Abstract
Anticancer properties of Garcinia indica–derived garcinol are just beginning to be elucidated. We have earlier reported its cancer cell–specific induction of apoptosis in breast cancer cells, which was mediated through the downregulation of NF-κB signaling pathway. To gain further mechanistic insight, here, we show for the first time that garcinol effectively reverses epithelial-to-mesenchymal transition (EMT), that is, it induces mesenchymal-to-epithelial transition (MET) in aggressive triple-negative MDA-MB-231 and BT-549 breast cancer cells. This was associated with upregulation of epithelial marker E-cadherin and downregulation of mesenchymal markers vimentin, ZEB-1, and ZEB-2. We also found that garcinol upregulates the expression of miR-200 and let-7 family microRNAs (miRNAs), which provides a molecular mechanism for the observed reversal of EMT to MET. Transfection of cells with NF-κB p65 subunit attenuated the effect of garcinol on apoptosis induction through reversal of MET to EMT. Forced transfection of p65 and anti-miR-200s could also reverse the inhibitory effect of garcinol on breast cancer cell invasion. Moreover, treatment with garcinol resulted in increased phosphorylation of β-catenin concomitant with its reduced nuclear localization. The results were also validated in vivo in a xenograft mouse model where garcinol was found to inhibit NF-κB, miRNAs, vimentin, and nuclear β-catenin. These novel findings suggest that the anticancer activity of garcinol against aggressive breast cancer cells is, in part, due to reversal of EMT phenotype, which is mechanistically linked with the deregulation of miR-200s, let-7s, NF-κB, and Wnt signaling pathways.
Introduction
Cancer is a major public health problem in the United States and worldwide. In particular, breast cancer continues to affect lives of millions of women. In the United States alone, it is estimated that 226,870 new cases of breast cancer will be reported in the year 2012 (1). This places breast cancer on top of the list of cancers that affect females. Although the last decade has seen a decline in the mortality associated with breast cancer, due to detection and intervention at an early stage, the estimation that 39,510 patients with breast cancer will die in the year 2012 (1) is still alarming. This calls for an urgent development of novel therapeutic agents for the targeted treatment of breast cancer.
We recently reported the anticancer activity of a naturally occurring chemical compound, garcinol, against breast cancer cells (2). Garcinol (Fig. 1A) is a polyisoprenylated benzophenone derivative that is obtained from Garcinia indica extracts (3). Early studies with garcinol focused on its antioxidant abilities (3–5), but gradually its potent anticancer activity was recognized as well (5–16). Despite the increasing interest in anticancer activity of garcinol, the exact molecular mechanism of its action is not yet known. A number of different mechanisms have been proposed, including inhibition of histone acetyltransferases (17) and induction of reactive oxygen species (15). We have previously reported regulation of NF-κB signaling pathway by garcinol as one of the mechanisms by which it inhibits cell proliferation and induces apoptosis in breast cancer cells (18). This was shown to occur through a direct inhibition of constitutive NF-κB activity as well as downregulation of NF-κB – regulated genes. NF-κB signaling cross-talk with a number of oncogenic path-ways leading to invasion and metastasis of cancer cells has been reported (19), and therefore, we designed the current study to further investigate the mechanistic details of anticancer action of garcinol that involves NF-κB signaling as the key factor.
Figure 1.
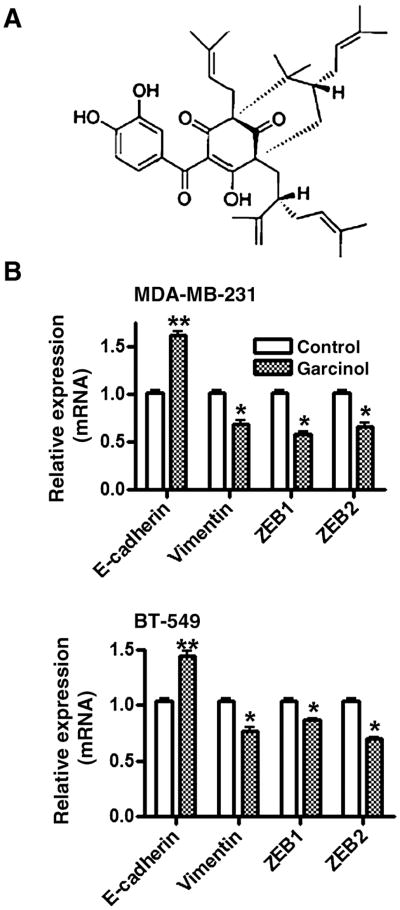
Chemical structure of garcinol (A) and effect of garcinol on the expression of EMT markers (B). Cells were treated with 25 μmol/L garcinol for 48 hours, and the expression of epithelial marker, E-cadherin and mesenchymal markers, vimentin, ZEB1, and ZEB2 was evaluated by real-time reverse transcription PCR. *, P < 0.05 and **, P < 0.01 versus respective vehicle controls.
