Abstract
The great apes include, in addition to Homo, the genera Pongo (orangutans), Gorilla (gorillas), and Pan, the latter comprising two species, P. troglodytes (chimpanzees) and P. paniscus (bonobos). Adult-onset hypothyroidism was previously reported in 4 individual nonhuman great apes. However, there is scarce information on normal serum thyroid hormone levels and virtually no data for thyroid autoantibodies in these animals. Therefore, we examined thyroid hormone levels and TSH in all nonhuman great ape genera including adults, adolescents, and infants. Because hypothyroidism in humans is commonly the end result of thyroid autoimmunity, we also tested healthy and hypothyroid nonhuman great apes for antibodies to thyroglobulin (Tg), thyroid peroxidase (TPO), and the TSH receptor (TSHR). We established a thyroid hormone and TSH database in orangutans, gorillas, chimpanzees, and bonobos (447 individuals). The most striking differences are the greatly reduced free-T4 and free-T3 levels in orangutans and gorillas vs chimpanzees and bonobos, and conversely, elevated TSH levels in gorillas vs Pan species. Antibodies to Tg and TPO were detected in only 2.6% of adult animals vs approximately 10% in humans. No animals with Tg, TPO, or TSHR antibodies exhibited thyroid dysfunction. Conversely, hypothyroid nonhuman great apes lacked thyroid autoantibodies. Moreover, thyroid histology in necropsy tissues was similar in euthyroid and hypothyroid individuals, and lymphocytic infiltration was absent in 2 hypothyroid animals. In conclusion, free T4 and free T3 are lower in orangutans and gorillas vs chimpanzees and bonobos, the closest living human relatives. Moreover, thyroid autoantibodies are rare and hypothyroidism is unrelated to thyroid autoimmunity in nonhuman great apes.
The great apes include, in addition to the genus Homo, the genera Gorilla (gorillas), Pongo (orangutans), and Pan (chimpanzees and bonobos). For simplicity, in this report we will use the term “great apes” to represent the nonhuman genera. Based on nucleotide divergences, humans are closest to chimpanzees, followed by gorillas, and are least similar to orangutans (1). Indeed, molecular studies demonstrate that chimpanzees and bonobos (Pan troglodytes and Pan paniscus, respectively) are the closest living relatives of humans (see, for example, Ref. 2). Great apes share some physiologic characteristics with humans such as conservation of glycosylation (3), similar cytokine and chemokine induction in chimpanzees (4), and the presence of Pyrin-only 2 protein in chimpanzees (5). However, differences have also been observed in thyroid hormone metabolism (3) and elevated levels of phytannic acid in erythrocytes (6, 7).
The thyroid disorders Graves' disease and Hashimoto's thyroiditis are the most common organ-specific autoimmune diseases affecting humans. Despite the closeness of great apes to humans, to our knowledge, thyroid autoimmunity has not been documented in the few reports of thyroid dysfunction in these animals. Hyperthyroidism treated in a gorilla with antithyroid drugs for many years was of unknown etiology (8). Also to our knowledge, hypothyroidism of adult onset has been reported in only 4 great apes: a chimpanzee (9), a gorilla (10, 11), and 2 orangutans (12, 13). Hypothyroidism in humans is, in most instances, the end result of autoimmunity against thyroid autoantigens, thyroglobulin (Tg) and thyroid peroxidase (TPO) (reviewed in Refs. 14 and 15). In humans, hypothyroidism with thyroid atrophy is rarely caused by antibodies to the thyrotropin receptor (TSHR) that block the action of TSH (16, 17). Autoantibodies to Tg and TPO were undetectable in the hypothyroid chimpanzee (9). No thyroid autoantibody measurements were reported for the other 3 hypothyroid great apes (10–13). Previously, because of this paucity of information in the literature, we contacted 9 primate centers and zoos in North America. At that time, we learned of about 1 gorilla in the Bronx zoo and 2 orangutans in the Atlanta zoo being treated with l-T4 for spontaneous thyroid dysfunction (18).
Because autoimmune thyroiditis is commonly subclinical in humans (see, for example Ref. 19) and may not be suspected in great apes, in the present study we greatly expanded our inquiries, not only for information, but specifically to obtain as many great ape sera as possible for analysis in our laboratory, an undertaking that spanned nearly 3 years. Our data on sera from more than 400 great apes, both euthyroid and hypothyroid, are the first to document autoantibodies to Tg and TPO in these animals, although their prevalence is low relative to humans. In order to correlate thyroid autoantibodies with thyroid dysfunction, we also assayed the sera for thyroid hormones and TSH. Remarkably, hypothyroidism, when present, was not associated with Tg or TPO autoantibodies, and all animals with autoantibodies were euthyroid. Aside from information on thyroid autoantibodies, our data on serum thyroid hormone and TSH levels in great apes, by far the largest and most comprehensive data set for these near-human genera, provides reference values, presently lacking, to assist in the interpretation of thyroid hormone levels in hypothyroid animals before and after treatment with l-T4.
Materials and Methods
Great ape sera
Blood samples are drawn from great apes in the course of routine physical examinations as well as from individual apes at times of ill health. Aliquots of “left-over” sera were generously provided to us as follows: 1) chimpanzee sera (Dana Hasselschwert, University of Louisiana at Lafayette); 2) sera from gorillas, orangutans, and bonobos (Ryan S. DeVoe, North Carolina Zoological Park; Hayley Murphy, Zoo Atlanta; Michael T. Barrie, Columbus Zoo and Aquarium; Jay Peterson, Brookfield Zoo; Jim Grillo, Audubon Zoo; Roy B. Burns, Louisville Zoo; Jennifer D'Agostino and Julia Jones, Oklahoma City Zoo; Marie-Josee Limoges, Granby Zoo; Syd Tanner, Little Rock Zoo; Donna Ialeggio, Philadelphia Zoo; Stephanie Braccini, Saint Louis Zoological Park; and Raven Jackson, Chimp Haven. A primate “archive”, including sera from 53 gorillas, 52 orangutans, and 18 bonobos, compiled to study antibodies to herpes virus (20), was generously given to us by Richard Eberle (Veterinary Pathology, Oklahoma State University).
Clinical data and information on age groups and great ape diet came from the above individuals and from: Francois Villinger (Yerkes National Primate Research Center); Rita McManamon (University of Georgia College of Veterinary Medicine); Maria Franke and Graham J. Crawshaw (Toronto Zoo); Lisa Argilla (Wellington Zoo); Gay Edwards Reinertz (Zoological Society of Milwaukee), Pam Dennis, Kristen Lucas, and Elena H. Less (Cleveland Metro-Parks Zoo); Maryanne Tocidlowski and Lauren L. Howard (Houston Zoo, Inc); Rachel Watkins Rogers (Zoo Miami); Megan Elder (Como Park Zoo); Dana Hatcher (Columbus Zoo and Aquarium), and Lori Perkins (chair of the Orangutan Species Specific Survival Plan, Zoo Atlanta). Other information, advice, and support were provided by: Gregory Brent (University of California Los Angeles); Catherine Bresee (Cancer Institute, Cedars-Sinai Medical Center); John D. Young (Cedars-Sinai Medical Center); and Elizabeth Ford (Association of Primate Veterinarians).
These studies were performed with the approval of the Orangutan Species Survival Plan (SSP) and the Gorilla SSP and in accordance with the regulations of the individual zoos, or the New Iberia Research Center that house the great apes. Sera and thyroid tissue from a deceased gorilla diagnosed with, and treated for, hypothyroidism in Canada (10, 11) was imported on a CITES I permit obtained from the US Fish and Wildlife Service.
Individual chimpanzees are indicated by identity (ID) numbers and individual gorillas and orangutans are indicated by the first 2 initials of their names followed by their studbook numbers [www.dewarwildlife.org/jrdavis-gorilla-studbook/; 2010 International Studbook of the Orangutan(Pongo Pygmaeus, Pongo Abelii) provided by Megan Elder, Como Zoo & Conservatory).
