Abstract
The ability to resist infections and respond to vaccinations is greatly reduced in the older adult population owing to a general decline in innate and adaptive immune functions with aging. Over the years several strategies such as increasing the vaccine dose, number of immunizations and using adjuvants have been evaluated to improve the immunogenicity and efficacy of vaccines in the older adult population. Murine ß-defensin 2 (Mbd2) has been shown to function as a molecular adjuvant by recruiting and activating immature dendritic cells (DCs), professional antigen-presenting cells (APC), to the site of the immunization. In this study, we evaluated the potential utility of Mbd2 to enhance the efficacy of an adenoviral vector-based H5N1 influenza vaccine expressing hemagglutinin (HA) and nucleoprotein (NP) (HAd-HA-NP) in an aged mouse model. Our results indicated that immunostimulation with an adenoviral vector expressing Mbd2 (HAd-Mbd2) activated DCs and significantly enhanced the humoral and cellular immune responses induced by HAd-HA-NP. Furthermore, immunostimulation with HAd-Mbd2 followed by immunization with HAd-HA-NP resulted in significantly lower virus titers in the lungs following challenge with a H5N1 influenza virus compared to the group immunized with HAd-HA-NP without immunostimulation. Overall, our results highlight the potential utility of Mbd2 as a molecular adjuvant to enhance the immunogenicity and protective efficacy of vaccines for the elderly.
Keywords: adenovirus, avian influenza, adenovirus vector-based vaccines, vaccine, influenza, pandemic influenza vaccine, Mbd2, murine beta defensin 2, aged mice
1. INTRODUCTION
The older adult population has increased considerably worldwide during the last century. It is estimated that by the year 2030, older adults (≥ 65 years) will comprise 20 % of US population (Howden and Meyer, 2011). With aging, the ability to resist infectious diseases and respond to preventive vaccinations decreases significantly (Aspinall et al., 2007). From 1976 to 2007 in the U.S., the estimated annual average of deaths resulting from respiratory or circulatory causes associated with seasonal influenza was about 23,600 (range 3,300 to 48,600) (MMWR, 2010). Persons aged 65 years and older account for 90% of deaths caused by influenza. Although, older adults are a major target group for annual seasonal influenza vaccination, currently licensed inactivated vaccines are less effective in this age group compared with healthy, younger adults(Fiore A.E. et al., 2012). Hence, there is a need to develop novel vaccine approaches which will be more effective in the older adult population.
Defensins are small antimicrobial peptides that are produced by the host in response to microbial infection (Oppenheim et al., 2003; Yang et al., 2002). Based on the size and disulfide bond formation, defensins are classified into α, β, and θ types (Oppenheim et al., 2003). ß-defensin 2 (bd2) belonging to the β-defensin class is mainly expressed by epithelial cells (Morrison et al., 1999). This defensin has been shown to chemoattract immature dendritic (DCs) cells and acts as an endogenous ligand for the toll like receptor 4 (TLR4) thereby inducing the expression of co-stimulatory molecules and the activation of DCs resulting in an enhancement in antigen-specific immune responses (Biragyn et al., 2002b).
Incidences of highly pathogenic avian H5N1 influenza virus infections in humans with a fatality rate of approximately 60% highlight the pandemic threat posed by these viruses (Hoelscher et al., 2008a; Vemula and Mittal, 2010). Although human-to-human transmission has been limited and infrequent, it is widely believed that a H5N1 virus could acquire a pandemic potential either by genetic reassortment with a human influenza virus or by mutations in the H5N1 virus genome (Imai et al., 2012; Russell et al., 2012). Vaccination remains the most efficient cost-effective strategy to combat the pandemic threat posed by H5N1 viruses (Singh et al., 2010).
In this study, we evaluated the potential of Mbd2 to enhance the efficacy of a human adenovirus (HAd)-based vaccine (HAd-HA-NP) carrying the hemagglutinin (HA) and nucleoprotein (NP) genes of a H5N1 influenza virus in aged mice. We chose to use vaccination against pseudotyped H5N1 virus in this proof-of concept study because the development of effective vaccines against this potential pandemic threat for all age groups remains a public health priority. Immunization of mice with a HAd vector (HAd-Mbd2) expressing Mbd2 prior to HAd-HA-NP vaccination resulted in a significant enhancement of antigen-specific humoral and cellular immune responses compared to vaccination without Mbd2 immunostimulation. Furthermore, Mbd2 treated and vaccinated mice had a significant reduction of virus titers in the lungs following challenge with a H5N1/PR8 reassortant/ pseudotyped virus.
2. MATERIALS AND METHODS
2.1. Cell lines and recombinant viruses
293 (human embryonic kidney cells expressing human adenovirus serotype 5 (HAd5) early region 1 (E1) gene products), 293Cre (293 cells that constitutively expresses Cre-recombinase enzyme; a gift from Merck Inc., Whitehouse Station, NJ) (Graham et al., 1977) and BHH2C (bovine-human hybrid clone 2C)(van Olphen and Mittal, 2002) were used. These cell lines were grown as monolayer cultures in Eagle’s minimum essential medium (MEM) (Life Technologies, Gaithersburg, MD) supplemented with 10% reconstituted bovine serum (Fetal Clone III; Hyclone, Logan, UT) and 50 μg/ml gentamycin. All adenovirus constructs were purified by cesium chloride density-gradient centrifugation and titrated by plaque assay on BHH2C as previously described (Bangari and Mittal, 2004). The construction and propagation of replication defective HAd-ΔE1E3 (HAd5 vector having deletions in the E1 and E3 regions) has been previously described (Tandon et al., 2012).