Earlier investigations from our laboratory have established that natural agents possess an ability to modulate the expression of microRNAs (miRNAs), especially the miR-200 and let-7 family of miRNAs that are involved in the regulation of epithelial-to-mesenchymal transition (EMT; ref. 20). Because the process of EMT itself involves the crucial role of NF-κB signaling in breast cancer cells (21), we questioned whether the mechanism of action of garcinol could involve the regulation of novel miRNAs and the resulting EMT to MET (mesenchymal-to-epithelial transition) through NF-κB signaling. Also, there is evidence to suggest the influence of nutraceuticals on multiple signaling pathways, including the Wnt signaling pathway (22), and that garcinol being a nutraceutical itself (16), it may regulate the Wnt signaling cross-talk with NF-κB signaling (23). Therefore, this study was designed to assess the effect of garcinol on Wnt signaling pathway and further assessed the mechanistic involvement of novel miRNAs, EMT, and NF-κB in the regulation of Wnt signaling. The mechanistic details were studied not only in vitro but also in vivo in a mouse xenograft model.
Materials and Methods
Cell lines and reagents
Human breast cancer cell lines MDA-MB-231 and BT-549 were cultured in Dulbecco's Modified Eagle's Medium and RPMI, respectively, with 10% FBS and penicillin/streptomycin. All cells were cultured in 5% CO2-humidified atmosphere at 37°C. Both of these cell lines are classified as triple negative because of the absence of estrogen receptor (ER), progesterone receptor (PR), and Her-2/neu (ErbB2). The cell lines have been tested and authenticated by the core facility (Applied Genomics Technology Center at Wayne State University; Detroit, MI) through short tandem repeat profiling using the PowerPlex 16 System from Promega. Antibodies were purchased from following sources—vimentin (Abcam), NF-κB p65 (Chemicon), β-catenin and p-β-catenin (Ser33/37/Thr41; Cell Signaling), glycogen synthase kinase (GSK-3-β; Millipore), cyclin D1 (Santa Cruz Biotechnology), and β-actin (Sigma-Aldrich).
Real-time reverse transcription PCR
Total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's instructions. Real-time PCR was used to quantify mRNA expression. Sequences of primers for E-cadherin, vimentin, ZEB1, ZEB2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were same as reported earlier (24) and the amount of RNA was normalized to GAPDH expression. For miRNA analysis, total RNA was isolated using the mirVana miRNA Isolation Kit (Ambion), as described previously (25). The levels of miRNAs were determined using miRNA-specific Taqman MGB probes from the Taqman MicroRNA Assay (Applied Biosystems). The relative amounts of miRNA were normalized to RNU6B.
Histone/DNA ELISA for detection of apoptosis
The Cell Death Detection ELISA Kit (Roche) was used to detect apoptosis in breast cancer cells treated with garcinol, as described previously (26). Briefly, cells were treated with garcinol or dimethyl sulfoxide (DMSO) control for 72 hours. After treatment, the cytoplasmic histone/DNA fragments from these cells were extracted and incubated in the microtiter plate modules coated with antihistone antibody. Subsequently, the peroxidase-conjugated anti-DNA antibody was used for the detection of immobilized histone/DNA fragments followed by color development with ABTS substrate for peroxidase. The spectrophotometric absorbance of the samples was determined by using Ultra Multifunctional Microplate Reader (TECAN) at 405 nm.
Transfection experiments
For transfection studies with p65 cDNA, cells were first seeded in a 6-well plate (200,000 cells per well), incubated at 37°C for 24 hours, and then transfected with NF-κB p65 cDNA or control empty vector using ExGen 500 (Fermentas). After 48 hours, the p65 cDNA-transfected cells were used in apoptosis and invasion assays or for assessing the expression of EMT marker vimentin. For transfection of anti-miRNAs, cells were seeded at 2.5 × 105 cells per well in 6-well plates and transfected with appropriate anti-miRs or miRNA-negative control at a final concentration of 600 to 200 nmol/L each of miR-200a, miR-200b, and miR-200c anti-miRNAs (Ambion) using DharmaFECT4 transfection reagent (Dharmacon). After 2 days of transfection, cells were split and transfected twice again before the use of cells for specified experiments.
Preparation of cytoplasmic and nuclear lysates
Cell pellet was suspended in lysis buffer (0.08 mol/L KCl, 35 mmol/L HEPES (pH 7.4), 5 mmol/L potassium phosphate (pH 7.4), 5 mmol/L MgCl2, 25 mmol/L CaCl2, 0.15 mol/L sucrose, 2 mmol/L phenylmethylsulfonyl fluoride, and 8 mmol/L dithiothreitol) and frozen at −80°C, as described previously (27). Small aliquot of the cells were checked for cell membrane breakage using trypan blue and cell suspensions were thawed and passed through 28-gauge needles 3 times followed by centrifugation. Supernatant was saved as cytoplasmic lysate, whereas the pellet was suspended in lysis buffer, and the nuclei were lysed by sonication. After centrifugation, supernatant was saved as nuclear lysate.
Western blot analysis
For Western blot analysis, cells were lysed in radio-immunoprecipitation assay (RIPA) buffer containing complete mini EDTA-free protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich; ref. 25). After resolution through 12% PAGE under denaturing conditions, proteins were transferred to nitrocellulose membranes, incubated with appropriate primary and horseradish peroxidase-conjugated secondary antibodies, and further visualized using chemiluminescence detection system (Pierce).