Great ape species
Eleven gorillas studied were western gorillas (Gorilla gorilla). Sera from Bornean and Sumatran orangutans (Pongo pygmeus and Pongo abelli, respectively) were available, as well as sera from some hybrid orangutans. Orangutan data from both species and hybrids were pooled. Data were analyzed separately for chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). For simplicity, the 4 groups of great apes are referred to by their common names.
Thyroid hormones
Sera were tested for total and free T4 (TT4, FT4), total and free T3 (TT3, FT3) and TSH using commercially available kits for humans (Siemans; formerly Diagnostic Products Corp). Values were determined using kit standards and reported as micrograms/dL (TT4), nanograms/dL (FT4, TT3), picograms/dL (FT3), and microunits/mL (TSH). Data for thyroid hormones and TSH were analyzed according to the appropriate age groups for each species of great ape (Supplemental Table 1 published on The Endocrine society's Journals Online web site at http://endo.endojournals.org) and according to sex for adult orangutans, gorillas, and chimpanzees; bonobo data were pooled for adult males and females.
Background to measuring thyroid autoantibodies in nonhuman great apes
Reports of thyroid autoantibody measurements, to our knowledge, have only been performed in one nonhuman great ape and in rhesus monkeys: 1) Autoantibodies to Tg or TPO were undetectable (assays not specified) in a hypothyroid chimpanzee (9); 2) In 3 rhesus macaques (Macaca mulatta) with compensated goitrous hypothyroidism, microsomal antibodies (TPOAbs) were absent (titer < 1:100) but Tg antibodies (TgAbs) were present in 1 animal (titer 1:1600) (21). Based on the publication date (1985), expression of the data as “titers” and the term “antimicrosomal,” it is likely that human hemagglutination assays were used; 3) In rhesus macaques with hyperthyroidism due to multinodular goiter, thyroid stimulating antibodies were tested by a clinical laboratory (Endocrine Sciences), presumably using human assays, and found to be undetectable (22).
Amino acid homology between nonhuman great ape Tg, TPO, and the TSHR is extremely high, greater than 97% in virtually all cases (88% for a partial Tg sequence in orangutans; Supplemental Table 2). Detection of TgAbs in a rhesus monkey is consistent with 92% homology between Tg in humans and Macaca mulatta (Supplemental Table 2). To put these high levels of homology in perspective, it is worth noting that, because mouse and human TPO are only 74% homologous, the use of murine TPO was critical for detecting spontaneously arising TPO antibodies in NOD.H2h4 mice (23).
Antibodies to Tg and TPO
Tg antibodies (TgAbs) and TPO antibodies (TPOAbs) were measured by ELISA. Human Tg was purchased from EMD Biosciences. Recombinant human TPO ectodomain was generated from CHO cells overexpressing TPO and purified by affinity chromatography as previously described (24). ELISA plates were coated with Tg at 5 μg/mL and TPO at 1 μg/mL. Great ape sera were diluted 1:100 and antibody binding was detected using horseradish peroxidase-labeled protein-A (Calbiochem). This approach had previously been used to demonstrate antibodies to herpes virus in all 4 species of great apes (20). The data for TgAbs and TPOAbs are expressed as optical density at 490 nm (OD490). Following this initial screen, sera with ELISA OD values > 0.8 were retested using goat antimonkey-IgG horseradish peroxidase (AbD Serotec) as the second antibody. Both assays included in-house standard human Hashimoto sera positive for TgAbs and TPOAbs as well as a negative human serum control. Data from different assays were normalized according to the TgAb (or TPOAb) standards.
Antibodies to the TSHR
The presence of antibodies to the TSHR was investigated by measuring inhibition of TSH binding to the receptor (TBI) using two kits: the TRAb kit based on porcine TSHR (Kronus) and a similar kit utilizing the human TSHR (Alpco). Sera were tested according to the kit protocols (50 μL for the TRAb kit; 100 μL for the human TSHR kit). All assays included, in addition to the kit standards, an “in-house” Graves'-positive serum, sera from hypothyroid great apes, and a large number of euthyroid control great ape sera. Data from the porcine TSHRs are expressed as TBI (% inhibition of TSH binding) and for the human TSHR as International Units/L.
Thyroid histology
Thyroid tissue obtained at autopsy was available from 2 hypothyroid gorillas (Rita McManamon, University of Georgia College of Veterinary Medicine; and Marie-Josee Limoges, Granby Zoo), and euthyroid gorillas (Francois Villinger, Yerkes National Primate Research Center; and Nancy Lung, Fort Worth Zoo). A histologic slide of thyroid tissue from a euthyroid gorilla was provided by Roy Burns (Louisville Zoo). All tissues had been fixed in formalin and processed to paraffin blocks, and serial sections were stained with hematoxylin and eosin.
Antibodies to hepatitis C virus (HCV) and serum vitamin D
Antibodies to HCV were measured using the human antihepatitis C virus antibody ELISA kit (TSZ ELISA), and vitamin D levels were measured using a 25-OH-Vitamin-D-ELISA kit (Eagle Biosciences, Inc.).
Statistics
Multiple comparisons were performed using ANOVA (ANOVA on ranks). In some cases, the statistical significance or differences were determined by Mann Whitney rank sum test or by Student's t test (when normally distributed). Correlation was tested by a nonparametric test, Spearman rank. Tests were performed using SigmaStat (Jandel Scientific Software). The Wald Binomial proportion test was used to determine the significance of a difference between our observed proportions of thyroid autoantibodies vs published rates in humans.
Results
Reference values for thyroid hormones and TSH in great apes without thyroid dysfunction
Evaluation of the clinical significance of thyroid autoantibodies in apparently normal animals is only possible in the context of normal reference values for thyroid hormones and TSH, with only limited data available at the onset of our study. In addition, it was possible that these values would differ among the great ape genera. We, therefore, determined serum TT4, FT4, T3, FT3 and TSH in more than 400 sera, excluding sera from the 11 great apes known to be hypothyroid.
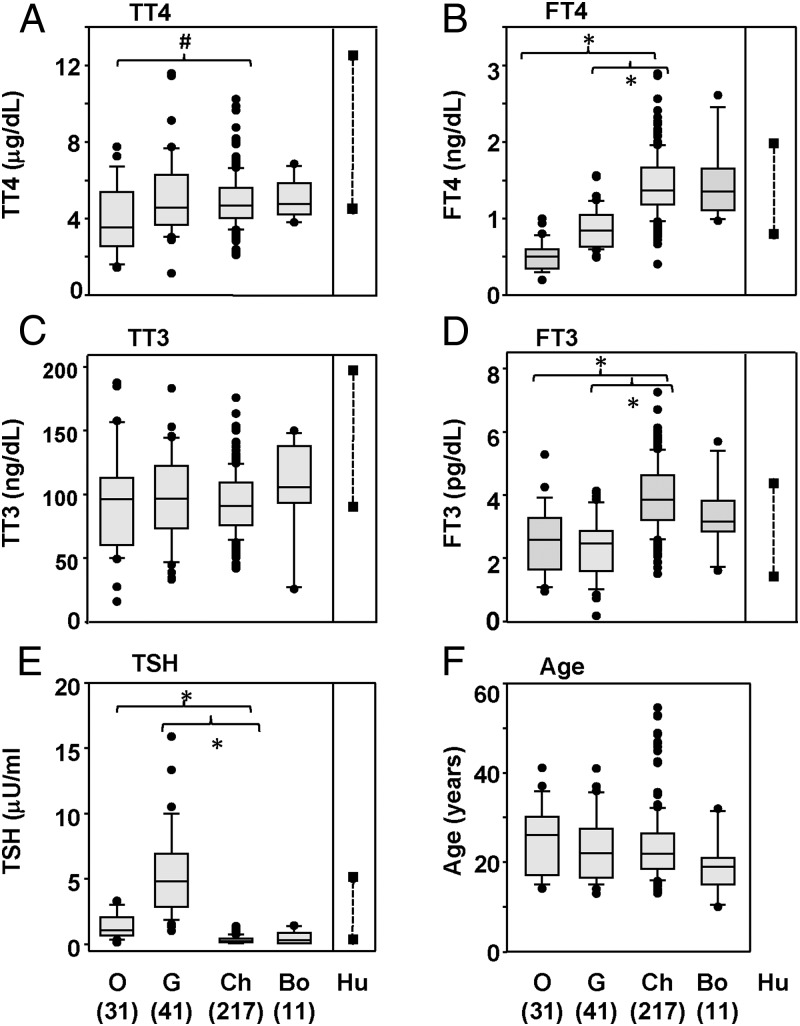
Serum TT4 and TT3 levels were comparable in adult gorillas, chimpanzees, and bonobos although TT4 values were slightly lower in orangutans than in chimpanzees (Figure 1, A and C; P < .05, ANOVA). In contrast, there were striking differences in free thyroid hormone levels for 2 of the 4 great ape genera: FT4 and FT3 levels were considerably lower in both orangutans and gorillas than in chimpanzees or bonobos (Figure 1, B and D; P < .05, ANOVA). TSH levels were significantly higher in orangutans, and even higher in gorillas, than in chimpanzees and bonobos (Figure 1E). TSH and FT4 levels were inversely correlated for the combined population of adult chimpanzees, gorillas, and orangutans (n = 285 animals; Spearmann Rank order correlation; rs − 0.457; P < .001). The adult ages for the 4 groups of great apes studied did not differ significantly (Figure 1F), despite slight differences in the adult age limits for the genera of great apes (Supplemental Table 1).
