pigments from plant and as well as the pigments synthesized by the larvae. These pigments render the cocoon its acquired fluorescent signature. 3. Cocoon pieces are reacted with aqueous water glass (Na2SiO3 + H2O) solution. During the extraction process, the fluorophores leaches out from the cocoon surface and bind or adhere on to the water glass surface. 4. The water glass crystals obtained after fluorophore extraction are reacted with methanol, which removes the fluorophores from the water glass surface. 5. The dried fluorophores are then dissolved in water. 6. This fluorophore solution is then applied to the diatom culture. The silicate shell of the diatom picks up the fluorophores and started to fluoresce. The red colored filled circles in diatom pictures indicates the chloroplast in the diatom.
Fluorescent cocoon
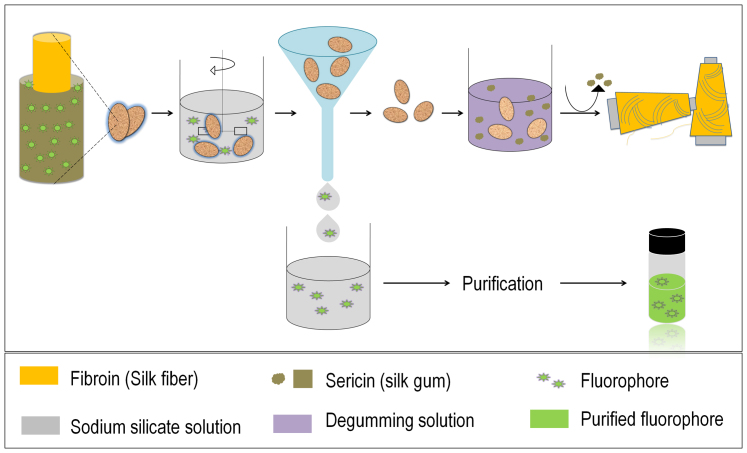
Figure 3 shows the confocal fluorescent image of the outer surface of silk cocoon membrane. The images indicate the fluorescent signature of the silk cocoon. The presence of the fluorophore could be seen in the interwoven silk threads of the cocoon.
Figure 3. Fluorescent cocoon.
Microscopic images of outer membrane of silk cocoon when the membrane was imaged after exposing it to (a) white light. (b) UV light. (c) blue light, 488 nm. (d) green light, 543 nm. Scale bar = 500 μm.
Water glass - fluorophore solution
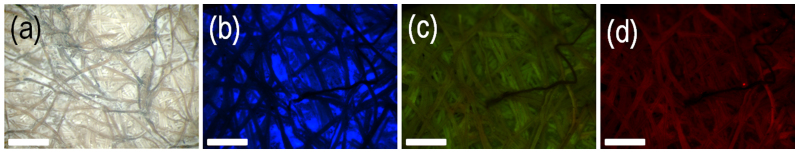
In the next step, we treated the cocoon pieces in aqueous solution of water glass. On treatment, we obtained a fluorescent solution as observed under UV light (Figure 4 a, inset i, ii). The fluorescent solution indicates that the fluorophores have leached out from the cocoon membrane into the solution. The UV-Visible absorption spectrum of the fluorescent solution containing the “fluorophores + water glass” is recorded (Figure 4 b). The maximum absorption was observed at a wavelength of 235 nm. Further fluorescence studies were conducted at an excitation wavelength of 235 nm and the maximum emission was observed at a wavelength of 420 nm. We did not observe significant emission following excitations at 488 nm as well as 543 nm (Figure 4 c).
Figure 4. Light absorption and emission properties of fluorophore.
(a) Optical image under UV light of (i) Blank aqueous water glass solution (ii) Water glass solution following the extraction of the fluorophores from the silk membrane. (b) UV-Visible absorption spectrum of fluorophore. (c) Fluorescence emission spectra of fluorophore at different excitation wavelengths.
“Water glass–fluorophore ferry”
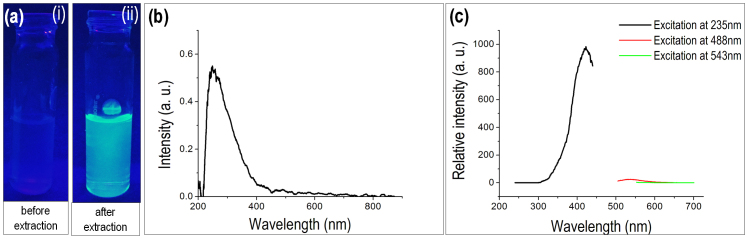
We further dried the “fluorophores + water glass” solution and observed the water glass crystals under light and fluorescent microscopes. In Figure 5 (a,b,c,d) we have shown the light and fluorescent microscopic images of pure sodium silicate crystals (without fluorophore) in white light, UV, blue (wavelength 488 nm) and green (wavelength = 543 nm) lights. In figure 5 (e,f,g,h) we have shown the microscopic images of sodium silicate crystal with extracted fluorescent compounds from silk cocoon at the above mentioned wavelengths respectively. The fluorescence signals indicate that fluorophore molecules have adhered or bind to the water glass.