Cell invasion assay
Cell invasion assay was conducted using 24-well Trans-well Permeable Supports with 8 μm pores (Corning), as described previously (25). Briefly, cells were suspended in serum-free medium and seeded into the Transwell inserts coated with growth factor reduced Matrigel (BD Bio-sciences). Bottom wells were filled with media containing complete media. After 24 hours, cells were stained with 4 μg/mL calcein (acetoxymethyl)-ester (calcein AM; Invitrogen) in PBS at 37°C for 1 hour and photographed under a fluorescent microscope. The cells were detached from inserts by trypsinization and fluorescence of the invaded cells was read in ULTRA Multifunctional Microplate Reader (TECAN).
In vivo studies
Female homozygous ICR severe combined immunodeficient (SCID) mice, ages 4 weeks, were used for our study. The animal experimental protocol was approved by the Committee on the Ethics of Animal Experiments of Wayne State University Institutional Users of Animal Care Committee. To initiate the xenografts, 5 × 106 MDA-MB-231 cells (in serum-free medium) were injected subcutaneously in the flank areas of SCID mice bilaterally. Animals were examined 3 times per week until they developed palpable tumors. Then, the animals were randomly divided into 2 groups of 6 animals each (12 tumors per group because of the bilateral tumors in each animal). Group I was assigned as control and received only sesame seed oil without garcinol, whereas group II mice were administered garcinol by oral gavage (5 mg/d/animal by oral gavage), 6 days per week for 4 weeks. Mice from both groups were sacrificed at the end of garcinol treatment. Tumors were harvested from each animal and processed for molecular analysis. There was no loss of weight or any adverse effects were observed in garcinol-administered mice, suggesting nontoxic nature of garcinol in mice.
Immunohistochemistry
Tissue sections were subjected to immunohistochemistry using specific antibodies for Ki-67, CD31, E-cadherin, vimentin, and β-catenin. Tissue sections were deparaffinized, fixed, and stained with appropriate antibodies, as described recently (28). Immunostained slides were blindly evaluated by a trained pathologist (S. Sethi) under a transmission light microscope. Areas of highest staining density were identified for evaluating the expression in tumors.
Data analysis
The experimental results presented in the figures are representative of 3 or more independent observations. The data are presented as the mean values ± SE. Statistical comparisons between the groups were done using one-way ANOVA. Values of P < 0.05 were considered to be statistically significant and individual P-values are reported in the figures, as appropriate.
Results
Garcinol causes reversal of EMT to MET in aggressive breast cancer cells
The inhibition of cell proliferation and induction of apoptosis by garcinol has been previously reported by our laboratory (2) and, therefore, we started our investigation by looking at the expression of markers of EMT, which is one of the characteristics that defines aggressiveness of breast cancer cells. We chose 2 breast cancer cell lines that represent mesenchymal phenotype, MDA-MB-231 and BT-549, for understanding the mechanism of anticancer effects of garcinol. We found that treatment with 25 μmol/L garcinol for 48 hours resulted in a significant (P < 0.05) downregulation of mesenchymal markers, vimentin, ZEB1, and ZEB2 in MDA-MB-231 as well as BT-549 cells (Fig. 1B) with concomitant upregulation of epithelial marker E-cadherin in both the cell lines (Fig. 1B), which was found to be highly significant (P < 0.01). These results suggest an effective switch from MET phenotype of breast cancer cells when exposed to garcinol.
Garcinol-mediated MET involves regulation of miRNAs
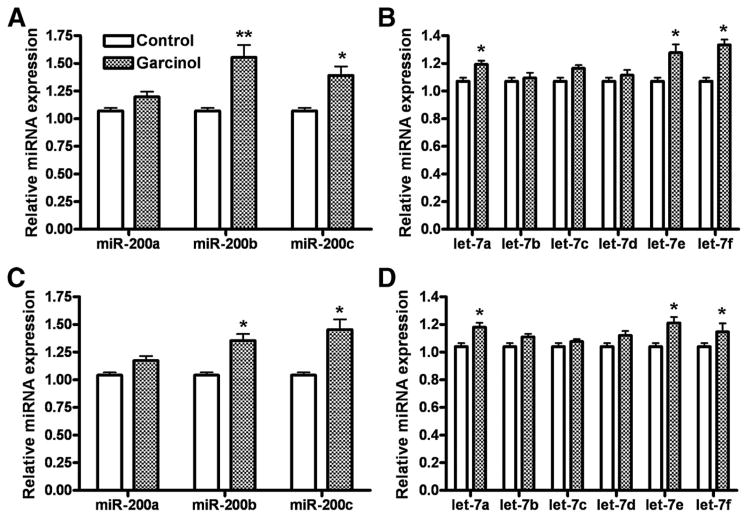
It is increasingly being realized that the phenomena of EMT, that defines the aggressiveness of cancer cells, is regulated by the loss or gain of miRNAs (29). In particular, 2 families of miRNAs, miR-200 family and let-7 family, have been implicated in the maintenance and regulation of EMT to MET switch (29). Because we observed a change in the expression of EMT markers after treatment with garcinol (Fig. 1B), we proceeded to quantitate the expression of miR-200 and let-7 miRNAs to evaluate whether these miRNAs could play a role in garcinol-mediated switch from MET phenotype. As shown in Fig. 2, we found that garcinol upregulated the expression of miR-200 family miRNAs in MDA-MB-231 cells (Fig. 2A) and BT-549 cells (Fig. 2C). Upregulation of miR-200b and miR-200c family members was determined to be significant. An evaluation of let-7 family miRNAs revealed that let-7a, let-7e, and let-7f were significantly upregulated by garcinol in MDA-MB-231 (Fig. 2B) and BT-549 cells (Fig. 2D). Because miR-200 and let-7 family miRNAs are determinants of the epithelial phenotype (29), these results are in agreement with the observation that garcinol induces the expression of epithelial markers, which provides a mechanism, by which this natural agent leads to the induction of MET phenotype in aggressive breast cancer cells making it less aggressive as presented below.