Figure 1.
Thyroid hormones and TSH in adult orangutans, gorillas, chimpanzees, and bonobos Values for TT4, FT4, TT3, FT3, TSH, and the ages of the great apes studied are depicted as box plots. This presentation shows the median, 25th, and 75th percentiles and, as error bars, the 10th and 90th percentiles; symbols indicate the values for “outliers,” O, G, Ch, and Bo indicate orangutans, gorillas, chimpanzees, and bonobos; the numbers of great apes studied are included in parentheses. Hu indicates the range for the RIA kits in euthyroid humans. Significant differences (tested by ANOVA; P < .05): For TT4, # indicates values lower in orangutans than in chimpanzees; for F-T4 and F-T3, * indicates values much lower in orangutans and gorillas than in chimpanzees; for TSH, * indicates values higher in gorillas and orangutans than in chimpanzees or bonobos.
Although we did not restandardize these RIAs human sera (for which reason we could not perform a statistical analysis) it was interesting to compare our data for great apes with those provided by the manufacturer for euthyroid humans (Figure 1, A–D). Serum TT4, FT4, and TT3 appeared to be higher in humans than in all 4 great ape genera (Figure 1, A and C). In contrast, chimpanzee and bonobo serum FT4 and FT3 values exceeded those in humans (Figure 1, B and D), and TSH values in gorillas were far higher than in humans (Figure 1E). A previous comparison of T4 and T3 in chimpanzees demonstrated differences between great ape and human thyroid hormone metabolism (3).
In general, our present data are comparable to earlier published studies with smaller numbers of great apes, as well as with unpublished values from 3 zoos (Supplemental Tables 3A and 3B). In particular, the lower FT4 and FT3 values that we found for gorillas vs bonobos was also observed in data from Columbus Zoo (Supplemental Table 3A). The biggest discrepancies in the various studies are for TSH in bonobos (Supplemental Tables 3A and 3B). Such differences could be explained by varying cross-reactivity between different human-specific TSH antibodies for great ape TSH.
Gender and age differences in thyroid hormone and TSH
We observed a few statistically significant differences between adult males vs adult females and/or adults vs teenagers plus infants within the 4 groups of great apes (Supplemental Figures 1 and 2). Because of the greater number of chimpanzee sera available to us for analysis, we could subdivide the thyroid hormone and TSH data into the following groups: adult males and females, old males and females (not included in the former groups), teenagers and infants (Supplemental Figure 3; age grouping from supplemental Table 1).
Compared with markedly lower FT4 and FT3, and elevated TSH, levels in orangutans and gorillas vs chimpanzees and bonobos (Figure 1), most gender and age differences were small (Supplemental Figures 1 and 2). The most striking observation was for TSH in chimpanzees in which the highest values were observed for infants and particularly teenagers (Supplemental Figure 3, panel C). These findings are consistent with human data showing highly variable values for TSH, FT4, and FT3 in infants and adolescents (25).
Autoantibodies to Tg and TPO are present in a small number of euthyroid great apes
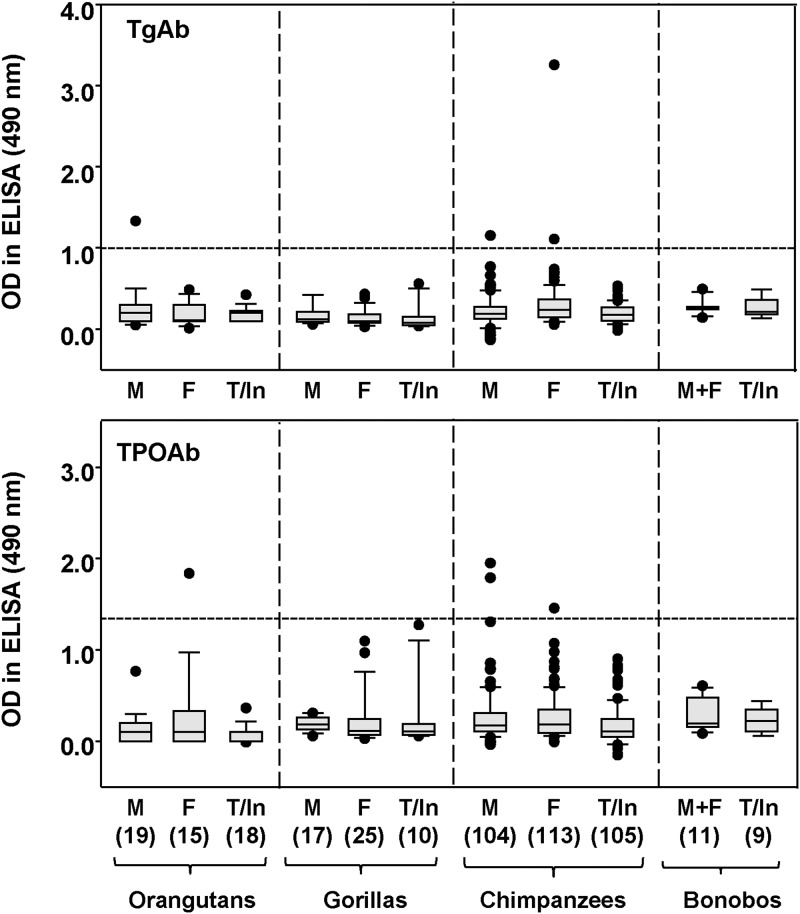
We screened unselected sera from the following great apes for autoantibodies to Tg or TPO: 52 orangutans, 52 gorillas, 322 chimpanzees, and 20 bonobos (including adults, adolescents, and infants). Signals for antibody binding to Tg- or TPO-coated ELISA plates, detected using Protein A, were generally low, but potentially positive in many instances (Figure 2). However, when potentially positive sera were retested (with appropriate negative controls) using goat antimonkey as the second antibody, 4 sera remained positive for TgAbs and a different set of 4 sera remained positive for TPOAbs (Table 1). Sera were only positive for TgAbs or TPOAbs at relatively low dilution (1:100), except for the single chimpanzee serum that had extremely high binding detected with Protein A to Tg (OD > 3.0).
Figure 2.