Figure 5. “Water glass-fluorophore ferry”.
Light and fluorescence microscopic images of (a–d) blank sodium silicate crystals, scale bar = 50 μm. (e–h) crystals of sodium silicate obtained after fluorophore extraction, scale bar = 100 μm. The nature of the light and their respective wavelengths at which the crystals were images: (a), (e) under white light. (b), (f) under UV light. (c), (g) under blue light, 488 nm. (d), (h) under green light, 543 nm.
Optimized protocol for the extraction of fluorophore
The detailed optimization procedure for extraction of the fluorophores from the silk cocoon membrane has been discussed in the supplementary information. We optimized the following three critical parameters: molar concentration of water glass, concentration of cocoon (w/v) and the reaction time. It was observed that at concentration of 0.125 M, water glass leaches maximum fluorophores from cocoon in comparison with other concentrations (figure S2a). Maximum yield of fluorescence was recorded to be at 1% (w/v) weight of cocoon/volume of water glass solution (figure S2b). Also it was observed that one hour of reaction time is sufficient enough to leach the fluorescent compounds from silk cocoon (figure S2c). We also have repeatedly treated same cocoon sample with new batches of fresh water glass solution. This result is represented in figure S2d, which indicate the lowering of quantum fluorescence yield following repeated treatments.
Comparative extraction studies
This molecule is soluble in aqueous alkaline medium as well as in alcohol. Since some of the previous studies have used methanol to leach out the fluorophores from cocoon membrane17,21,25, so we evaluated the efficiency of the methanol versus water glass based strategy. Results for both quantitative (figure S3a, Supplementary information) and qualitative estimation of comparison between untreated cocoon control (Figure S3b, Supplementary information), water glass treated cocoon (figure S3c, Supplementary information) and methanol treated cocoon (figure S3d, Supplementary information) based strategy showed that water glass based strategy is more efficient and more robust.
Photobleaching studies
The compound was observed to be fluorescently stable and active for three hours under constant excitation at 235 nm wavelength of light. Figure S4a (Supplementary information) shows a constant fluorescence emission spectrum recorded at wavelength of 420 nm for 3 hours.
Heat and pressure stability studies
Figure S4b (Supplementary information) presents the fluorescence emission spectra of autoclaved and non autoclaved fluorophores extracted using water glass strategy. The results indicate the fluorophores remains stable even after autoclaving.
Thin layer chromatography (TLC)
To analyze the chemical nature of the fluorophores content of the extract, we subjected it to thin layer chromatography using methanol as a solvent. The chromatography plate was observed under UV light. A single visible fluorescent band appeared slightly above the starting point presented in figure 6a.
Figure 6. Analysis of the chemical nature of the fluorophores.
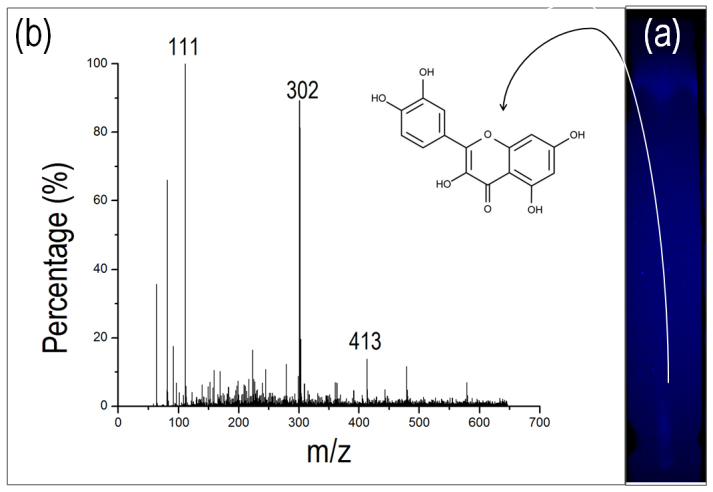
(a) Thin layer chromatography of fluorophore sample showing a distinct fluorescent band in the UV light. This fluorescent band was isolated and subjected for MS analysis. (b) Mass spectrogram of fluorophore showing distinct peaks at molecular mass of 111, 302 and 413.
Mass spectroscopy
The fluorescent band from TLC was scratched out and leached back in methanol. This solution was then analyzed by mass spectrophotometer and the results are displayed in figure 6b. The spectrogram yielded a peak at mass 302, which is equivalent to mass of quercetin molecule. Normally it stays as dihydrate with molecular weight as 338 but free molecule matches the strong peak at 302. It also shows, peaks at mass 111 and 413 indicating that the quercetin moiety might have some chemical modification of molecular mass 111.