2.2. Generation of replication-defective HAd-Mbd2
The Cre-recombinase-mediated site-specific recombination system was used to construct a replication-defective HAd5 vector expressing Mbd2 (HAd-Mbd2). The entire cDNA clone of the Mbd2 gene was synthesized (Integrated DNA technologies, Skokie, IL) and inserted into a pDC311 transfer plasmid (Microbix, Inc., Toronto, Ontario, Canada) (Bangari and Mittal, 2004) under the control of a cytomegalovirus (CMV) promoter and the bovine growth hormone (BGH) polyadenylataion (polyA) signal to generate pDC311-Mbd2. This pDC311-Mbd2 was co-transfected with pBHGloxΔE1E3Cre (a plasmid containing almost the entire HAd5 genome and a loxP site except for the packaging signal, E1 and E3 deletions) in 293Cre cells to generate the infectious recombinant virus HAd-Mbd2. This recombinant virus was plaque purified, and its genome was analyzed by restriction enzyme digestions to confirm the presence of the Mbd2 gene cassette and the absence of any other major deletion or insertion.
2.3. Western blot analysis
The procedure was essentially the same as described previously(Pandey et al., 2012). In brief, 293 cells were mock-infected or infected with either an empty vector (HAd-ΔE1E3) or HAd-Mbd2 at an multiplicity of infection (MOI) of 20 plaque forming units (pfu) per cell. Cell supernatants were collected 48 hours (h) post-infection and were analyzed by Western blot using a monoclonal antibody against Mbd2 (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:500 dilution and HRP-conjugated donkey anti-goat IgG (Santa Cruz Biotechnology) at a 1:3000 dilution as a the primary and secondary antibodies, respectively. Mock or HAd-ΔE1E3-infected cell supernatants served as negative controls.
2.4. Transwell migration assay
The bioactivity of Mbd2 expressed by HAd-Mbd2 was determined by evaluating the ability of cell supernatants from HAd-Mbd2-infected 293 cells to attract immature mouse DCs. Mouse immature DCs were isolated as described elsewhere (Nair et al., 2003). Briefly, bone marrow was collected from the tibias and femurs of 6-8 week-old BALB/c mice. Erythrocytes were lysed with ACK RBC lysis buffer (Lonza, Walkersville, MD) for 5 minutes at 37°C. The precursors were plated in Roswell Park Memorial Institute medium (RPMI; GIBCO, Grand Island, NY) containing 5% Fetal Clone III supplemented with 15 ng/mL of granulocyte macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ) and 10 ng/mL interleukin (IL)-4 (Peprotech, Rocky Hill, NJ,). Three days later, the floating cells were removed, and the plates were replenished with fresh RPMI medium containing GM-CSF and IL-4-. Non adherent cells were harvested on Day 5. To confirm the phenotype of the immature DCs, expression of cell surface markers (such as CD80, CD86, CD40, and MHCII along with CD11c) were analyzed by flow cytometry. The migration of immature DCs was assessed using 5 μm pore transwells (Costar, Cambridge, MA). Immature DCs (50 μl from a 106/ml suspension) were added to the upper compartment. Supernatants from either mock-infected, HAd-Mbd2- or HAd-ΔE1E3-infected 293 cells were collected at 48 h post-infection, and 600 μl of these supernatants were added to the lower compartment. For a positive control, monocyte chemotactic protein-1 (MCP1) (Abcam, Cambridge, MA) was added to the lower compartment. Cells were incubated for 2 h at 37°C in a CO2 incubator. The immature DCs that migrated to the lower compartment were collected and counted. To evaluate the percentage of migration, the number of migrated DCs was divided by the total number of cells at the time of harvest.
2.5. In vivo activation of DCs by Mbd2
All animal studies were conducted following guidelines and approvals from Institutional Biosafety Committee and Institutional Animal Care and Use Committee at Purdue University. Aged (18 month-old) female BALB/c mice were procured from the National Institute of Aging, Bethesda, MD and the young 6-8 week old female BALB/c mice were procured from Harlan Sprague Dawley Inc., Indianapolis. Animals (3 mice/group) were inoculated intramuscularly (i.m.) with either phosphate-buffered saline (PBS), 1 × 108 pfu of HAd-Mbd2, or HAd-ΔE1E3. Seven days post-inoculation, animals were euthanized, and the spleen and inguinal lymph nodes were collected. Spleen and lymph nodes were mechanically disrupted and then digested using 1mg/ml collagenase D (Sigma Aldrich). The cells were then passed through a 70 μ cell strainer, washed in PEB buffer (PBS containing 10 mM EDTA and 0.1% BSA), and the red blood cells were depleted by ACK lysis buffer (Lonza, Walkersville, MD). The cells from spleens and lymph nodes (pooled from three animals) were washed and used for DC purification by positive selection using CD11c microbeads (Miltenyi Biotec, Auburn, CA). Fcγ receptors were blocked by anti-mouse CD16/CD32 before incubation with the immunomagnetic beads. The enriched CD11c+ cells were stained with allophycocyanin (APC)-conjugated anti-CD11c and flouro-isothiocyanin (FITC)-conjugated anti-CD11b antibodies. These cells were then stained with a biotinylated anti-CD80, anti-CD86, or anti-CD40 antibody and later with phycoerythrin (PE)-conjugated streptavidin. Appropriate isotype-matched immunoglobulins were used as negative controls. Flow cytometric analyses were done using BD FACSCantoII, (BD Bioscience, San Jose, CA) and FACSDiva software. Data were processed using WinMDI software.