TgAbs and TPOAbs in great apes OD490 values in ELISA were measured using plates coated with human Tg or human TPO and antibody binding was detected using horseradish peroxidase-labeled protein A. The data are shown as box plots: median, 10th, 25th, 75th, and 90th percentiles; symbols indicate values for “outliers.” Values for males (M), females (F), and adolescents/teenagers or infants (T/Inf) are provided separately. O, G, Ch, Bo, and Hu: orangutans, gorillas, chimpanzees, and bonobos; the numbers of great apes studied is in parentheses. The broken lines represents the “cut-off” for positivity using antimonkey IgG (labeled with horseradish peroxidase) described in the text.
Table 1.
Great Apes with TgAbs or TPOAbs (ELISA OD) or High TBI Have Thyroid Hormone and TSH Levels Within the Normal Range.
| Great ape Identification | M/F | Age | TT4 (μg/dL) | FT4 (ng/dL) | TT3 (ng/dL) | FT3 (pg/mL) | TSH (μU/mL) | TgAbs (ELISA OD) | TPOAbs | TBI (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| TgAb positive | ||||||||||
| Orangutans | ||||||||||
| Be 2604 | M | 18 | 3.2 | 0.6 | 97.1 | 1.5 | 1.1 | 0.86 | neg | 12.4 |
| Chimpanzees | ||||||||||
| 97A005 | F | 15 | 2.4 | 1.3 | 115.1 | 4.1 | 1.4 | 0.70 | neg | 4.6 |
| A224 | F | 46 | 6.0 | 1.3 | 62.0 | 2.8 | 0.3 | 1.73 | neg | 11.4 |
| CB0627 | M | 31 | 5.1 | 1.6 | 130.9 | 5.0 | 0.3 | 0.80 | neg | 8.4 |
| TPOAb positive | ||||||||||
| Orangutans | ||||||||||
| Fe no. 2157 | F | 23 | 5.7 | 0.7 | 158 | 3.7 | 0.5 | neg | 1.41 | 14.3 |
| Chimpanzees | ||||||||||
| 93A002 | F | 19 | 4.3 | 1.6 | 80 | 2.5 | 0.9 | neg | 0.96 | 10.3 |
| 91A021 | M | 20 | 5.7 | 1.2 | 111 | 3.5 | 0.5 | neg | 1.20 | 0.0 |
| X161 | M | 36 | 8.9 | 2.3 | 97 | 3.4 | 0.2 | neg | 1.15 | 8.4 |
| High TBI | ||||||||||
| Chimpanzees | ||||||||||
| A8A002 | F | 5 | 6.5 | 2.6 | 121 | 8.6 | 0.3 | neg | neg | 28.8 |
| A192D | F | 29 | 6.5 | 1.4 | 96 | 2.8 | 0.2 | neg | neg | 25.3 |
| 93A007 | F | 18 | 4.3 | 1.2 | 76 | 3.0 | 0.2 | neg | neg | 25.2 |
| A3A020 | M | 8 | 4.1 | 1.5 | 127 | 5.0 | 0.5 | neg | neg | 24.8 |
| 95A016 | M | 16 | 6.6 | 2.8 | 104 | 7.1 | 0.1 | neg | neg | 31.2 |
Orangutans: initials, studbook numbers; chimpanzees: identity numbers. Antibody binding to ELISA plates coated with Tg or TPO detected using horseradish peroxidase-conjugated antimonkey IgG; neg, negative by ELISA using protein A. Boldface indicates positive levels of TgAb, or TPOAb or TBI values above the normal range in humans.
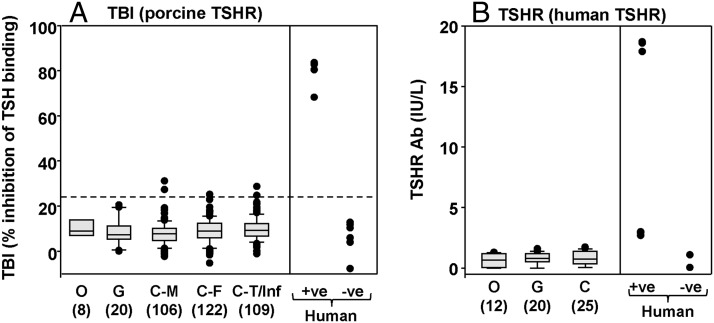
Antibody interaction with the TSHR was tested using commercially available TSH binding inhibition (TBI) kits, one utilizing the porcine, the other the human TSHR. With the porcine TSHR assay, and using the mean + 2 SD for normal human serum binding as the “cut-off,” none of the sera from euthyroid orangutans and gorillas were positive (Figure 3A). Five of 337 chimpanzee sera were marginally positive, with none comparable to serum from a TSHR antibody positive Graves' patient. However, using the human TSHR kit, no great ape serum attained even a “low positive” value in the assay (Figure 3B).
Figure 3.
Inhibition of TSH binding to the porcine TSHR (panel A) or to the human TSHR (panel B) in great apes The data are shown as box plots: median, 10th, 25th 75th, and 90th percentiles; symbols indicate values for outliers. O, G, Ch, Bo, and Hu: orangutans, gorillas, chimpanzees, bonobos, and humans; the number of great apes studied is in parentheses; separate data are provided for males (M), females (F), and adolescents/teenagers or infants (T/Inf). The broken line in panel A represents the mean + 2 SD for normal human serum included in the same assays. +ve, positive; −ve, negative, for TSHR antibodies.
Most important, thyroid hormone and TSH levels were all within the normal range for great ape sera positive for TgAbs or TPOAbs by ELISA and in the 5 chimpanzee sera with marginally positive TBI values (Table 1). Similar small numbers of males and females had detectable TgAbs or TPOAbs. No serum was positive for both TgAbs and TPOAbs and none of the sera with borderline positive TBI levels were positive for TgAbs or TPOAbs. Overall, TgAbs or TPOAbs were present in 8 of 305 (2.6%) adult nonhuman great apes. Approximately 10% of adults humans are positive for TgAbs or TPOAbs (see, for example. Ref. 26). Using the Wald Binomial Proportion test, 2.6% (95% confidence interval [CI] 0.8%; 4,4%) in nonhuman great apes is significantly different from the assumed 10% rate in humans (P < .001).
Thyroid hormone and TSH levels in great apes with thyroid dysfunction
Inquiries about thyroid dysfunction in great apes in captivity (contact information from the Gorilla-SSP and the Orangutan-SSP) yielded responses from 32 North American zoos, and information came indirectly from one zoo in New Zealand. No cases of hyperthyroidism (other than iatrogenic) had been observed. There is a single report of hyperthyroidism of unknown etiology in a male gorilla (Benno), born in the wild and initially kept in a German zoo (26). Unfortunately, Benno (studbook no. 0119; www.dewarwildlife.org/jrdavis-gorilla-studbook/) had no offspring that would have been interesting to follow for possible thyroid dysfunction.
Goiter has been described in hypothyroid rhesus monkeys (21). We are not aware of reports on goiter in nonhuman great apes but one of the authors (C.L.C.) recently observed goiter in a 21-year-old female chimpanzee that was euthanized due to bacterial peritonitis. At autopsy, the thyroid gland was diffusely enlarged and nodular; the total thyroid weight was 44.8 g compared with 10.3 g in a 31-year-old normal female (∼4 times normal size).
We were informed of 11 adult great apes and one with congenital hypothyroidism currently being treated with l-T4. Sera were available from 10 of these 11 adults including an orangutan (Ma; Studbook no. 449) previously reported (13). We also analyzed sera from a deceased hypothyroid gorilla (Ca; Studbook no. 0305) (9, 10) drawn from the animal subsequent to these reports. Before treatment with l-T4, sera (when available) had elevated TSH and low TT4 levels (Table 2). After treatment, some great apes had elevated TT4 and undetectable TSH levels. These findings illustrate the difficulty of adjusting the l-T4 dose to restore euthyroidism in the absence of established normal values and guidelines for thyroid hormone levels in normal great apes, particularly in the case of TSH, for which there are no assays specific for great ape TSH.