Fluorescent diatom
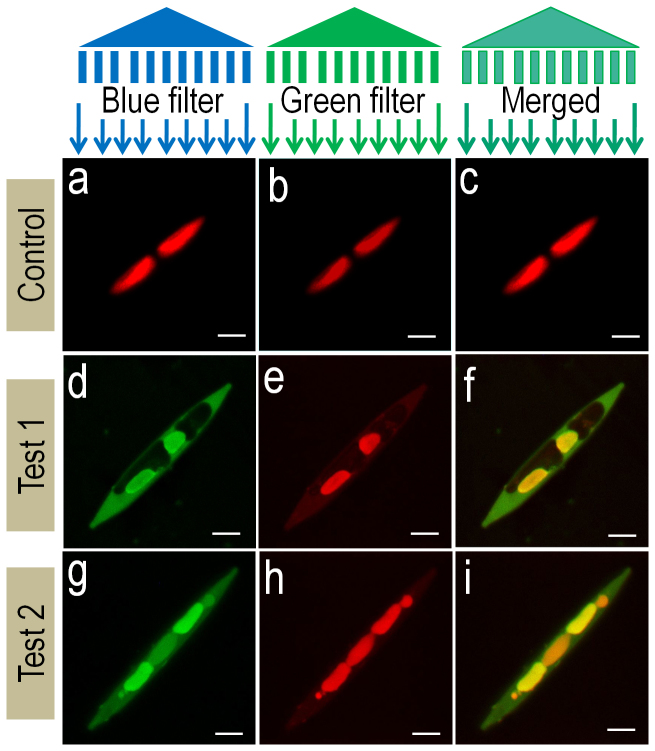
Diatoms cover a major group of algae commonly recognized by its characteristic silicate cell wall, a frustule. These diatoms are purified from different sources (Supplementary information, Figure S5 provides the necessary details of diatom purification and culture). Nitzschia is a diatom genus which can be identified by its characteristic frustule geometry and position of chloroplast. This diatom has a spindle shaped bilaterally symmetrical frustule with two distinctly placed chloroplasts at each end. The frustule and chloroplast can be visualized using normal light microscopy. Chloroplast can also be visualized in fluorescence microscopy. It is observed that chloroplast has a characteristic red optical fluorescence when observed at wavelengths ranging from UV to green light (Figure S6 a–f, Supplementary information). This property of chloroplast is due to auto-fluorescence of chlorophyll molecule (Figure 7a–7c). On the other hand, diatom silicate frustule does not show optical fluorescence at any wavelength. Frustule can be visualized using scanning electron microscopy (Figure S6 a, d, d.inset). Our objective was to evaluate the ability of the silk cocoon fluorophore, to binds naturally occurring silicate matrix or the glass house covering of the diatom. Such a technique will help us to visualize the silicate shell, independent of the chlorophyll molecule. Extracted fluorophore fluoresces differently when excited by UV, blue and green light (figure 5 f, g, h). This property distinguishes it from the auto-fluorescence of chlorophyll. The difference in fluorescence patterns of control and fluorophore treated diatom can be clearly observed in figure 7a–7c and figure 7d–7i respectively. When normal Nitzschia was visualized in fluorescence microscope, only chloroplast appeared red in every filter with no fluorescence from silicate frustules (Figure 7 a–c and supplementary information, Figure S6). But when the fluorophore was added to the Nitzschia culture, frustules started showing fluorescence as that of the fluorophore as seen in figure 7f and 7i. This offers an opportunity to observe the fluorescence of both frustules and chloroplast separately.
Figure 7. Fluorescent diatom.
Fluorescence confocal microscopic images of Nitzschia. (a–c) Control represents N. palea without fluorophore addition and (d–i) test 1 and test 2 represents Nitzschia with fluorophore addition. Light filter and its wavelengths used for imaging - (a), (d), (g) blue filter, 543 nm. (b,e,h) green filter, 488 nm. (c), (f), (i) are the merged images from respective groups. Scale bar = 5 μm.
Discussion
The key finding of this study is the lateral shift and conservation of the flavonoid molecules across the diverse life forms which have adapted in different ecosystems. In this study, we showed that the flavonoid molecules, which are synthesized by the plants for plethora of applications including UV protection27,28,29,30, transferred from the plants to the larva via food. Inside the body of the larva, the flavonoid gets chemically modified which might have some unexplored evolutionary significance10,11,12,13. Then this modified molecule becomes part of the cocoon through silk gland exudation. In the cocoon, it probably serves the role of UV protection coating21 as it also does in the plants30. In natural condition, the presence of this molecule on the surface of cocoon, may also be related to its antioxidant property, as it could readily donate hydrogen to scavenge free radicals27,28,29,30. Thus, why it is so much embedded on the surface of cocoon may have some biological role to protect the cocoon from reactive oxygen species (ROS) generated in the environment. On isolating these flavonoid fluorophores, they exhibited the ability to fluorescently label the silicate shell of diatom. Hence, we observed that through food chain the flavonoid molecules are transported from the plant kingdom to insect kingdom and then it was used to label the glass casing of diatoms. This understanding surely raises the possibility of using such simple ubiquitous biocompatible flavonoid fluorescent molecules as fluorescent probes for different bio-imaging applications.