2.6. Immunogenicity and protection study in aged mice
Aged mice (8 animals/group) were inoculated i.m. with 1 × 108 pfu of HAd-Mbd2. Seven days post-immunostimulation, the animals were immunized i.m. with 1 × 108 pfu of HAd-HA-NP. Control groups received PBS, followed by HAd-Mbd2 or HAd-HA-NP vaccine on Day 7. The vector control group received 1 × 108 pfu of HAd-ΔE1E3 twice in place of immunostimulation and vaccine. Four weeks post-immunization, blood samples were collected by retro-orbital puncture to evaluate the development of HA-specific antibodies. Three mice per group were euthanized, and the spleens were collected to evaluate the induction of HA-518 or NP-147-specific cell-mediated immune responses. Five mice from each group were challenged with 100 MID50 (50% mouse infectious dose) of VNH5N1-PR8/CDC-RG (a reassortant virus strain containing the HA gene (with a modified basic amino acid cleavage site) segment, the neuraminidase (NA) gene segment from the A/Vietnam/1203/2005 (H5N1) virus and the six internal gene segments of A/Puerto Rico/8/34 (H1N1) virus generated by reverse genetics (RG) technology) (Subbarao et al., 2003). Mouse infectious dose 50 (MID50) titers were determined by inoculating groups of young (6-8 week-old) BALB/c mice i.n. with 10-fold dilutions serial of the virus. MID50 titers were calculated by the method of Reed and Muench and were expressed as the egg infectious dose 50 (EID50) values. The reassortant virus is neither lethal nor does it produce clinical disease and weight loss in mice; therefore, the protective efficacy was monitored by virus clearance from the lungs. Three days post-challenge, these mice were euthanized, and the lungs were collected to determine virus titers in the embryonated eggs.
2.7. Hemagglutination inhibition Assay
Sera from all mice were treated with a receptor-destroying enzyme from Vibrio cholerae (Denka Seiken, Tokyo, Japan) at 37°C for 16 h to destroy non-specific serum inhibitor activity. The hemagglutination inhibition (HI) antibody titers were determined using VNH5N1-PR8/CDC-RG virus and 0.5% horse red blood cells (HRBC) as described (Pandey et al., 2012).
2.8. NP-147 epitope-specific pentamer staining
Splenocytes were isolated and stained as previously described with a murine MHC-encoded allele Kd–specific pentamer for immunodominant NP-147 epitope (Proimmune Inc., Bradenton, FL)-conjugated with PE and an anti-CD8 antibody (BD PharMingen, San Jose, CA.)-conjugated with APC according to the manufacturer’s instructions (Hoelscher et al., 2006; Hoelscher et al., 2008b). B cells were removed by staining splenocytes with anti-CD19 FITC (FITC; BD PharMingen, San Jose, CA) and gating them out in analysis. Flow cytometric analyses were done using BD FACSCantoII, (BD Bioscience) to identify the percentage of NP-147 specific CD8+ T cells among the total splenic CD8+ T cells (where 500,000 total events were collected).
2.9. ELISpot assay
96-well filter plates (Millipore, Bedford, MA) were coated with an anti-mouse interferon gamma (IFN-γ) antibody (BD Bioscience) and incubated at 4°C overnight. Splenocytes (3.3 × 105 to 1 × 106 cells per well) from each mouse were cultured in the presence of the HA-518 and NP-147 peptide (3μg/ml) in RPMI medium supplemented with 10% Fetal Clone III for 60 h and developed according to an ELISpot protocol (Hoelscher et al., 2007). The spots were counted using Bioreader 5000 (BIOSYS, Miami, FL.
2.10. Statistical analysis
Student’s t-test was used for determination of significance, which was set at P ≤0.05
3. RESULTS
3.1. Generation and characterization of HAd5 vector expressing Mbd2
The full length coding region of the Mbd2 gene under the control of a CMV promoter and the BGH polyA was inserted in the E1 region of HAd5 genome in the E1-parallel orientation using the Cre-recombinase-mediated site-specific recombination (Figure 1a). Western blot analysis was done to confirm the expression of Mbd2 in HAd-Mbd2-infected 293 cells. A band corresponding to 6 kDa was visible in the HAd-Mbd2-infected 293 cell supernatant (Figure 1b). The HAd5 vector with deletions of the E1 and E3 regions (HAd-ΔE1E3) served as a negative control.
Figure 1.
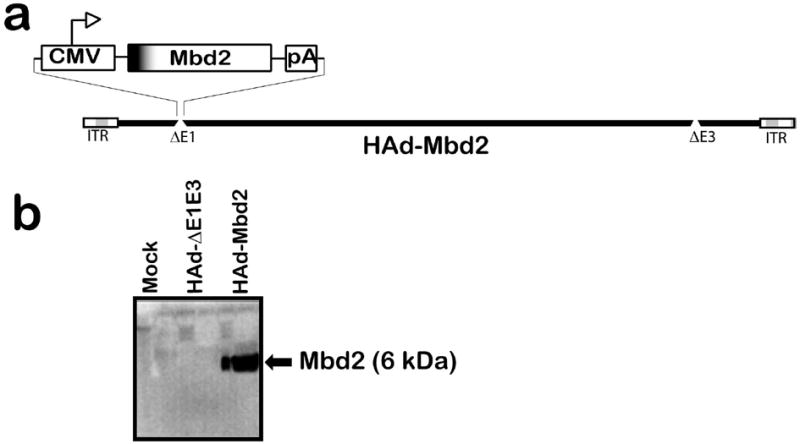
(a) Diagrammatic representation of HAd-Mbd2 [HAd-ΔE1E3 carrying the murine ß-defensin 2 (Mbd2) gene cassette in the E1 region]. ITR, inverted terminal repeat. (b) Expression of Mbd2 in 293 cells infected with HAd-Mbd2. Mock (PBS), HAd-ΔE1E3, or HAd-Mbd2 infected 293 cells were harvested 48 h post-infection, and cell supernatants were analyzed by Western blot using a mouse monoclonal antibody against Mbd2.
3.2. Mbd2 expressed by HAd-Mbd2 activates DCs
To evaluate the functionality of Mbd2 expressed by HAd-Mbd2, the culture supernatants from HAd-Mbd2, HAd-ΔE1E3 or mock infected 293 cells were tested for their ability to chemoattract murine bone marrow–derived immature DCs. The purity of murine bone marrow–derived DCs and their immature phenotype was confirmed by the expression of CD11c+ and the low levels of CD40, CD86 and MHC class II expression (data not shown). The percent migration of the immature bone marrow-derived DCs induced by supernatants from HAd-Mbd2 infected cell was significantly (P<0.001) higher than that induced by supernatants from the vector control (Figure 2). The result clearly suggests that HAd-Mbd2 induced the chemotaxis of the immature DCs.