Table 2.
Thyroid Hormone and TSH Levels in Great Apes Before and After Treatment With l-T4 for Hypothyroidism.
| Before l-T4 | After l-T4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | TT4 (μg/dL) | FT4 (ng/dL) | TT3 (ng/dL) | FT3 (pg/mL) | TSH (μU/mL) | Age (yr) | TT4 (μg/dL) | FT4 (ng/dL) | TT3 (ng/dL) | FT3 (pg/ml) | TSH (μU/ml) | ||
| Orangutans | |||||||||||||
| Ma (F) | 35 | 5.0 | 0.5 | 71 | 1.6 | 6.7 | 43 | 14.3 | 2.4 | 184 | 4.7 | <0.2 | |
| 42 | 3.7 | 0.3 | 56 | 1.3 | 2.8 | 45 | 9.2 | 1.5 | 35 | 0.6 | <0.2 | ||
| 48 | 7.5 | 0.7 | 42 | 1.2 | <0.2 | ||||||||
| Mi (F) | 13 | 3.1 | 0.4 | 84 | 2.4 | 0.4 | 18 | 7.3 | 0.8 | 153 | 5.1 | 0.4 | |
| Bi (F) | 40 | 1.6 | 0.3 | 70 | 2.1 | 5.6 | 41.0 | 7.5 | 1.5 | 89 | 1.9 | 0.8 | |
| 41.3 | 3.8 | 0.8 | 55 | 1.5 | 0.4 | ||||||||
| Gorillas | |||||||||||||
| An (M) | 26 | 3.2 | 0.6 | 98 | 1.8 | 8.0 | 27 | 5.4 | 1.2 | 107 | 2.4 | <0.2 | |
| 32 | 6.5 | 1.7 | 60 | 1.8 | 4.3 | ||||||||
| Ni (F) | 20 | 0.9 | 11.3 | 20 | 9.1 | 1.5 | 119 | 2.0 | <0.2 | ||||
| 28 | 9.3 | 1.9 | 83 | 2.1 | 2.2 | ||||||||
| Ca (F) | 27 | 9.6 | 1.3 | 128 | 2.5 | <0.2 | |||||||
| 28 | 12.0 | 1.3 | 97 | 1.9 | <0.2 | ||||||||
| Ki (F) | 28 | 16.0 | 3.1 | 73 | 2.7 | <0.2 | |||||||
| 33 | 9.8 | 1.9 | 67 | 1.6 | 3.3 | ||||||||
| Nd (F) | 17 | 3.0 | |||||||||||
| Bonobos | |||||||||||||
| Su (F) | 19 | 3.8 | 0.9 | 53 | 1.0 | 0.4 | 30.0 | 18.3 | 1.5 | 186 | 5.1 | <0.2 | |
| La (F)a | 19 | 4.8 | 1.2 | 98 | 1.6 | 0.3 | 30.5 | 9.0 | 2.2 | 95 | 3.5 | <0.2 | |
| 31.0 | 7.0 | 1.0 | 106 | 2.7 | 0.9 | ||||||||
| Chimpanzee | |||||||||||||
| Va | 42 | 9.4 | 1.8 | 1.1 | |||||||||
Studbook numbers: orangutans Ma 449; Mi 2500; Bi 1106; gorillas An 0891; Ni 1306; Ca 0305; Ki 0680; Nd 1423; bonobos Su 115; La 116. Boldface indicates values outside 95% confidence limits for euthyroid great apes of the same genus. Italics indicate pretreatment data for gorilla Ni from Michael Barrie, Columbus Zoo (serum not available to us).
Receiving l-T4 to improve fertility.
Hypothyroid great apes lack thyroid autoantibodies and thyroid lymphocytic infiltration
Before and/or after treatment with l-T4, sera from hypothyroid great apes were negative for binding to Tg or TPO in ELISA and were negative for TSHR antibodies in 2 different assays (Table 3).
Table 3.
Antibodies to the TSHR are Negative in Gorillas, Orangutans, Bonobos, and a Chimpanzee Before (B) and/or After (A) Treatment (Rx) with l-T4 for Hypothyroidism.
| Age (yr) | Rx | TgAb (OD in ELISA) | TPOAb (OD in ELISA) | TSHR Ab (TBI %) | TSHR Ab (IU/L) | |
|---|---|---|---|---|---|---|
| Orangutans | ||||||
| Ma (F) no. 449 | 35 | B | 0.41 | 0.25 | 20.9a | 0.06 |
| 50 | A | 0.25 | 0.18 | 6.2b | 0.06 | |
| Mi (F) no. 2500 | 13 | B | 0.42 | 0.36 | 9.6 | 1.0 a |
| 18 | A | 0.36 | 0.36 | 7.5 | 0.4 b | |
| Bi (F) no. 1106 | 40 | B | 0.07 | 0.05 | 15.5 a | 1.2 a |
| 41 | A | 0.24 | 0.22 | 7.8 b | 0.9 | |
| Gorillas | ||||||
| An (M) no. 0891 | 17 | B | 0.10 | 0.28 | 16.6 | 0.5 |
| Ni (F) no. 1306 | 28 | A | 0.16 | 0.22 | 10.2 | 0.5 |
| C (F) no. 0305 | 27 | A | 0.18 | 0.03 | 9.6 | 0.06 |
| 28 | A | 0.13 | 0.09 | 12.3 | 0.06 | |
| 31 | A | 0.08 | 0.10 | nd | 0.06 | |
| Ki (F) no. 0680 | 29 | A | −0.03 | 0.16 | 11.5 | 0.5 |
| 33 | A | −0.06 | 0.16 | 0.4 | 0.2 | |
| Nd (F) no. 1423 | 17 | A | 0.06 | 0.16 | 7.1 | nd |
| Bonobos | ||||||
| Su no. 115 | A | 0.26 | 0.12 | 14.5 a | 0.7 | |
| La no. 116 | 19 | B | 0.26 | 0.61 | 15.6 a | 0.1 |
| Chimpanzee | ||||||
| Va | 42 | A | −0.10 | 0.06 | 7.1 | nd |
highest TBI;
lowest TBI; nd, not determined. All sera were negative for antibody binding to Tg to Tg or TPO in ELISA.