A relevant debate could be made to derive fluorophore directly from the plants, instead of isolating it from the silk cocoon. This point is extremely important to address, since the genesis of the fluorophore is from the flavonoids like quercetin, which are present in the plants then consumed by the larvae and further secreted out by the larvae to construct the silk cocoon. On investigation, we found that silk larva has a significant role in making fluorophore. We compared the fluorescence and staining abilities of pure quercetin with the isolated silk cocoon fluorophore. We found that pure quercetin did not have same fluorescence as the cocoon fluorophore, nor it could stain diatom. This supports the theory that quecetin is being modified in the gut of the larvae, which renders its enhanced fluorescence and staining property21.
Currently PDMPO, HCK-123, NBD-N2, silane-FITC complexes, and rhodamine are among the commercially available fluorescent dyes which have been used for staining biosilica of diatoms (Table 1)34,35,36,37. Interestingly, all of the listed dyes are synthetically derived while some of them are mutagenic38. On the other hand, fluorophore used in this study is obtained from a natural source and in addition to having a remarkable fluorescence, this fluorophore also show UV shielding property21. A comparative study between the rhodamine and silk cocoon fluorophore in staining diatom is documented in the supplementary information (Part V, Figure S7). A cost analysis of the different synthetic fluorophores and the natural cocoon fluorophore is given in the table S2 (Part V, Table S2) which clearly indicates that if commercially exploited for large scale production, cocoon derived fluorophore could be a obtained at a fairly cheap price with respect to the other synthetic fluorophores. In the supplementary section, the economics of the proposed silk fluorophore industry is given.
Table 1. Details of commercially available biosilica staining dyes.
| Sr. No. | Compound Name | Excitation/Emission (nm) | Source |
|---|---|---|---|
| 1 | PDMPO | 329/440§ | Synthetic |
| 2 | HCK-123 | 485/564§ | Synthetic |
| 3 | NBD-N2,N3 | 465/537§ | Synthetic |
| 4 | Methoxysilane-FITC | 498/516§ | Synthetic |
| 5 | Rhodamine 123 | 506/528§ | Synthetic |
| 6 | Cocoon Fluorophore | 235/420 | Natural |
PDMPO: 2-(4-pyridyl)-5-((4-(2-dimethylaminoethylaminocarbamoyl)methoxy)-phynyl)oxazole. HCK: Hemopoietic cell kinase. NBD: 7-nitrobenzo-2-oxa-1,3-diazole. FITC: Fluorescein isothiocyanate.
§values obtained from product details supplied by Life Technologies™.
Fluorophore obtained from silk cocoon has an optical advantage that it could be fluorescently visualized at different wavelengths. Such unique property gives user a flexibility to use multiple probes for labeling in an experiment. Reason behind silk cocoon fluorescence is presence of organic compounds like flavonoids and carotenoids10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26 while our mass spectrometric analysis showed presence of flavonoid quercetin at high concentration. Also we do not completely rule out the possibility of the presence of other compounds, which are at a much lower concentration and could not be detected. As in this case, cocoon extract have major portion of quercetin, we draw a conclusion that it is acting as a fluorophore.
Quercetin covers a major group of plant phenolics containing around 4000 naturally occurring compounds27,28,29,30. The presence of quercetin in silk cocoon is known since 1934 as reported by Japanese scientist Oku10,11. This compound has a characteristic yellow fluorescenece under UV light when it is glucosylated at 5-O position21. In our experiments we have observed similar characteristic fluorescence.
Another interesting feature of this family of flavonoid fluorophores is that they are extremely stable against light, heat and pressure. This kind of stability is logically realizable. A cocoon has to withstand the vagaries of nature from 3 weeks to as long as 9 months, and during this period, it needs to protect the developing new life form1,2,3. Hence for it evolutionary success, it has to equip itself with all the stable tools from the chemical arsenal of nature27,28,29,30. Fluorescent flavonoids are probably, one of those potent tools in the chemical armory of nature, which has the ability to withstand harsh natural conditions. Hence it is not surprising to find the extremely stable nature of these groups of fluorophores against varied intensity of light, natural UV exposure, varying heat of tropical and sub-tropical semi arid regions and pressure.