Figure 2. In vitro biofunctional assay to characterize HAd-Mbd2.
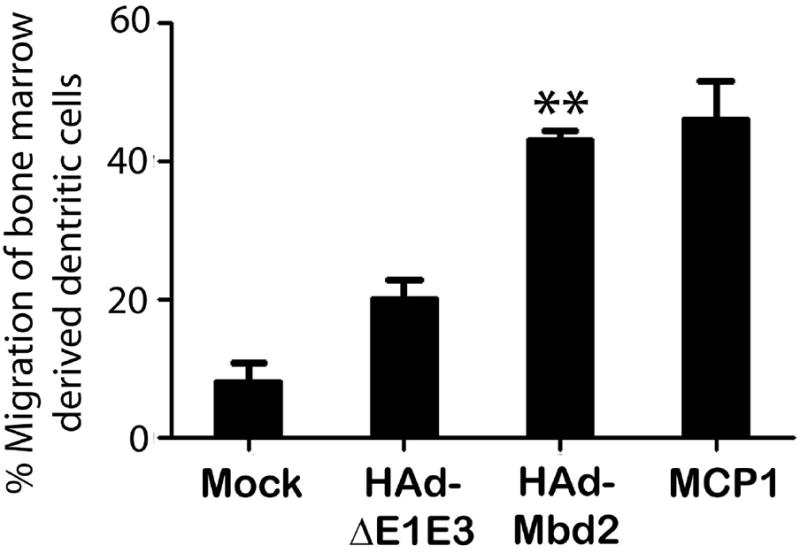
Migration of immature dendritic cells (DCs) induced by the supernatants from HAd-Mbd2, empty vector (HAd-ΔE1E3) or mock (PBS)-infected 293 cells was examined using a 5-μm pore transwells assay. In the positive control, MCP1 protein was added to the lower compartment. The rate of migration was expressed in percentages of migrated cells as a fraction of the total number of cells placed in the upper compartment. Data represent mean ± SD of three experiments. **; P<0.001, compared to HAd-ΔE1E3 control group.
To evaluate the effect of HAd-Mbd2 on in vivo DC activation, young and aged mice were inoculated with HAd-Mbd2. Control mice received either PBS or a similar dose of HAd-ΔE1E3. Seven days post-inoculation, mice were euthanized, and DCs were purified from the spleen and pooled lymph node cells. The purity of the DCs preparation was 90% (data not shown). The DCs isolated from both spleen and inguinal lymph nodes from both young and aged mice showed significant (P<0.05) increases in the expression levels of the CD40, CD80 and CD86 activation markers in response to HAd-Mbd2 compared to the mice inoculated with an empty vector (Figure 3a and 3b). These results suggest that DCs from both young and aged mice developed an activated phenotype when stimulated with HAd-Mbd2.
Figure 3. In vivo activation of dendritic cells by murine ß-defensin 2 (Mbd2).
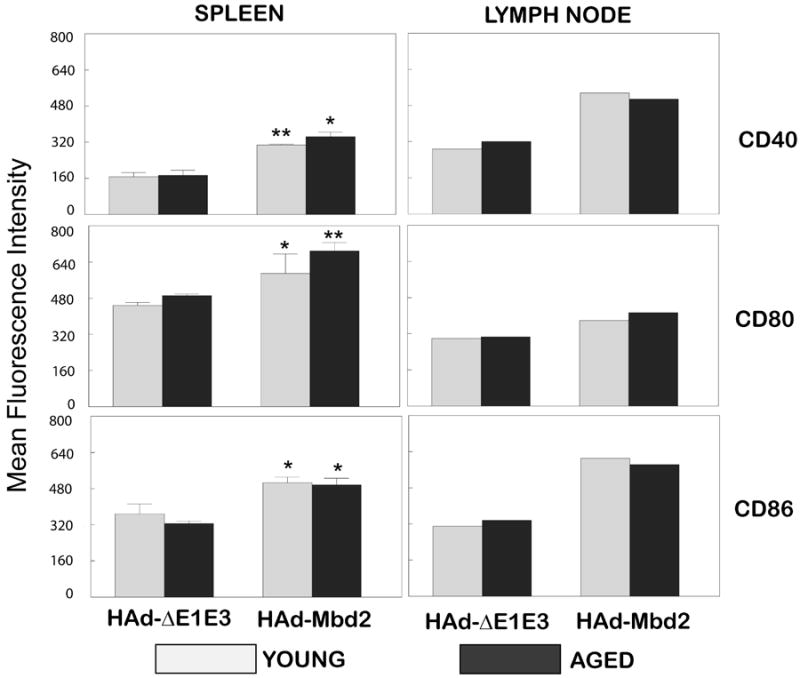
Young (6-8 week old) and aged (18 month old)- mice were inoculated intramuscularly (i.m.) either with PBS, 1 × 108 plaque forming units (pfu) of HAd-Mbd2 or HAd-ΔE1E3 (empty vector). Seven days post-inoculation, animals were euthanized and DCs from the spleen (3a) and pooled inguinal lymph nodes were positively selected using CD11c microbeads (Miltenyi Biotec, Auburn, CA). The enriched CD11c+cells were stained with allophycocyanin (APC)-conjugated anti-CD11c and flouro-isothiocyanin (FITC)-conjugated anti-CD11b antibodies. These cells were then stained with a biotinylated anti-CD80, anti-CD86, or anti-CD40 antibody and later with phycoerythrin (PE)-conjugated streptavidin. Appropriate isotype-matched immunoglobulins were used as negative controls. Flow cytometric analyses were done using BD FACSCantoII, (BD Bioscience, San Jose, CA) and FACSDiva software. Data were processed using WinMDI software. The fluorescence intensity data for CD40, CD80 and CD86 expression on DCs from the spleens are represented as mean ± standard deviation from three mice/group. *; P<0.05, **; P≤0.001, compared to HAd-ΔE1E3 control group.