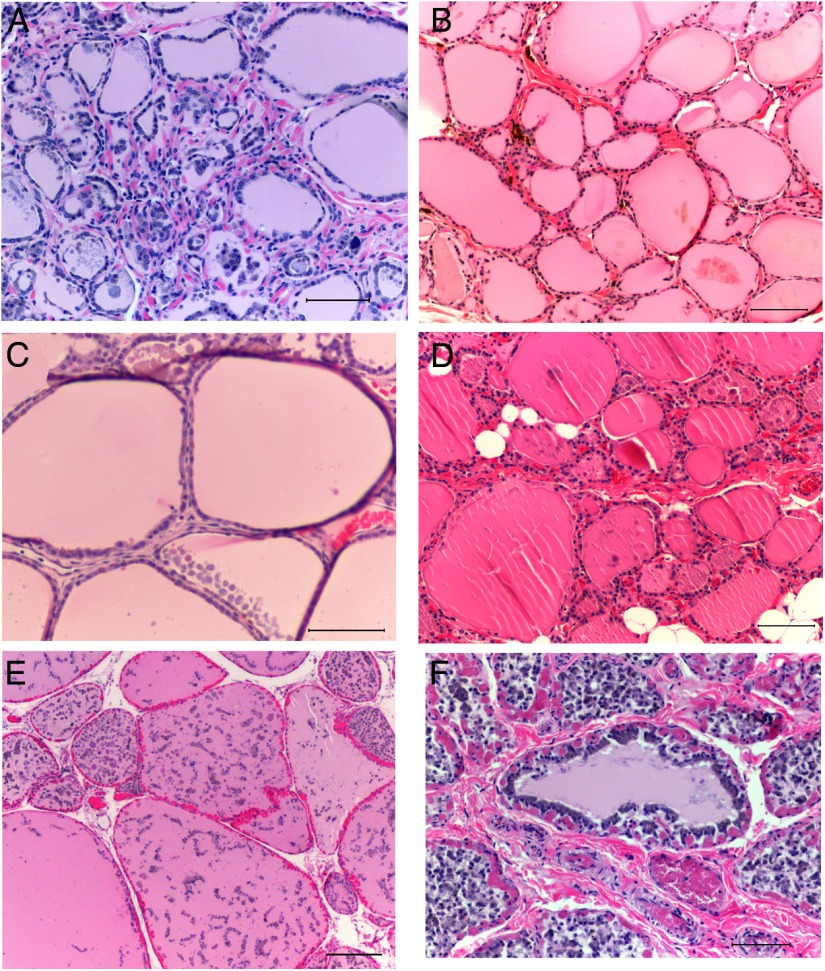
Thyroid histology was examined in autopsy tissues available from 2 gorillas receiving l-T4 for hypothyroidism (Figure 4, A and B). As controls, thyroid tissue was examined from 4 great apes without thyroid dysfunction (2 gorillas, 2 orangutans; Figure 4, C–F). All sections reveal variable degrees of autolysis, as expected from material obtained at necropsy. For both normal and hypothyroid great apes, microscopic findings were similar and showed variably sized follicles filled with colloid. None of the tissues had any evidence of interstitial inflammatory cells.
Figure 4.
Thyroid histology at autopsy from 2 gorillas treated for hypothyroidisim with lT4 (panels A and B) and from 2 gorillas and 2 orangutans without thyroid dysfunction (panels C–F) Sections were stained with hematoxylin and eosin; bars represent 100 mm. A, Gorilla Pa no. 0191 (age 41 years); B, Gorilla Ca no. 0305 (age 31 years); C, Gorilla Jo no. 0268 (age 49 years); D, Gorilla Tu no. 0217(age 45 years); E, Orangutan To no. 1510 (age 22 years); F, Orangutan Ji no. 1510 (age 22 years).
Factors that could lead to hypothyroidism
Because thyroid autoantibodies were absent in hypothyroid great apes, we sought other potential factors that could play a role in hypothyroidism. Information on dietary iodine and/or goitrogens was not available for the animals that we studied. However, we tested for HCV and vitamin D insufficiency. Antibody binding to HCV in hypothyroid great apes was not significantly different from that observed in euthyroid great apes (Table 4), unlike the elevated levels in 2 chimpanzees exposed to HCV (D. Hassleschwert, personal communication). Neither of these chimpanzees had detectable TgAbs or TPOAbs. In addition, 25-OH Vitamin D levels in hypothyroid great apes were not reduced compared with values in euthyroid animals in the present study (Table 4) or in previous reports (27–29).
Table 4.
Antibodies to Hepatitis C (HCV Ab) and Vitamin D Levels (25-OH-VitD) in Gorillas, Orangutans, and Chimpanzees Before (B) and After (A) Treatment (Rx) with l-T4 for Hypothyroidism.
| Sex | Age (yr) | Rx | HCV Ab (pg/mL) | 25-OH-Vit D (ng/mL) | |
|---|---|---|---|---|---|
| Gorilla An (no. 0891) | M | 17 | B | 276 | 7.4 |
| 32 | A | 218 | 27.1 | ||
| Gorilla Ni (no. 1306) | F | 20 | B | 263 | 29.3 |
| 28 | A | 318 | 38.8 | ||
| Gorilla Ca (no. 0305) | F | 26 | A | nd | nd |
| Orangutan Ma (no. 449) | F | 35 | B | 175 | 13.0 |
| 50 | A | 183 | 21.2 | ||
| Orangutan Mi (no. 2500) | F | 13 | B | 175 | 50.3 |
| 40 | A | 185 | 62.1 | ||
| Orangutan Bi (no. 110) | F | 15 | B | 437 | 40.2 |
| 36 | A | 103 | 22.7 | ||
| Bonobo Su (no. 115) | F | 19 | B | 289 | 34.9 |
| Bonobo La (no.116) a | F | 19 | B | 505 | 22.9 |
| 30 | A | 484 | nd | ||
| Euthyroid great apes | |||||
| Mean + se. (n = 21) present study | 331 + 40b | ||||
| (n = 31) present study | 30.3 + 2.5 | ||||
| Catarrhini (n = 21) | 33.8 + 5.3 c | ||||
| Orangutans (n = 9) | 15.6 + 3.9 d | ||||
| Gorillas (n = 25) | 16.7 + 1.2 d | ||||
| Chimpanzees (n = 14) | 13.1 + 1.4 d | ||||
| Chimpanzees (indoor) | 15.3 + 4.9 e | ||||
| Chimpanzees (in and outdoor) | 20.6 + 6.3 e | ||||
Previous data for Vitamin D levels in great apes are included. nd, not determined.
On l-T4 to assist in fertility;
, excluding two chimpanzees immunized against HCV (3052 and 2955 pg/ml HCV antibody; personal communication, Dana Hasselschwert, New Iberia Research Center);
, Catarrhini including 6 gorillas, 5 orangutans and 10 old world monkeys (Ref. 27);
, Ref 28;
, Ref 29; nd, not determined.
Discussion
To address the question of the occurrence of thyroid autoimmunity in great apes, we studied sera from 10 adults and one deceased animal receiving l-T4 treatment. This group included great apes from each of the 4 genera: 3 orangutans, 5 gorillas, 1 chimpanzee, and 2 bonobos. All were being treated for adult-onset hypothyroidism except for one bonobo which was receiving l-T4 for reproductive/fertility purposes. Hypothyroidism in 2 animals, an orangutan (13) and a gorilla (10, 11), had been reported previously.
Importantly, none of the hypothyroid great apes had TgAbs or TPOAbs, indicating that the etiology of this hypothyroidism is unlikely to be autoimmune thyroiditis. The inability to detect TgAbs or TPOAbs in these hypothyroid great apes cannot be attributed to false assay negatives because these autoantibodies were clearly detected in a number of euthyroid great apes, although with a prevalence of 2.6% of adults, significantly lower than the approximately 10% that would be expected in the adult human population (26). No serum had antibodies to both Tg and TPO. However, because of the small number of thyroid autoantibody-positive great apes, it is not possible to conclude a difference in the mechanisms leading to thyroid autoimmunity in humans vs great apes. In euthyroid humans, TgAbs or TPOAbs represent markers of subclinical disease, as indicated by their correlation with thyroid lymphocytic infiltration observed at autopsy (30, 31). We examined thyroid tissue from 2 deceased gorillas receiving l-T4 for hypothyroidism. Both lacked lymphocytic infiltrates, consistent with the absence of thyroid autoantibodies and the likelihood that the etiology of the hypothyroidism was unrelated to thyroid autoimmunity.
Turning to hyperthyroidism, our inquiries yielded no reports of hyperthyroidism. Because we did not receive responses from all institutions, we cannot conclude that hyperthyroidism in great apes has not been observed in North America. However, hyperthyroidism in these animals is extremely rare and appears to be confined to a single report of hyperthyroidism of unknown etiology in a male gorilla (26). Although none of the great apes that we studied had a history of hyperthyroidism, we assayed most sera for TSHR autoantibodies using TSH binding inhibition assays. We were interested in the possible presence of TSH-blocking antibodies in the hypothyroid great apes. However, none of the sera from euthyroid or hypothyroid animals were clearly positive, even when using the human TSHR kit. Therefore, whether Graves' disease occurs in great apes remains an open question, although the answer is likely to be negative.