The major technological finding of this study is the use of “water glass technology”31,32,33 to extract the fluorophores from diverse group of natural systems. This approach has the potential to speed up the isolation, purification and screening of fluorophores, while maintaining the functional integrity of the molecules. Surely, this technology could opens up a wide range of possibilities for developing cheaper fluorophores from natural systems. However the mechanism by which water glass is acting on the cocoon membrane to isolate the fluorophores is not clear to us. Here, we are proposing a possible mechanism. The aqueous solution of water glass is highly basic in nature. Such basic solution readily solublizes quercetin type of molecules by deprotonation rendering its solubility and thus leaching it out from the surface of cocoon. Apart from it, silicate has the inherent ability to form complexes or adducts with the elements present on cocoon surface and this property of silicate may help in the rapid extraction of the fluorophores.
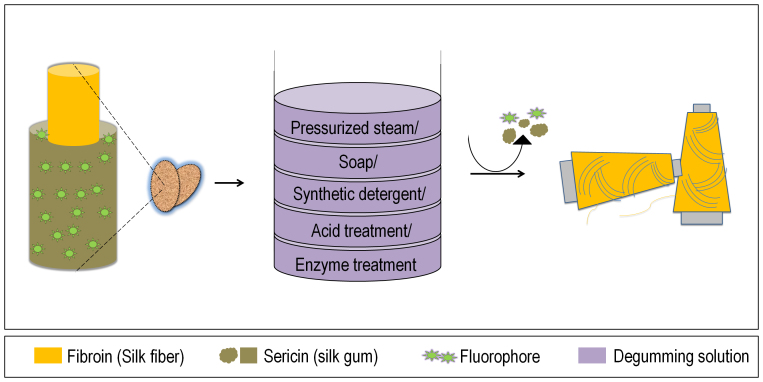
Looking at the commercial feasibility of this fluorophore, seems it has a potential to give rise to a new industry. At present commercial silk cocoon industries follow a common protocol summarized in figure 8. Structural architecture of silk cocoon is made of two proteins, fibroin and sericin. Fibroin is the silk fiber which is commercially available as silk, while sericin is gelatinous gummy protein holding fibroin fibers together39. Compounds such as fluorophores are distributed in the cocoon. Commercially silk fibers are obtained through a degumming40 and reeling process in which cocoons are cooked by any of the five methods given in the figure 8. This process essentially remove the sericin, a waste product, from the silk39,40. Degumming process also removes the fluorophore like compounds.
Figure 8. Conventional silk processing.
Raw silk consist fibroin (silk fiber) and sericin (silk gum) proteins. In order to remove silk gum, cocoons are treated with one of the five degumming solutions mentioned in the figure. In this process fluorophore get wasted along with the sericin.
Our technology of extracting and utilizing fluorophores from silk cocoon not only reclaims an application from a waste but also keeps silk fiber unaffected. To confirm the fluorophore extraction efficacy of sodium silicate with respect to any of the degumming method, we boiled cocoon in water and confirmed the presence of fluorophores in that water. Further quantitative and qualitative studies confers that sodium silicate based strategy is more effective. The biggest advantage of sodium silicate based strategy is fluorophores are extracted without a fraction of sericin impurity. Sodium silicate solution does not cause any damage to the silk fiber. The integrity of both sericin and fibroin remain intact after brief treatment of sodium silicate solution, while we able to extract maximum amount of fluorophore. On the other hand, while boiling treatment is given to the cocoons, a large fraction of sericin is removed along with some fluorophore (Refer supplementary information, Part VI). Based on the present industrial methodology for obtaining silk fibers we propose a new process flow which has the potential to give rise to a commercial biotechnological product along with silk fiber (Figure 9). Further we are presenting an overall survey of the ‘rural economy of silk cottage industry’ in the state of Chattishgarh, India from where we have obtained the supply of silk cocoon. This survey has been has been developed in consultation with ‘Directorate of Rural Industries, Sericulture sector, Government of Chhattishgarh, Chhattishgarh, India’ (Refer to supplementary information, Part VII). The survey attempts to highlight the possibility of developing a new cottage industry of silk cocoon fluorophore for bio-imaging applications.
Figure 9. Proposed modern industrial process flow.
Raw silk cocoons are treated with sodium silicate solution, fluorophore is leached out leaving raw silk's structural architecture intact. This sodium silicate extract is then separated and processed to obtain commercial fluorophore. While the sodium silicate treated cocoons are the processes conventionally to degum the sericin and obtain commercial grade silk fiber. Using this strategy we can obtain additional commercial product from waste without affecting the conventional integrity of the process.
Further, we have made a successful attempt to label the silicate frustule of diatom with the isolated fluorophore. In this study, we have explored one of the commercial aspects of this compound as a dye and opened up a new commercial dimension in the existing silk industry.
Methods
Source of cocoon
Tropical Tussar (Tussah) silk cocoons were used for this study. These are non mulberry silk, generated by the silkworm, Antheraea mylitta.