3.3. Immunostimulation with HAd-Mbd2 enhances humoral immune responses in aged mice vaccinated with HAd-HA-NP
The development of robust HI antibody titers is an important indicator of the immunogenicity and protective efficacy of an influenza vaccine. To evaluate if HAd-Mbd2 immunostimulation would enhance the antibody responses induced by the HAd-HA-NP vaccine in aged mice, serum samples collected from immunized mice were analyzed for HI antibody titers. As shown in Table 1, aged mice immunostimulated with HAd-Mbd2 and then vaccinated with HAd-HA-NP developed significantly (P≤0.05) higher HI titers compared to those vaccinated with HAd-HA-NP without immunostimulation. HI titers in aged mice vaccinated with either HAd-HA-NP without HAd-Mbd2 immunostimulation or HAd-ΔE1E3 were below the level of detection. Single inoculation of young mice with the same dose of HAd-HA-NP elicited robust HI titers (unpublished data).
Table 1.
Post-immunization hemagglutination inhibition (HI) antibody titers and lung viral titers 3 days post-challenge with a homologous H5N1 reassortant virus in vaccinated aged mice.
| Group | Immunostimulation or Pre-immunization | Immunization | HI titers (Geometric mean) | Lung virus titer (Log10 EID50/ml ±SD) |
|---|---|---|---|---|
| Vector Control | HAd-ΔE1E3 | HAd-ΔE1E3 | ≤10 | 6.50±0.10 |
| Without immunostimulation | PBS | HAd-HA-NP | ≤10 | 4.60±0.50 |
| With Immunostimulation | HAd-Mbd2 | HAd-HA-NP | 50 | 3.10± 0.30 |
Aged mice (18 month-old) were inoculated intramuscularly (i.m.) either with PBS or with 1 × 108 plaque-forming units (pfu) of HAd-Mbd2 [adenovirus vector expressing murine ß-defensin 2 (Mbd2)]. Seven days later, animals were immunized with 1 × 108 pfu of HAd-HA-NP [adenovirus vector expressing hemagglutinin (HA) and nucleoprotein (NP) of a H5N1 influenza virus]. Animals that were similarly inoculated with 1 × 108 pfu of HAdΔE1E3 on days 0 and 7 served as negative controls. Serum samples were obtained from all animals four weeks after the last inoculation and analyzed for A/Vietnam/1203/2004–specific hemagglutination inhibition (HI) titers by HI assay using 1% horse red blood cells. The titers are shown as geometric mean values. Four weeks after the last immunization, mice from each group were challenged with 100 MID50 (50% mouse infectious dose) of reverse genetics-derived A/Puerto Rico/8/1934 (H1N1) [PR8] containing HA and NA gene fragments of A/Vietnam/1203/04 (H5N1) [VNH5N1-PR8/CDC-RG]. Three days post-challenge, mice were euthanized, and the lungs were collected. The lung viral titers were determined to evaluate the protective efficacy of the vaccine. The detection limit of the lung viral titer was ≤1.5 Log10EID50 /ml. EID, egg infectious dose.
3.4. Immunostimulation with HAd-Mbd2 enhances cellular immune responses in aged mice vaccinated with HAd-HA-NP
The cell-mediated immune (CMI) responses play an important role in influenza virus clearance and thus contribute to recovery from the infection (Thomas et al., 2006). To assess the effect of Mbd2 on the cellular immune responses induced by the HAd-HA-NP vaccine, aged mice were immunized with HAd-Mbd2 and, seven days later, immunized with HAd-HA-NP vaccine. Control groups received PBS followed by either HAd-Mbd2 or HAd-HA-NP vaccine on Day 7. Four weeks after immunization, spleens were collected and analyzed by pentamer staining and interferon-gamma (IFN-γ)-specific ELISpot assay to monitor the frequency and functionality of influenza-specific CD8+ T cells. The frequency of NP-147 epitope-specific CD8+ T cells in splenocytes of aged mice in both the mock-treated HAd-Mbd2 group (data not shown) or the HAd-HA-NP vaccine group were at the background levels similar to the vector control group (Figure 4). However, the aged mice immunostimulated with HAd-Mbd2 prior to vaccination had a significantly higher level of NP-147 epitope-specific CD8+ T cells compared to the PBS-HAd-HA-NP (P<0.0001) vaccine group (Figure 4). Similarly, the frequency of IFN-γ secreting HA-518 or NP-147 epitope-specific CD8+ T in the spleens of the mock-treated vaccine group were at background levels similar to the vector control group that did not receive the vaccine. Immunostimulation of aged mice resulted in a significantly higher frequency of IFN-γ secreting HA-518 or NP-147 epitope-specific CD8+ T cells when compared to either the HAd-ΔE1E3- or mock-HAd-HA-NP vaccine group (Figure 5).
Figure 4. Effect of murine ß-defensin 2 (Mbd2) on the frequency of NP-147 epitope-specific CD8+ T cells in the spleens of HAd-HA-NP vaccinated aged mice.