Our study raises two interesting questions. First, why is the prevalence of thyroid autoantibodies lower in great apes than in humans? Second, what is the basis for hypothyroidism in great apes? Regarding the first question, there is evidence that great apes have lower immune/autoimmune responses than humans. In particular, chimpanzees have reduced lymphocyte activity (32) and do not develop rheumatoid arthritis or type 1 diabetes (33). A major cause of reduced immune responses involves the presence in great apes and other mammals, but not in humans, of a sialic acid variant (Neu5Gc) (34). The ability of Neu5Gc to down-regulate immune responses is illustrated by enhanced T lymphocyte function in mice lacking the enzyme required to generate this compound (35). Other factors responsible for reduced thyroid autoimmunity could include the absence of the relevant susceptibility genes previously mentioned (18) and limited genetic diversity in captive great apes (36).
Turning to the second question, in the absence of thyroid autoimmunity, what is the basis for hypothyroidism in nonhuman great apes? We considered possible roles for iodide intake, dietary goitrogens, HCV infection, and vitamin D insufficiency. Iodine generally causes hypothyroidism in humans because of a failure in thyroid iodine autoregulation associated with an underlying thyroid disorder, such as subclinical thyroid autoimmunity (reviewed in Ref. 37). An inexpensive dietary component of captive great apes is cabbage or other Brassica family members that contain the goitrogen thiocyanate. A very high thiocyanate intake caused hypothyroidism in a woman (38), and lesser amounts of thiocyanate together with limited dietary iodine intake lead to hypothyroidism (for example Ref. 39). The complex diets for most captive great apes include some high-fiber “greens” such as kale that contain thiocyanate (D. Hatcher, Columbus Zoo and Aquarium; personal communication). Although the iodine intake by captive great apes is currently not known, the rarity of reports on goiter suggests that it is unlikely to be grossly deficient. However, a study to specifically address the issue of iodide and thiocyanate in great apes has recently been initiated (M. Barrie, G. Brent, personal communication). Based on available information, together with well-established observations in humans, we speculate that moderately low iodine levels, together with thiocyanate in the diet, could play a role in long-term hypothyroidism.
We directly addressed possible roles for HCV infection and vitamin D levels in great ape hypothyroidism. HCV infection is associated with thyroid autoimmunity and hypothyroidism in humans (40), particularly after interferon-a therapy (40, 41). Moreover, HCV infection can cause hypothyroidism in the absence of thyroid autoimmunity (for example Refs. 42 and 43). Testing for HCV antibodies excluded this possibility for the hypothyroid great apes that we studied. However, great apes are subject to other viral infections, such as herpes virus (20) and alphavirus or flavivirus (44), that may contribute to the development of hypothyroidism. We assayed vitamin D in great ape sera because the immunomodulatory effects of vitamin D are well known (45)and because relative vitamin D deficiency has been associated with Hashimoto's thyroiditis (46) although it is not associated with the early stages of thyroid autoimmunity (47). However, we found no evidence that vitamin D levels were lower in hypothyroid vs euthyroid nonhuman great apes. In addition, the diets of most great apes housed in zoos include multivitamins (28).
An important corollary of our study is the provision of a large database (52 orangutans, 53 gorillas, 20 bonobos, and 322 chimpanzees) for thyroid hormones, TSH, and thyroid autoantibodies in euthyroid great apes. Smaller numbers of chimpanzees have previously been investigated for TT4 and TT3 (48) and for total and free T4 and T3 (3). One chimpanzee and small numbers of healthy gorillas were tested for thyroid hormones as controls to diagnose hypothyroidism (9–11) and to evaluate the application to primates of a human immunometric assay for TSH (49).
Striking differences were observed between the 4 great ape species in terms of FT4, FT3, and TSH. Orangutans had the lowest FT4 and FT3 levels, followed by gorillas, and the highest levels were observed in chimpanzees and bonobos. The pattern was reversed for TSH, being lowest in chimpanzees and bonobos and higher in orangutans and gorillas. Moderate gender differences were observed, notably higher TT4 in female than in male orangutans and gorillas and higher FT4 in male than in female chimpanzees. A similar pattern of differences in basal salivary amylase and salivary cortisol was observed for orangutans and gorillas vs chimpanzees (50).
In conclusion: First, we established a database for thyroid hormone and TSH levels in orangutans, gorillas, chimpanzees, and bonobos. The most striking differences between these 4 great ape genera are greatly reduced FT4 and FT3 levels in orangutans and gorillas, and elevated TSH levels in gorillas, compared with chimpanzees and bonobos. Second, autoantibodies to Tg and TPO are detectable in only 2.6% of adult great apes, significantly lower than the approximately 10% in humans. No great apes with thyroid autoantibodies exhibit thyroid dysfunction and neither thyroid autoantibodies nor thyroid lymphocytic infiltration are present in hypothyroid great apes. Finally, hypothyroidism in the closest surviving human relatives may involve dietary components including goitrogens but is unrelated to thyroid autoimmunity.
Acknowledgments
We thank the veterinarians, curators, and animal management supervisors from the following institutions (listed alphabetically) for their invaluable assistance in providing sera, thyroid tissue, clinical information, and age group data for great apes: Audubon Zoo (New Orleans, LA), J. Grillo, M. Tomingas; Brookfield Zoo (Chicago, IL), J. Peterson and M. Warneke; Chimp Haven (Keithville, LA), R. Jackson; Cleveland MetroParks Zoo (Cleveland, OH), K. Lucas; Columbus Zoo and Aquarium (Columbus OH), M.T. Barrie, A.Meinelt, D. Hatcher; Como Park Zoo (Saint Paul, MN), M. Elder; Fort Worth Zoo (Fort Worth, TX), N. Lung; Granby Zoo (Quebec Canada), M.J. Limoges, P. Leggett; Houston Zoo, Inc (Houston, TX), M. Tocidlowski, L.L. Howard; Little Rock Zoo (Little Rock, AK), S. Tanner, K. Rainwater; Louisville Zoo (Louisville, KY), R.B. Burns, C. Smolinski; New Iberia Research Center, University of Louisiana at Layfayette (Lafayette, LA), D. Hasselschwert, M. Louis; North Carolina Zoological Park (Asheboro, NC), R.S. DeVoe, C. Goldston, D. Hill; Philadelphia Zoo (Philadelphia, PA), D. Ialeggio, J. Blough; St. Louis Zoo (St Louis, MO), S. Braccini; Toronto Zoo (Scarborough, Ontario, Canada), G.J. Crawshaw, M. Franke; University of Georgia College of Veterinary Medicine, UGA Zoo and Exotic Animal Pathology Service (Athens, GA), R. McManamon; Wellington Zoo (Wellington, New Zealand), L. Argilla; Oklahoma City Zoo (Oklahoma City, OK), J. D'Agostino, J. Jones; Veterinary Pathology, Oklahoma State University (Stillwater, OK), R. Eberle; Yerkes National Primate Research Center, Pathology and Laboratory Medicine (Atlanta, GA), F. Villinger; Zoo Atlanta (Atlanta, GA), H. Murphy and S. Earheart; Zoological Society of Milwaukee (Milwaukee, WI), G.E. Reinertz; Zoo Miami (Miami FL), R. W. Rogers. Assistance with a CITES I application was provided by A. Shoemaker (Columbia, SC). Other information and support about great apes came from Dr. John D. Young (Comparative Medicine, Cedars-Sinai Medical Center, Los Angeles, CA) and Dr. Elizabeth Ford (Association of Primate Veterinarians). We thank Dr. Gregory Brent (Veterans Affairs Medical Center and University of California Los Angeles) for information about the effects of thiocyanate on thyroid function; Catherine Bresee (Cancer institute, Cedars-Sinai Medical Center, Los Angeles, CA) for statistical advice; and Kris Alpi (William R and Kenan Jr Library of Veterinary Medicine, North Carolina State University, Raleigh NC) for primate literature updates. We are grateful to Kronus (Boise, ID) for providing TRAb kits at reduced cost.