Figure 2.
Effect of garcinol on the expression of miR-200 family in MDA-MB-231 (A) and BT-549 (C), and on let-7 family in MDA-MB-231 (B) and BT-549 (D) breast cancer cells. Cells were treated with 25 μmol/L garcinol for 48 hours, and analysis was done by real-time reverse transcription PCR. *, P < 0.05 and **, P < 0.01 versus respective vehicle controls.
Overexpression of NF-κB abrogates the anticancer activity of garcinol
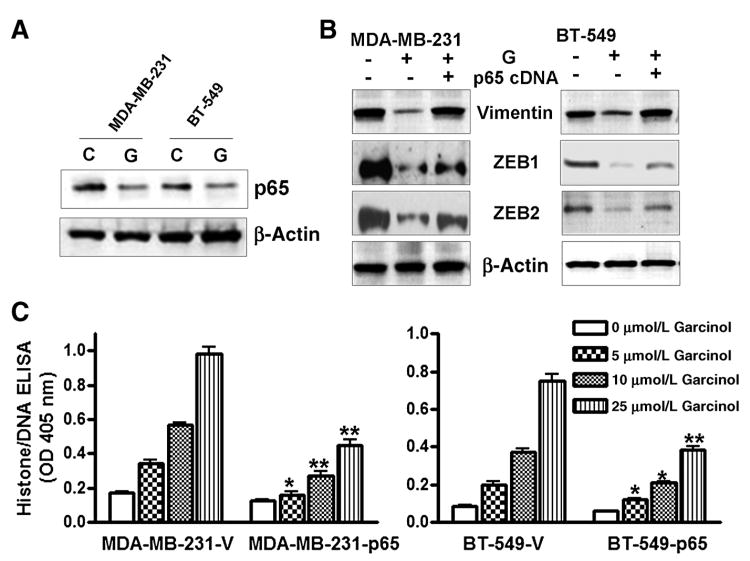
Our earlier results have shown downregulation of NF-κB activity by garcinol treatment (2), and here, we tested the effect of garcinol on the expression of NF-κB, as determined by the expression of its p65 subunit. We found that exposure of cells to garcinol significantly downregulated the expression of NF-κB p65 (Fig. 3A). To further test whether this downregulation of NF-κB could be functionally linked with the reversal of EMT to MET phenotype, we questioned whether overexpression of p65 can protect cells against garcinol-mediated downregulation of mesenchymal markers. Using vimentin, ZEB1, and ZEB2 as markers for mesenchymal phenotype, we studied their expression in garcinol-treated cells, with and without overexpression of NF-κB p65. As expected, expression of vimentin, ZEB1 as well as ZEB2 was significantly downregulated by garcinol in both MDA-MB-231 and BT-549 cells (Fig. 3B), whereas transfection of p65 restored their expression, suggesting a critical role of NF-κb signaling in garcinol-mediated MET. We also tested whether p65 over-expression can reverse the apoptosis-inducing activity of garcinol, and found that garcinol-induced apoptosis was significantly reduced in both MDA-MB-231 and BT-549 cells that were subjected to forced overexpression of NF-κB p65 (Fig. 3C). Such inhibition of garcinol activity was observed at all the concentrations tested.
Figure 3.
A, garcinol downregulated NF-κB expression. Overexpression of NF-κB reversed the effect of garcinol (G) in B, mesenchymal markers vimentin, ZEB1, and ZEB2, and garcinol-induced apoptosis in both the cell lines tested (MDA-MB-231 and BT-549; C).*, P < 0.05 and **, P < 0.01 versus respective vehicle controls. OD, optical density.
Mechanistic role of NF-κB and miRNAs in invasion of breast cancer cells
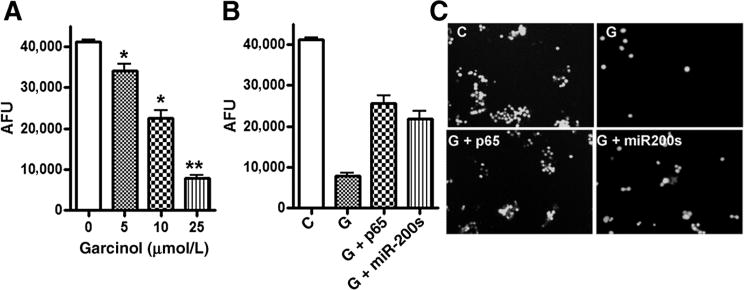
To test whether the observed effects of garcinol on the phenotype of breast cancer cells can be validated functionally, we conducted invasion assays. Treatment of invasive MDA-MB-231 cells by increasing doses of garcinol significantly reduced the number of cells that could invade through the Matrigel-coated wells (Fig. 4A). To ascertain a mechanistic role of NF-κB and miRNAs in the inhibition of invasion by garcinol, we either overexpressed NF-κB p65 or antagonized the expression of miR-200s in MDA-MB-231 cells, and studied the effect of garcinol. We found that overexpression of NF-κB as well as inhibition of miR-200s could significantly attenuate the garcinol-mediated inhibition of invasion (Fig. 4B and C). These results strongly suggest that downregulation of NF-κB signaling and upregulation of miR-200s is the potential molecular mechanism(s) by which garcinol inhibits invasion of aggressive breast cancer cells.