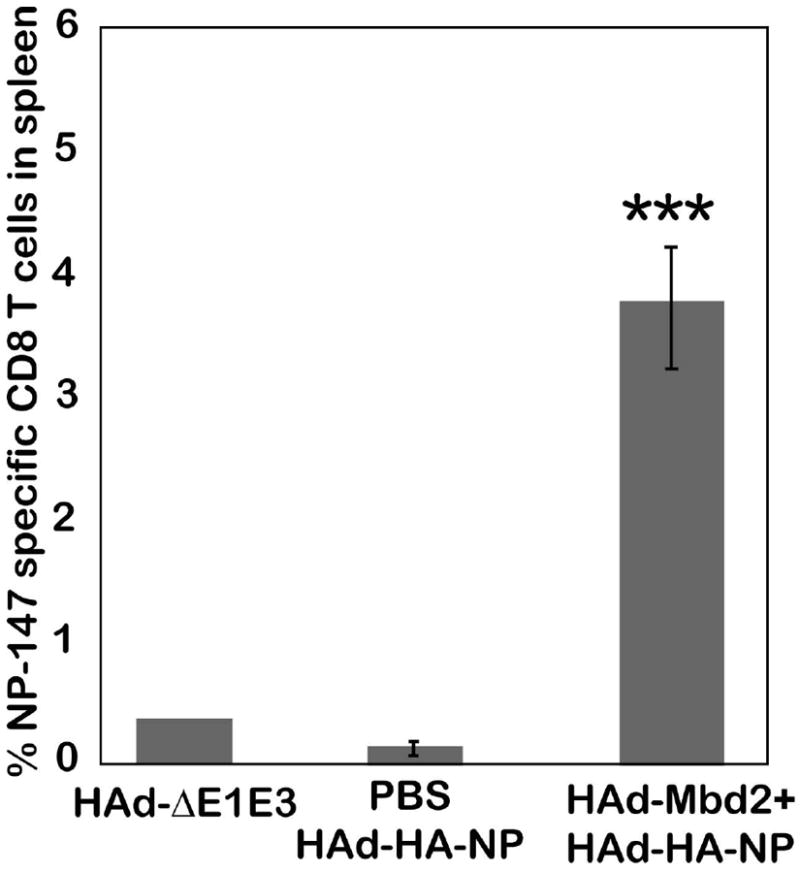
Aged mice (18 month-old) were inoculated intramuscularly (i.m.) either with phosphate-buffered saline or 1 ×108 plaque-forming units (pfu) of HAd-Mbd2 [adenovirus vector expressing murine ß-defensin 2 (Mbd2)]. Seven days later, animals were immunized with 1 × 108 pfu of HAd-HA-NP [adenovirus vector expressing hemagglutinin (HA) and nucleoprotein (NP) of a H5N1 influenza virus]. Animals similarly inoculated with 1 × 108 pfu of HAd-ΔE1E3 on days 0 and 7 to serve as negative controls. Mice were euthanized four weeks after the last immunization, and the spleens were collected. Single cell suspensions were prepared by passage through screens, and 2 × 106 cells were stained with a murine MHC-encoded allele kd–specific pentamer for immunodominant NP-147 epitopes-conjugated with phycoerythrin (PE) and also with an anti-CD8+ antibody-conjugated with allophycocyanin (APC) and an anti-CD19 antibody-conjugated with flouro-isothiocyanin (FITC). Flow cytometric analysis was done to identify the number of NP-147 specific CD8+ T cells. Data were collected using BD FACSCanto II (BD Bioscience, CA) and FACSDiva software was used for analysis. The data represent mean± standard deviation (SD) from six animals per group. ***; P<0.0001, compared to HAd-ΔE1E3 or PBS-HAd-HA-NP control group.
Figure 5. Effect of murine ß-defensin 2 (Mbd2) on the frequency of HA-518 and NP-147 epitope-specific IFN-γ secreting CD8+ T cells in the spleens of HAd-HA-NP vaccinated aged mice.
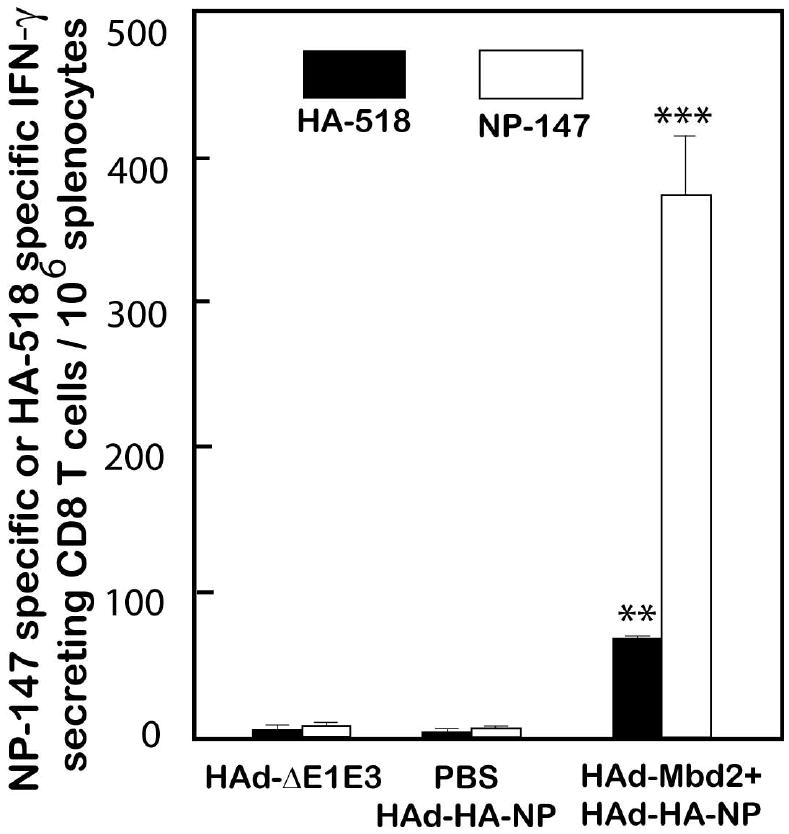
Aged mice (18 month-old) were inoculated intramuscularly (i.m.) either with PBS or 1 × 108 plaque-forming units (pfu) of HAd-Mbd2 (adenovirus vector expressing Mbd2). Seven days later, animals were immunized with 1 × 108 pfu of HAd-HA-NP [adenovirus vector expressing hemagglutinin (HA) and nucleoprotein (NP) of a H5N1 influenza virus]. Animals similarly inoculated with 1 × 108 pfu of HAd-ΔE1E3 on days 0 and 7 to serve as negative controls. Mice were euthanized four weeks after the last immunization, and the spleens were collected. Single cell suspensions were prepared by passage through screens, and 1 × 106 cells were cultured in the presence of HA-518 or NP-147 peptide on anti-interferon-γ antibody-coated 96-well filter plates and developed according to an ELISpot protocol. The ELISpot plates were read using Bioreader 5000 (BIOSYS Miami, FL). The bars represent mean ± standard deviation (SD) from 3 animals/group. ** ; P<0.001, *** ; P<0.0001, compared to HAd-ΔE1E3 or PBS-HAd-HA-NP control group.