Extraction of fluorophores from silk cocoon membrane
Obtained cocoons were surface cleaned by blowing dry air. Cleaned cocoons were then taken into sterile environment. Sterile condition was maintained in laminar air flow cabinet. Using a sharp scissor cocoons were cut and the contained pupae were removed. The pupae free cocoon membranes were further cut into small pieces of 0.5 cm × 0.5 cm size. For extraction studies, sterile cocoon pieces were immersed in water glass solution and stirred using magnetic stirrer. After completion of stirring, the mixture is filtered through whatman's filter paper and the filtrate was collected. This filtrate was evaporated and extract powder was obtained. For purification of fluorophore extract powder was mixed in methanol and stirred for 1 hour. After stirring suspended extract powder was removed by a brief centrifugation. The supernatant is collected and evaporated to get pure fluorophore. fluorophore is weighed and dissolved in water. Fluorophore solution was stored in amber colored bottle. (Refer to supplementary information for further details, Figure S1a, S1b).
UV spectroscopic measurements
UV-Vis spectrum of the sample used in this study was taken by Ocean optics UV-Vis-NIR spectrophotometer.
Fluorescence studies
Fluorescence spectrum of each sample was obtained using Perkin Elmer LS 55 fluorescence spectrophotometer. The fluorescence was recorded at excitation wavelength of 235 nm and emission wavelength 420 nm.
Optimization of parameters in extraction protocol
(Supplementary information, Figure S2a–d); All the chemicals used in this study were procured from HiMedia, India. A stock solution of 1 M water glass was prepared and its lower concentration solutions were prepared by diluting the stock solution. Cocoon used in the procedure was cut into small pieces of approximately 0.5 cm × 0.5 cm size.
Molar concentration of sodium silicate: A fixed quantity of cocoon was added to solutions having different concentrations of water glass. Solutions of 1 M, 0.5 M, 0.25 M, 0.125 M, 0.0625 M, 0.0312 M and 0.0156 M water glass were used for this study. All other parameters such as duration of the treatment, temperature and cocoon quantity were kept constant.
Concentration of cocoon (w/v): Cocoon with 1%, 2%, 3%, 4%, 5% and 6% concentration was used in 0.125 M water glass solution. The study was performed at room temperature and for a fixed duration.
Duration of the treatment: Reaction was carried off 1% (w/v) of cocoon with 0.125 M water glass and was stirred on magnetic stirrer for different durations. Duration of 1, 2, 3, 4, 5 and 6 hours were given. All the study was conducted at room temperature.
Thin layer chromatography
The dried extract powder was subjected to thin layer chromatography using 95% methanol as a solvent. Thin layer of silica gel was created using silica gel GF 254. In total 5 μg of extract powder, dissolved in water, was loaded on the plate.
Mass spectrometry
Mass was determined using Waters-Q-TOF Premier-HAB213 electrospray ionization-mass spectrometer. It was operated at 1: TOF MS ES + mode. Methanol was used as a solvent. The capillary voltage was 3 kV; cone voltage was 5 V while and source temperature was kept 100°C.
Purification of fluorophore
Once the reaction is over the solution was filtered through whatman filter paper. The filtrate was then completely evaporated till the yellowish-white powder is obtained. This powder then mixed and stirred in methanol solution for one hour. After one hour, a clear methanol solution is obtained by briefly centrifuging the mixture. Once a clear methanol solution is obtained, it is evaporated completely and the final product is obtained. This product is soluble in water.
Photobleaching studies
Photobleaching study study was performed using Perkin Elmer LS 55 fluorescence spectrophotometer. The sample was excited at wavelength of 235 nm for 3 hours continuously. The fluorescence spectrum was recorded at 420 nm.
Heat stability
Heat stability was determined by autoclaving the sample. The temperature during autoclaving was 121°C and pressure was 15 lbs. Autoclaving was performed for 15 minutes. The fluorescence spectrum of both samples, autoclaved and control, were recorded.
Staining diatom silicate frustules
A pure culture of Nitzschia palea was grown and used for this study. The detailed protocol for the culture and purification of Nitzschia palea species of diatom has been discussed in the supplementary information, table S1, figure S5, figure S6. The fluorophores derived from the cocoon was dissolved in distilled water at a concentration of 5 mg per ml and used as a dye solution. Diatom cell suspension and dye solution were mixed in the ratio of 1:1 and this mixture was incubated for one hour. After incubation the mixture was heat fixed on a glass slide and used for microscopy.
Confocal fluorescence microscopy
Two types sample were imaged in this study. One of them was ‘control’ which had untreated diatom obtained from directly from the culture. Second sample had fluorescently labeled diatom as discussed above. Microscopy was performed using Leica TCS SP5 microscope. Two excitation wavelengths, 488 nm and 543 nm, were used for the imaging.