Figure 4.
A, garcinol dose dependently inhibited the invasion of MDA-MB-231 cells. B and C, effect of NF-κB p65 and anti-miR-200 transfection on garcinol-induced inhibition of invasion of MDA-MB-231 cells. C, control (vehicle treatment); G, cells treated with 25 μmol/L garcinol; G + p65, cells transfected with NF-κB p65 followed by 25 μmol/L garcinol treatment, G + miR-200s, cells transfected with anti-miR-200s (anti-miR-200a + anti-miR-200b + anti-miR-200c) followed by garcinol treatment. Bar graphs represent quantitation of the degree of invasiveness as determined by fluorescence values under each condition. *, P < 0.05 and **, P < 0.01 versus vehicle control. AFU, arbitrary fluorescence units.
Role of Wnt signaling pathway in garcinol-mediated effects
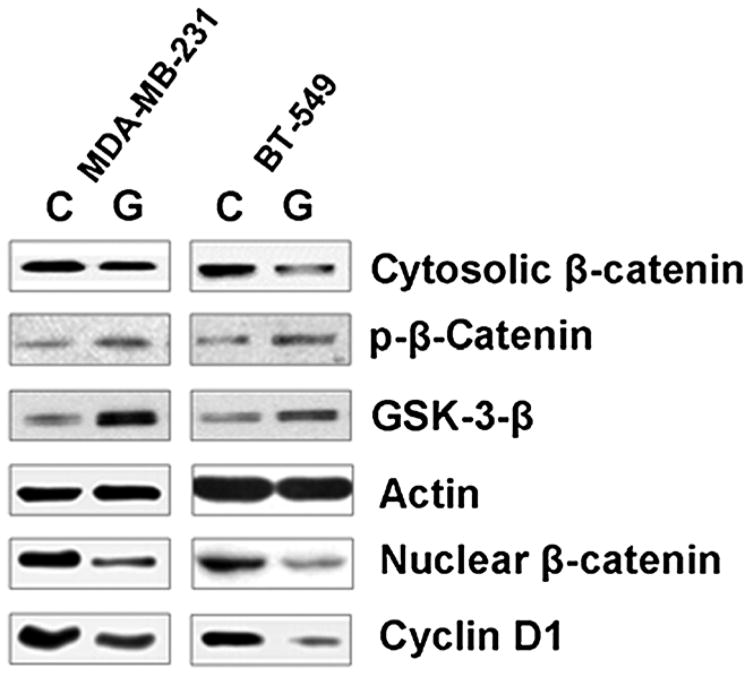
In view of the cross-talk between NF-κB and Wnt signaling pathways in human cancers (23, 30), we further studied the effect of garcinol treatment on Wnt signaling pathway to elucidate the mechanism of anticancer action of garcinol. We observed an inhibition of β-catenin in garcinol-treated MDA-MB-231 as well as BT-549 cells (Fig. 5). β-Catenin levels were found to be decreased in cytosolic fraction and, much more significantly, in the nuclear fraction. An increase in the phosphorylated form of β-catenin was also found after garcinol treatment (Fig. 5). Because phosphorylated β-catenin is targeted for degradation, this might help explain the observed reduced levels of β-catenin in garcinol-treated cells. GSK-3β, the factor that phosphorylates β-catenin, was found to be upregulated by garcinol (Fig. 5). This establishes the complete mechanism by which garcinol regulates Wnt signaling, resulting in the inhibition of nuclear translocation of β-catenin. To further verify an effective inhibition of β-catenin by garcinol, we evaluated the expression of cyclin D1, a downstream target of β-catenin. We found a significant downregulation of cyclin D1 expression in both the cell lines after garcinol treatment (Fig. 5). These observations indicate a potent inhibitory action of garcinol on Wnt signaling pathway.
Figure 5.
Effect of garcinol on Wnt signaling. MDA-MB-231 cells were treated with 25 μmol/L garcinol for 48 hours and the expression of various Wnt signaling factors was evaluated by Western blotting. C, control (vehicle-treated) cells; G, garcinol-treated cells. β-Actin was used as the internal loading control.