3.5. Immunostimulation with HAd-Mbd2 enhances efficacy of HAd-HA-NP against H5N1/PR8 reassortant virus challenge in aged mice
In order to evaluate if immunostimulation with Mbd2 could enhance the protective efficacy of the HAd-HA-NP vaccine in aged mice, animals were inoculated with 1 × 108 pfu of HAd-Mbd2 and, seven days later, vaccinated with 1 × 108 pfu of HAd-HA-NP. Four weeks following immunization, mice were challenged intranasally (i.n.) with 100 MID50 of VNH5N1-PR8/CDC-RG H5N1 virus. The protection efficacy was determined by lung virus titers on three days after challenge. As expected, mice inoculated with HAd-ΔE1E3 had the maximum virus titers in the lung samples (Table 1). Mock-treated mice immunized with HAd-HA-NP had slightly reduced but still significant virus titers in the lungs following H5N1 virus challenge. However, mice pretreated with HAd-Mbd2 before HAd-HA-NP vaccination had lung virus titers substantially lower compared to the HAd-ΔE1E3 group (Table 1).
4. DISCUSSION
The inability to fight infection and decreased response to influenza vaccination in the older adult population is attributed to “immunosenecence”, a general decline in the innate and adaptive immune functions due to aging (Aspinall et al., 2007). This often results in an increased disease burden and serious complications following infections among older adults compared to their younger counterparts. This inequality underscores the importance to explore novel influenza vaccine approaches taking into consideration the limitations of the immune system in the older adults. In this study, we evaluated the ability of Mbd2 to enhance the efficacy of the HAd-HA-NP vaccine in an aged mouse model. Pretreatment of mice with HAd-Mbd2 prior to HAd-HA-NP vaccination resulted in a significant enhancement of antigen-specific humoral and cellular immune responses compared to the group vaccinated with HAd-HA-NP without Mbd2. Furthermore, significantly lower virus titers were observed in the lungs of the vaccinated mice immunostimulated with HAd-Mbd2 following challenge with a reassortant H5N1 virus (VNH5N1-PR8/CDC-RG).
Mbd2 has previously been shown to interact with immature DCs through the chemokine receptor-6 (CCR6) and to act as an endogenous ligand for TLR4 inducing the up-regulation of costimulatory molecules CD40 and CD86 thus resulting in DC maturation (Biragyn et al., 2001; Park et al., 2011). Consistent with these findings, Mbd2 expressed by the HAd-Mbd2 in our study was bioactive and chemoattracted bone marrow-derived immature DCs.
Moreover, DCs isolated from the spleens and the draining lymph nodes from the HAd-Mbd2-inoculated aged mouse group exhibited a more mature phenotype with an up regulation of costimulatory molecules CD40, CD80, and CD86. Consistent with earlier reports, DCs isolated from the mice (both young and aged) treated with the empty vector (HAd-ΔE1E3) demonstrated a more mature phenotype compared to the mock (PBS) treated mice (Bayer et al., 2011; Lietz et al., 2012). Due to their inherent immunogenicity, Ad vectors are known to activate innate immune responses by TLR dependent and independent pathways (Muruve, 2004; Yamaguchi et al., 2007).
Aged mice immunostimulated with HAd-Mbd2 prior to vaccination with HAd-HA-NP demonstrated a significant increase in the frequency of NP-147 epitope- specific CD8+ T cells. In our study, we chose to administer the vaccine seven days post-inoculation with HAd-Mbd2 taking into consideration three factors - the time needed for the adenovirus vector to express Mbd2 (48-72 h), the time needed for Mbd2 to exert its effect on immature DCs, and the time needed for TLR stimulation resulting in the generation of a proinflammatory milieu conducive for the vaccine-specific immune responses. Significantly higher numbers of IFN-γ producing HA-518 and NP-147 epitope-specific CD8+ T cells were also observed in the vaccinated mice immunostimulated with HAd-Mbd2. Consistent with the cellular immune response data, HAd-Mbd2 immunostimulation also resulted in an enhancement in anti-HA HI antibody titers.
Mbd2 has previously been demonstrated to enhance antigen-specific cellular immune responses. It has also been shown to mediate tumor regression either when fused to tumor vaccine antigens or when over expressed in irradiated tumor cells (Biragyn et al., 1999; Biragyn et al., 2001; Ruffini et al., 2004). Moreover, a DNA vaccine encoding the HIV-1 envelope protein linked to Mbd2 was shown to enhance antigen-specific immune responses in a mouse model (Biragyn et al., 2002a). Additional studies will be needed to further improve the development of humoral immune responses to the vaccine in response to immunostimulation in order to provide complete protection against the challenge virus. Some potential strategies may include optimizing the dose of the immunostimulant vector and the vaccine vector, and optimizing the timing of immunostimulation. Moreover, defensins (both alpha and beta) have been tested extensively as immunostimulants in pre-clinical studies, and there have been no reports of any adverse effects (Lapteva et al., 2009; Mei et al., 2012; Park et al., 2011; Yang et al., 2004). It would be important to monitor the effect of immunstimulation with Mbd2 on the induction of immune responses following vaccination with an inactivated influenza vaccine.