Author Contributions
T.S.K. discovered for the first time that Tussar silk cocoon has fluorescent properties, conceived the idea of using water glass for extraction of the fluorophore and planned the experiments, conducted the experiments and writing the manuscript. This work is T.S.K.'s doctoral research work and part of his thesis. He is the co-corresponding author. I.T. helped in the fluorophore isolation optimization studies. N.K.S. helped in microscopy, generated critical ideas, and helped in analyzing the data. K.B. helped in fluorescence spectroscopy and mass spectroscopy studies. S.S. assisted us in understanding the interaction of the fluorophore and sodium silicate S.K.S. helped in spectroscopy and other opto-electronics studies, screening the cocoons, helps in understanding the silk cocoon reeling process and initiated the interaction with the silk farmers in the government sericulture farms for future industry-institute collaboration. M.D. Conceived the idea and planned the experiments, writing the manuscript, supported the experiments.
Supplementary Material
Supplementary Information
Acknowledgments
The work was supported by IITK start-up grant (IITK/BSBE/20100206) (2010-2011) of MD. TSK is funded by IIT K PhD fellowship program. This work is part of TSK's doctoral thesis. Authors specially thank to Prof. P Sinha, Prof. A K Thakur and their PhD scholars for facilitating confocal microscopy and fluorescence spectroscopy work respectively. We thank Ms. Shraddha Singh and Prof. D S Katti for the SEM facility of BSBE, which was used for imaging the silicate shell of Diatom. We are exceptionally thankful to Mr. P Kagkachalam of Chemistry, IIT K for helping us with the MS facility. We specially thank Prof. K. Murugesh Babu of Department of Textile Technology, Bapuji Institute of Engineering & Technology, Davangere, PIN 77004, India for helping us to understand the current status of silk industry. A special thanks to ‘Directorate of Rural Industries, Sericulture Sector, Government of Chhattishgarh, Chhattishgarh, India’ for providing all the necessary information regarding the rural economics of silk cottage industry.
References
- Trouvelot L. The American silk worm. Am. Nat. 1, 30–38 (1867). [Google Scholar]
- Packard A. S. Textbook of Entomology. (The Macmillan company, New York, 1903). [Google Scholar]
- Roy M. et al. Carbondioxide gating in silk cocoon. Biointerphases 7:45, 1–11 (2012). [DOI] [PubMed] [Google Scholar]
- Danks H. V. The roles of insect cocoons in cold conditions. Eur. J. Entomol. 101, 433–438 (2004). [Google Scholar]
- Horrocks N. P., Vollrath F. & Dicko C. The silkmoth cocoon as humidity trap and waterproof barrier. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 164, 645–652 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang J., Rajkhowa R., Li J., Liu X. & Wang X. Silkworm cocoon as natural material and structure for thermal insulation. Mater. Des. 49, 842–849 (2013). [Google Scholar]
- Kaur J. et al. Photo-protection by Silk Cocoons. Biomacromolecules; 10.1021/bm401023h (2013). [Google Scholar]
- Shao Z. & Vollrath F. Surprising strength of silkworm silk. Nature 418, 741 (2002). [DOI] [PubMed] [Google Scholar]
- Roy M. et al. Graphene oxide from silk cocoon: a novel magnetic fluorophore for multi-photon imaging. 3 Biotech; 10.1007/s13205-013-0128-2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku M. Studies on cocoon pigment in the silkworm, Bombyx mori, quercetin glycosides in mulberry leaves. Nippon. Nogei-kaguku Kaishi. (in Japanese) 10, 1029–1038 (1934). [Google Scholar]
- Oku M. The chemical studies on the pigments in the cocoon filaments of Bombyx mori. Nippon. Nogeikagaku Kaishi. (in Japanese) 10, 1014–1028 (1934). [Google Scholar]
- Harizuka M. Physiological genetics of the carotenoids in Bombyx mori, with special reference to the pink cocoon. Bull. Seric. Exp. Stn. Japan. 14, 141–156 (1953). [Google Scholar]
- Hayashiya K., Sugimoto S. & Fujimoto N. Studies on the pigments of cocoon. The qualitative test of the pigments of green cocoon. J. Seric. Sci. Jpn. 28, 27–29 (1959). [Google Scholar]
- Fujimoto N. & Hayashiya K. Studies on the pigments of cocoon.(IX) The precursor of the pigments of green cocoon in the silkworm, Bombyx mori. J. Seric. Sci. Jpn. 41, 383–386 (1972). [Google Scholar]
- Fujimoto N. On the physiological functions of the genes concerning the formation and the translocation of the pigments of green cocoon in silkworm larvae. J. Seric. Sci. Jpn. 32, 338–342 (1972). [Google Scholar]