In vivo anticancer activity of garcinol
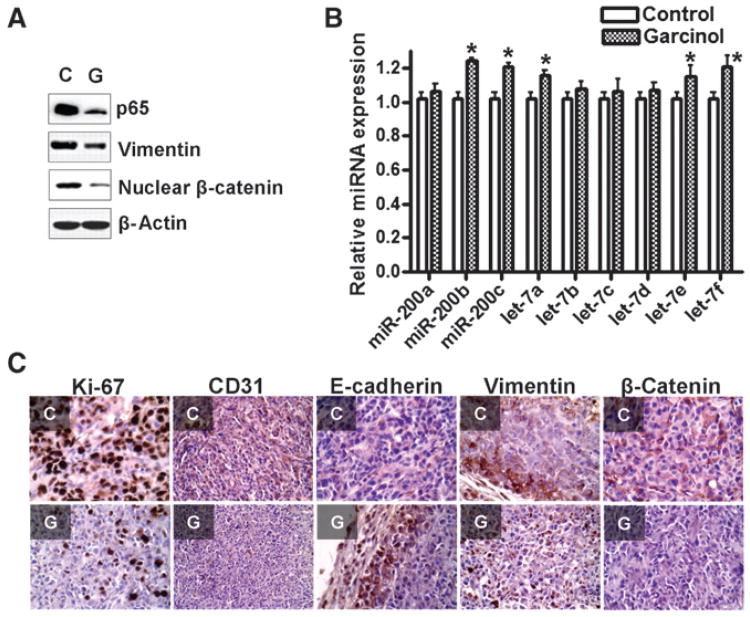
To verify whether our in vitro cell culture findings could be recapitulated in vivo, we used a xenograft mouse model to study the anticancer activity of garcinol. Tumor remnants were processed for present studies. Analyses of proteins, by Western blotting, revealed downregulation of p65, vimentin, and nuclear β-catenin in tumors of garcinol-administered mice (Fig. 6A). Furthermore, we found differential expression of various miR-200 and let-7 family miRNAs. The levels of miR-200b, miR-200c, let-7a, let-7e, and let-7f were significantly upregulated in the mice that received garcinol (Fig. 6B). We also conducted immunohistochemical (IHC) analysis to study the in vivo effects of garcinol on tumor growth from our xenograft model (Fig. 6C). Ki-67 and CD31 immunostaining revealed increased rate of proliferation and vascular density, respectively, in control tumors and both of which were significantly inhibited in garcinol-treated mice (Fig. 6C). Immunostain for EMT markers confirmed the reversal of EMT by garcinol wherein garcinol-treated tumors had upregulated E-cadherin and decreased vimentin (Fig. 6C). Finally, β-catenin expression in the tumor remnant was also found to be inhibited in garcinol-treated mice (Fig. 6C). These results are in full agreement with our observations in vitro, suggesting the mechanistic role of NF-κB, miRNAs, and Wnt signaling as molecular targets of garcinol in mediating its biologic activity.
Figure 6.
Garcinol inhibits NF-κB/EMT/miRNAs/Wnt signaling in vivo. MDA-MB-231 cells were injected in SCID mice subcutaneously and mice were randomized into 2 groups: control and garcinol-treated (n = 6 in each group). Tumor remnants were used to isolate proteins, RNA, and for IHC analyses. A, downregulation of NF-κB, mesenchymal marker vimentin, and nuclear β-catenin in tumor remnants as determined by Western blot analysis. C, control tumor; G, tumor from garcinol-treated animal. B, induction of several miR-200 and let-7 family miRNAs in tumors of garcinol-administered mice. C, IHC staining for proliferation marker Ki-67, vascular density marker CD31, epithelial marker E-cadherin, mesenchymal marker vimentin, and β-catenin in representative tumor samples. CD31 stains are shown at ×20 magnification, whereas all others are shown at ×40 magnification. *, P < 0.05 in treated tumors against vehicle control.
Discussion
The major conclusions of our present study are: (i) garcinol induces MET in aggressive breast cancer cells consistent with decreased mesenchymal markers and upregulated epithelial marker and miRNAs; (ii) forced overexpression of NF-κB abrogates garcinol-induced MET, induction of apoptosis, and inhibition of invasion; and (iii) garcinol also inhibits Wnt signaling pathway in breast cancer cells. In addition to in vitro assays, we carried out experiments in a xenograft mouse model in which we confirmed that the observed modulatory effects of garcinol on EMT, miRNAs, and Wnt signaling in vitro can be recapitulated in vivo as well.
We have earlier reported an antiproliferative and apoptosis-inducing effect of garcinol against ER-positive MCF-7 cells as well as in the triple-negative MDA-MB-231 cells (2). Our results were significant because we found the activity of garcinol to be cancer cell–specific without any effect on the normal breast epithelial MCF10A cells. Breast cancers that express ER, PR, and Her-2/neu (ErbB2) can be treated using targeted therapy; however, a major challenge lies for those tumors that are negative for these receptors (triple negative). About 15% to 21% of breast cancers fall under this category and they are known to be highly aggressive. A growing body of literature suggests that naturally occurring chemical compounds are potent anticancer agents (31). Owing to their pleiotropic nature, they are particularly suited to target difficult to treat cancers, such as triple-negative breast cancers. In addition to the breast cancer, we also showed that garcinol can inhibit the cell growth and induce apoptosis in prostate cancer cells, irrespective of their androgen receptor status as well as in pancreatic cancer cells (18); however, the molecular mechanism of garcinol has not been fully elucidated.
These earlier investigations with garcinol (2, 18) indicated its anticancer activity that was not dependent on the presence or absence of any receptors, suggesting the pleiotropic (multitargeting) activity of garcinol. We have identified NF-κB signaling as a major player that was affected by garcinol in breast, prostate, as well as in pancreatic cancer cells (2, 18). The role of NF-κB signaling pathway in the progression of human cancers is well recognized (32). However, NF-κB signaling cross-talks with multiple pathways (19), and the current study was designed to further understand the intricate details of mechanistic pathways that cross-talk with NF-κB signaling and further define the anticancer activity of garcinol. Specifically, we focused on 2 processes, EMT and Wnt signaling because both of them are connected to NF-κB signaling.