5. CONCLUSIONS
Our results demonstrate the potential of Mbd2 to enhance the efficacy of H5N1 vaccines in aged mice. Although the precise mechanism for the improvement in immune responses to influenza vaccine in aged mice immunostimulated with HAd-Mbd2 remains to be further explored, it may be due to the increased expression of co-stimulatory molecules and the production of proinflammatory cytokines by DCs thus enabling the generation of more robust adaptive immune responses. Overall, our results highlight the potential utility of Mbd2 as a molecular adjuvant to enhance the immunogenicity and protective efficacy of vaccines for the elderly.
HIGHLIGHTS.
HAd-Mbd2 activated dendritic cells in aged mice
HAd-Mbd2 enhanced vaccine induced humoral and cellular immune responses in aged mice
Vaccinated aged mice had reduced virus titers following H5N1 challenge
Mbd2 could be a potential adjuvant for enhancing efficacy of vaccines for elderly
Acknowledgments
This work was supported by a Public Health Service grant AI059374 from the National Institute of Allergy and Infectious Diseases and a Showalter Award. We are thankful to J. Kovach for her excellent secretarial assistance.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aspinall R, et al. Challenges for vaccination in the elderly. Immun Ageing. 2007;4:9. doi: 10.1186/1742-4933-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangari DS, Mittal SK. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. doi: 10.1016/j.virusres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Bayer W, et al. Improved vaccine protection against retrovirus infection after co-administration of adenoviral vectors encoding viral antigens and type I interferon subtypes. Retrovirology. 2011;8:75–90. doi: 10.1186/1742-4690-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biragyn A, et al. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood. 2002a;100:1153–1159. doi: 10.1182/blood-2002-01-0086. [DOI] [PubMed] [Google Scholar]
- Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002b;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- Biragyn A, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- Biragyn A, et al. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat Biotechnol. 1999;17:253–258. doi: 10.1038/6995. [DOI] [PubMed] [Google Scholar]
- Fiore AE, et al. Inactivated influenza vaccines. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. Elsevier; Saunders, USA: 2012. [Google Scholar]
- Graham FL, et al. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hoelscher M, et al. Vaccines against epidemic and pandemic influenza. Expert Opin Drug Deliv. 2008a;5:1139–1157. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- Hoelscher MA, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelscher MA, et al. New pre-pandemic influenza vaccines: An egg- and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin Pharmacol Ther. 2007;82:665–671. doi: 10.1038/sj.clpt.6100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelscher MA, et al. A broadly-protective vaccine against globally dispersed clade 1 and clade 2 H5N1 viruses. J Inf Dis. 2008b;197:1185–1188. doi: 10.1086/529522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden LM, Meyer JA. Age and sex compostion - 2010 Census briefs. Available at http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf.
- Imai M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486:420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapteva N, et al. Attraction and activation of dendritic cells at the site of tumor elicits potent antitumor immunity. Mol Ther. 2009;17:1626–1636. doi: 10.1038/mt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietz R, et al. Codelivery of the chemokine CCL3 by an adenovirus-based vaccine improves protection from retrovirus infection. J Virol. 2012;86:1706–1716. doi: 10.1128/JVI.06244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei HF, et al. beta-defensin 2 as an adjuvant promotes anti-melanoma immune responses and inhibits the growth of implanted murine melanoma in vivo. PLoS One. 2012;7:e31328. doi: 10.1371/journal.pone.0031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MMWR. Estimates of death associated with seasonal influenza: United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057–1062. [PubMed] [Google Scholar]
- Morrison GM, et al. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 1999;442:112–116. doi: 10.1016/s0014-5793(98)01630-5. [DOI] [PubMed] [Google Scholar]
- Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- Nair S, et al. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol. 2003;171:6275–6282. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, et al. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl 2):ii17–ii21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A, et al. Impact of preexisting adenovirus vector immunity on immunogenicity and protection conferred with an adenovirus-based H5N1 influenza vaccine. PLoS ONE. 2012;7:e33428–e33428. doi: 10.1371/journal.pone.0033428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, et al. Induction of TLR4-dependent CD8+ T cell immunity by murine beta-defensin2 fusion protein vaccines. Vaccine. 2011;29:3476–3482. doi: 10.1016/j.vaccine.2011.02.061. [DOI] [PubMed] [Google Scholar]
- Ruffini PA, et al. Genetic fusions with viral chemokines target delivery of nonimmunogenic antigen to trigger antitumor immunity independent of chemotaxis. J Leukoc Biol. 2004;76:77–85. doi: 10.1189/jlb.1003481. [DOI] [PubMed] [Google Scholar]
- Russell CA, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012;336:1541–1547. doi: 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, et al. Avian influenza pandemic preparedness: developing prepandemic and pandemic vaccines against a moving target. Expert Rev Mol Med. 2010;12:e14–e14. doi: 10.1017/S1462399410001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao K, et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003;305:192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- Tandon M, et al. Sequential administration of bovine and human adenovirus vectors to overcome vector immunity in an immunocompetent mouse model of breast cancer. Virus Res. 2012;163:202–211. doi: 10.1016/j.virusres.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PG, et al. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Olphen AL, Mittal SK. Development and characterization of bovine x human hybrid cell lines that efficiently support the replication of both wild-type bovine and human adenoviruses and those with E1 deleted. J Virol. 2002;76:5882–5892. doi: 10.1128/JVI.76.12.5882-5892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula SV, Mittal SK. Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther. 2010;10:1469–1487. doi: 10.1517/14712598.2010.519332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, et al. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum Gene Ther. 2007;18:753–762. doi: 10.1089/hum.2007.016. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]