- Ito T. & Kobayashi M. The Silkworm – An Important Laboratory Tool [Tazima, Y. (ed.)] (Kodansha, Tokyo, 1978). [Google Scholar]
- Tamura Y., Nakajima K.-i., Nagayasu K.-i. & Takabayashi C. Flavonoid 5-glucosides from the cocoon shell of the silkworm, Bombyx mori. Phytochemistry 59, 275–278 (2002). [DOI] [PubMed] [Google Scholar]
- Kurioka A. & Yamazaki M. Purification and identification of flavonoids from the yellow green cocoon shell (Sasamayu) of the silkworm, Bombyx mori. Biosci. Biotech. Bioch. 66, 1396–1399 (2002). [DOI] [PubMed] [Google Scholar]
- Sakudoh T. et al. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the Yellow blood gene. Proc. Natl. Acad. Sci. U.S.A. 104, 8941–8946 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama C., Kosegawa E., Tamura Y. & Nakamura M. Analysis of flavonoids in the cocoon layer of the silkworm regional races by LC-MS. Sanshi-Konchu. Biotec. 78, 57–63 (2009). [Google Scholar]
- Daimon T. et al. The silkworm Green b locus encodes a quercetin 5-O-glucosyltransferase that produces green cocoons with UV-shielding properties. Proc. Natl. Acad. Sci. U.S.A. 107, 11471–11476 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaohua Y. & Liqun X. Studies on inheritance of fluorescent colour of cocoons in silkworm, Bombyx mori. Acta. Sericol. Sin. 3 (1997). [Google Scholar]
- Yu Z. et al. A preliminary research on fluorescence pigment of the sex-limited fluorescence silkworm race. Acta. Sericol. Sin. 23, 64–67 (1997). [Google Scholar]
- Yu Z., Gu Y., Zhang F. & Shi R. Studies on difference of fluorescent pigment between female and male larvae of cocoon colour sex distinguished fluorescent silkworm variety. Acta. Sericol. Sin. 26, 123–124 (2000). [Google Scholar]
- Zhang Y. et al. Mechanism of fluorescent cocoon sex identification for silkworms Bombyx mori. Sci. China Life Sci. 53, 1330–1339 (2010). [DOI] [PubMed] [Google Scholar]
- Xiaolong H. et al. Elementary research of the formation mechanism of sex-related fluorescent cocoon of silkworm, Bombyx mori. Mol. Biol. Rep. 39, 1395–1409 (2012). [DOI] [PubMed] [Google Scholar]
- Harborne J. B. Flavonoids in the environment: structure-activity relationships. Prog. Clin. Biol. Res. 280, 17–27 (1988). [PubMed] [Google Scholar]
- Formica J. & Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 33, 1061–1080 (1995). [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. A., Miller N. J. & Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical. Bio. Med. 20, 933–956 (1996). [DOI] [PubMed] [Google Scholar]
- Buer C. S., Imin N. & Djordjevic M. A. Flavonoids: new roles for old molecules. J. Integr. Plant Biol. 52, 98–111 (2010). [DOI] [PubMed] [Google Scholar]
- Richardson A. The Action of Sodium Silicate When Used in Soaps. Ind. Eng. Chem. 15, 241–243 (1923). [Google Scholar]
- Vail J. G. & Wills J. H. Soluble Silicates: their properties and uses. Vol. 1, (Reinhold, New York, 1952). [Google Scholar]
- Weldes H. H. & Lange K. R. Properties of soluble silicates. Ind. Eng. Chem. 61, 29–44 (1969). [Google Scholar]
- Shimizu K., Del Amo Y., Brzezinski M. A., Stucky G. D. & Morse D. E. A novel fluorescent silica tracer for biological silicification studies. Chemistry & biology 8, 1051–1060 (2001). [DOI] [PubMed] [Google Scholar]
- Desclés J. et al. New tools for labeling silica in living diatoms. New Phytologist 177, 822–829 (2008). [DOI] [PubMed] [Google Scholar]
- Annenkov V. et al. Novel fluorescent dyes based on oligopropylamines for the in vivo staining of eukaryotic unicellular algae. Anal. Biochem. 407, 44–51 (2010). [DOI] [PubMed] [Google Scholar]
- Kucki M. & Fuhrmann-Lieker T. Staining diatoms with rhodamine dyes: control of emission colour in photonic biocomposites. J.R. Soc. Interface 9, 727–733 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestmann E. R., Douglas G. R., Matula T. I., Grant C. E. & Kowbel D. J. Mutagenic activity of rhodamine dyes and their impurities as detected by mutation induction in Salmonella and DNA damage in Chinese hamster ovary cells. Cancer Res. 39, 4412–4417 (1979). [PubMed] [Google Scholar]
- Sen K. & Babu K. Studies on Indian silk. I. Macrocharacterization and analysis of amino acid composition. J. Appl. Polym. Sci. 92, 1080–1097 (2004). [Google Scholar]
- Shukla S., Patel R. & Saligram A. Silk degumming process: a comparison of efficiencies. Am. Dyestuff Rep. 81, 22–22 (1992). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information