EMT is a phenomenon that is accompanied by increased cell motility and invasion, and involves loss of epithelial cell–cell junction and actin cytoskeleton reorganization. EMT is also regulated by miR-200 and let-7 family of miRNAs (20). These miRNAs are expressed in epithelial and less-differentiated cells, and their expression is lost or downregulated in more aggressive cancer cells that underwent EMT. Wnt is another signaling pathway of interest in the study of aggressive cancers (22). The canonical Wnt pathway is associated with a series of events that occur when Wnt proteins binds to cell surface receptors, ultimately resulting in the translocation of β-catenin to the nucleus. A complex set of factors including GSK-3β are involved in this signaling, which promote the proteolytic degradation of β-catenin, but once this complex is inhibited, β-catenin enters the nucleus leading to the expression of multiple prosurvival signals, such as induced expression of cyclin D1 among others.
Our findings suggest a novel effect of garcinol on EMT and Wnt signaling pathways. Garcinol treatment of breast cancer cells with EMT phenotype led to its reversal to MET phenotype. This effect of garcinol was, in part, mediated through the upregulation of miR-200 and let-7 family miRNAs. While all these signaling pathways/factors independently influence the aggressiveness of breast cancer cells, it is interesting to note that NF-κB serves as a converging point of multiple signaling cascades. There is strong evidence suggesting a cross-talk between NF-κB and EMT/Wnt signaling (21, 23, 30). Our recently published work, involving an experimental metastasis model, has revealed a connection between EMT and miR-200 family (25), which is a direct evidence in support of the role of miR-200 family leading to EMT in aggressive breast cancers. Interestingly, there is also evidence to support regulation of Wnt signaling by miR-200 family. Specifically, β-catenin has been suggested to be a target of miR-200a (33, 34). Our results indicate a significant effect of garcinol on miR-200b, miR-200c, and β-catenin, but not on miR-200a. Taken together, our observation also revealed that several let-7 family miRNAs are also regulated by garcinol, therefore it is reasonable to speculate that the regulation of these miRNAs (miR-200 and let-7) by garcinol could be relevant to its observed effects on EMT. The modulation of Wnt signaling, however, could be an outcome of a direct action of garcinol on GSK-3β and/or β-catenin because the involvement of miRNAs in Wnt signaling regulation by garcinol is yet not conclusive, suggesting that further in-depth investigation is warranted.
An exhaustive survey of literature reveals that even with emerging anticancer reports of garcinol, not much is known about its in vivo effects. A majority of garcinol reports have only focused on its in vitro activity. Here, we not only have identified novel targets of garcinol, but also recapitulated our in vitro findings with in vivo results. On the basis of our in vitro and in vivo observations, garcinol deserves to join the elite group of nutraceuticals that are useful to regulate the expression of miRNAs (35). This opens an entire new area of research, which can potentially help us uncover novel targets/mechanisms of regulation of cellular signaling pathways by such novel anticancer agents, although further mechanistic characterization of garcinol along with its anticancer activity against human malignancies is warranted.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Authors' Contributions: Conception and design: A. Ahmad, S.H. Sarkar, B. Bao, D. Kong, S.B. Padhye, F.H. Sarkar
Development of methodology: A. Ahmad, S.H. Sarkar, B. Bitar, S. Ali, S. Sethi, D. Kong, F.H. Sarkar
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A. Ahmad, S.H. Sarkar, A. Aboukameel, S. Sethi, B. Bao, F.H. Sarkar
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A. Ahmad, B. Bitar, S. Ali, A. Aboukameel, S. Sethi, B. Bao, F.H. Sarkar. Writing, review, and/or revision of the manuscript: A. Ahmad, S. Sethi, S. Banerjee, F.H. Sarkar
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): A. Ahmad, S.H. Sarkar, B. Bitar, Y. Li, F.H. Sarkar
Study supervision: A. Ahmad, B. Bitar, F.H. Sarkar
Took photomicrographs and evaluated microscopically the histopathology slides: S. Sethi
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad A, Wang Z, Ali R, Maitah MY, Kong D, Banerjee S, et al. Apoptosis-inducing effect of garcinol is mediated by NF-kappaB signaling in breast cancer cells. J Cell Biochem. 2010;109:1134–41. doi: 10.1002/jcb.22492. [DOI] [PubMed] [Google Scholar]
- 3.Padhye S, Ahmad A, Oswal N, Sarkar FH. Emerging role of garcinol, the antioxidant chalcone from Garcinia indica Choisy and its synthetic analogs. J Hematol Oncol. 2009;2:38. doi: 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi F, Saito M, Ariga T, Yoshimura Y, Nakazawa H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem. 2000;48:2320–5. doi: 10.1021/jf990908c. [DOI] [PubMed] [Google Scholar]
- 5.Pan MH, Chang WL, Lin-Shiau SY, Ho CT, Lin JK. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J Agric Food Chem. 2001;49:1464–74. doi: 10.1021/jf001129v. [DOI] [PubMed] [Google Scholar]
- 6.Hong J, Kwon SJ, Sang S, Ju J, Zhou JN, Ho CT, et al. Effects of garcinol and its derivatives on intestinal cell growth: inhibitory effects and autoxidation-dependent growth-stimulatory effects. Free Radic Biol Med. 2007;42:1211–21. doi: 10.1016/j.freeradbiomed.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Kohno H, Shimada R, Kagami S, Yamaguchi F, Kataoka S, et al. Prevention of colonic aberrant crypt foci by dietary feeding of garcinol in male F344 rats. Carcinogenesis. 2000;21:1183–9. [PubMed] [Google Scholar]
- 8.Liao CH, Sang S, Ho CT, Lin JK. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J Cell Biochem. 2005;96:155–69. doi: 10.1002/jcb.20540. [DOI] [PubMed] [Google Scholar]
- 9.Koeberle A, Northoff H, Werz O. Identification of 5-lipoxygenase and microsomal prostaglandin E2 synthase-1 as functional targets of the anti-inflammatory and anti-carcinogenic garcinol. Biochem Pharmacol. 2009;77:1513–21. doi: 10.1016/j.bcp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto K, Akao Y, Kobayashi E, Ito T, Ohguchi K, Tanaka T, et al. Cytotoxic benzophenone derivatives from Garcinia species display a strong apoptosis-inducing effect against human leukemia cell lines. Biol Pharm Bull. 2003;26:569–71. doi: 10.1248/bpb.26.569. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida K, Tanaka T, Hirose Y, Yamaguchi F, Kohno H, Toida M, et al. Dietary garcinol inhibits 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in rats. Cancer Lett. 2005;221:29–39. doi: 10.1016/j.canlet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Hong J, Sang S, Park HJ, Kwon SJ, Suh N, Huang MT, et al. Modulation of arachidonic acid metabolism and nitric oxide synthesis by garcinol and its derivatives. Carcinogenesis. 2006;27:278–86. doi: 10.1093/carcin/bgi208. [DOI] [PubMed] [Google Scholar]
- 13.Prasad S, Ravindran J, Sung B, Pandey MK, Aggarwal BB. Garcinol potentiates TRAIL-induced apoptosis through modulation of death receptors and antiapoptotic proteins. Mol Cancer Ther. 2010;9:856–68. doi: 10.1158/1535-7163.MCT-09-1113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chen CS, Lee CH, Hsieh CD, Ho CT, Pan MH, Huang CS, et al. Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast Cancer Res Treat. 2010;125:73–87. doi: 10.1007/s10549-010-0821-3. [DOI] [PubMed] [Google Scholar]
- 15.Cheng AC, Tsai ML, Liu CM, Lee MF, Nagabhushanam K, Ho CT, et al. Garcinol inhibits cell growth in hepatocellular carcinoma Hep3B cells through induction of ROS-dependent apoptosis. Food Funct. 2010;1:301–7. doi: 10.1039/c0fo00134a. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad A, Padhye S, Sarkar FH. Role of novel nutraceuticals garcinol, plumbagin and mangiferin in the prevention and therapy of human malignancies: mechanisms of anticancer activity. In: Sarkar FH, editor. Nutraceuticals and cancer. New York: Springer; 2012. pp. 179–99. [Google Scholar]
- 17.Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, et al. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004;279:33716–26. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad A, Wang Z, Wojewoda C, Ali R, Kong D, Maitah MY, et al. Garcinol-induced apoptosis in prostate and pancreatic cancer cells is mediated by NF-kappaB signaling. Front Biosci (Elite Ed) 2011;3:1483–92. doi: 10.2741/e349. [DOI] [PubMed] [Google Scholar]
- 19.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–12. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar FH, Li Y, Wang Z, Kong D. The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer. Cancer Metastasis Rev. 2010;29:383–94. doi: 10.1007/s10555-010-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh M. Regulation of WNT3 and WNT3A mRNAs in human cancer cell lines NT2, MCF-7, and MKN45. Int J Oncol. 2002;20:373–7. [PubMed] [Google Scholar]
- 24.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, et al. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–21. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, et al. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res. 2011;71:3400–9. doi: 10.1158/0008-5472.CAN-10-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad A, Banerjee S, Wang Z, Kong D, Sarkar FH. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J Cell Biochem. 2008;105:1461–71. doi: 10.1002/jcb.21966. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–50. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 28.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2011;3:90–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad A, Ali AS, Ali S, Wang Z, Kong D, Sarkar FH. MicroRNAs: targets of interest in breast cancer research. In: Mulligan JA, editor. MicroRNA: expression, detection and therapeutic strategies. New York: Nova Publishers; pp. 2011pp. 59–78. [Google Scholar]
- 30.Ougolkov A, Zhang B, Yamashita K, Bilim V, Mai M, Fuchs SY, et al. Associations among beta-TrCP, an E3 ubiquitin ligase receptor, beta-catenin, and NF-kappaB in colorectal cancer. J Natl Cancer Inst. 2004;96:1161–70. doi: 10.1093/jnci/djh219. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–23. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 33.Xia H, Ng SS, Jiang S, Cheung WK, Sze J, Bian XW, et al. miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem Biophys Res Commun. 2010;391:535–41. doi: 10.1016/j.bbrc.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 34.Saydam O, Shen Y, Wurdinger T, Senol O, Boke E, James MF, et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol. 2009;29:5923–40. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Kong D, Wang Z, Sarkar FH. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res. 2010;27:1027–41. doi: 10.1007/s11095-010-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]