This work was supported by the National Institutes of Health Grants DK54684 (to S.M.M.) and DK19289 (to B.R).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FT3
- free T3
- FT4
- free T4
- HCV
- hepatitis C virus
- SSP
- species survival plan
- TBI
- inhibition of TSH binding to the receptor
- Tg
- thyroglobulin
- TgAb
- Tg antibody
- TPO
- thyroid peroxidase
- TPOAb
- TPO antibody
- TSHR
- TSH receptor
- TT3
- total T3
- TT4
- total T4.
References
- 1. Scally A, Dutheil JY, Hillier LW, et al. Insights into hominid evolution from the gorilla genome sequence. Nature. 2012;483:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prüfer K, Munch K, Hellmann I, et al. The bonobo genome compared with the chimpanzee and human genomes. Nature. 2012;486:527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gagneux P, Amess B, Diaz S, et al. Proteomic comparison of human and great ape blood plasma reveals conserved glycosylation and differences in thyroid hormone metabolism. Am J Phys Anthropol. 2001;115:99–109 [DOI] [PubMed] [Google Scholar]
- 4. Brinkworth JF, Pechenkina EA, Silver J, Goyert SM. Innate immune responses to TLR2 and TLR4 agonists differ between baboons, chimpanzees and humans. J Med Primatol. 2012;41:388–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atianand MK, Fuchs T, Harton JA. Recent evolution of the NF-κB and inflammasome regulating protein POP2 in primates. BMC Evol Biol. 2011;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watkins PA, Moser AB, Toomer CB, et al. Identification of differences in human and great ape phytanic acid metabolism that could influence gene expression profiles and physiological functions. BMC Physiol. 2010;10:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moser AB, Steinberg SJ, Watkins PA, et al. Human and great ape red blood cells differ in plasmalogen levels and composition. Lipids Health Dis. 2011;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schneider HE. Hyperthyreosis in male lowland gorilla (Gorilla gorilla gorilla). Erkrankungen der Zootiere. 1989;31:243–244 [Google Scholar]
- 9. Miller RE, Albert SG, Boever WJ. Hypothyroidism in a chimpanzee. J Am Vet Med Assoc. 1983;183:1326–1328 [PubMed] [Google Scholar]
- 10. Lair S, Crawshaw GJ, Mehren KG, Pare J. Clinical investigation of hypothyroidism in a western lowland gorilla (Gorilla gorilla gorilla). In: Proceedings from the American Association of Zoo Veterinarians Annual Conference; Houston, TX, October 26–30, 1997;67–70 [Google Scholar]
- 11. Lair S, Crawshaw GJ, Mehren KG, Perrone MA. Diagnosis of hypothyroidism in a western lowland gorilla (Gorilla gorilla gorilla) using human thyroid-stimulating hormone assay. J Zoo Wildl Med. 1999;30:537–540 [PubMed] [Google Scholar]
- 12. Suedmeyer WK. Hypothyroidism in a orangutan (Pongo pygmaeus pygmaeus): Clinical management and long-term monitoring. In: Proceedings of the American Association of Zoo Veterinarians Annual Conference; Houston, TX, October 26–30, 1997;71–72 [Google Scholar]
- 13. Anonymous Orang treated for thyroid condition. Laboratory Primate Newsletter 2005;44(1):13 [Google Scholar]
- 14. McLachlan SM, Rapoport B. Thyroid peroxidase as an autoantigen. Thyroid. 2007;17:939–948 [DOI] [PubMed] [Google Scholar]
- 15. McLachlan SM, Rapoport B. Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies? Thyroid. 2004;14:510–520 [DOI] [PubMed] [Google Scholar]
- 16. Orgiazzi J, Williams DE, Chopra IJ, Solomon DH. Human thyroid adenyl cyclase-stimulating activity in immunoglobulin G of patients with Graves' disease. J Clin Endocrinol Metab. 1976;42:341–354 [DOI] [PubMed] [Google Scholar]
- 17. Endo K, Kasagi K, Konishi J, et al. Detection and properties of TSH-binding inhibitor immunoglobulins in patients with Graves' disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1978;46:734–739 [DOI] [PubMed] [Google Scholar]
- 18. McLachlan SM, Alpi K, Rapoport B. Review and hypothesis: does Graves' disease develop in non-human great apes? Thyroid. 2011;21:1359–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prentice LM, Phillips DIW, Sarsero D, Beever K, McLachlan SM, Rees Smith B. Geographical distribution of subclinical autoimmune thyroid disease in Britain: a study using highly sensitive direct assays for autoantibodies to thyroglobulin and thyroid peroxidase. Acta Endocrinol (Copenh). 1990;123:493–498 [DOI] [PubMed] [Google Scholar]
- 20. Eberle R, Hilliard JK. Serological evidence for variation in the incidence of herpesvirus infections in different species of apes. J Clin Microbiol. 1989;27:1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olson LC, Palotay JL, Haines JE, Hanada J, Bergquist DY. Compensated, goitrous hypothyroidism in rhesus macaques. Lab Anim Sci. 1985;35:629–634 [PubMed] [Google Scholar]
- 22. Brammer DW, Juneau PL, Chrisp CE, et al. Spontaneous hyperthyroidism in an aged male and female Macaca mulatta. J Med Primatol. 1998;27:273–277 [DOI] [PubMed] [Google Scholar]
- 23. Chen CR, Hamidi S, Braley-Mullen H, et al. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology. 2010;151:4583–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo J, McLachlan SM, Hutchison S, Rapoport B. The greater glycan content of recombinant human thyroid peroxidase of mammalian than of insect cell origin facilitates purification to homogeneity of enzymatically active protein remaining soluble at high concentration. Endocrinology. 1998;139:999–1005 [DOI] [PubMed] [Google Scholar]
- 25. Verburg FA, Kirchgässner C, Hebestreit H, et al. Reference ranges for analytes of thyroid function in children. Horm Metab Res. 2011;43:422–426 [DOI] [PubMed] [Google Scholar]
- 26. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–499 [DOI] [PubMed] [Google Scholar]
- 27. Adams JS, Gacad MA, Baker AJ, Gonzales B, Rude RK. Serum concentrations of 1,25-dihydroxyvitamin D3 in Platyrrhini and Catarrhini: a phylogenetic approisal. Am J Primatol. 1985;9:219–224 [DOI] [PubMed] [Google Scholar]
- 28. Crissey SD, Barr JE, Slifka KA, et al. Serum concentrations of lipids, vitamins A and E, vitamin D metabolites and carotenoids in nine primate species at four zoos. Zoo Biol. 1999;18:551–564 [Google Scholar]
- 29. Videan EN, Heward CB, Fritz J, Murphy J, Cortez C, Su Y. Relationship between sunlight exposure, housing conditions, and serum vitamin D and related physiologic biomarker levels of captive chimpanzees (Pan troglodytes). Comp Med. 2007;57:402–406 [PubMed] [Google Scholar]
- 30. Yoshida H, Amino N, Yagawa K, et al. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46:859–862 [DOI] [PubMed] [Google Scholar]
- 31. Paschke R, Vogg M, Swillens S, Usadel KH. Correlation of microsomal antibodies with the intensity of the intrathyroidal autoimmune process in Graves' disease. J Clin Endocrinol Metab. 1993;77:939–943 [DOI] [PubMed] [Google Scholar]
- 32. Soto PC, Stein LL, Hurtado-Ziola N, Hedrick SM, Varki A. Relative over-reactivity of human versus chimpanzee lymphocytes: implications for the human diseases associated with immune activation. J Immunol. 2010;184:4185–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leelayuwat C, Zhang WJ, Abraham LJ, Townend DC, Gaudieri S, Dawkins RL. Differences in the central major histocompatibility complex between humans and chimpanzees. Implications for development of autoimmunity and acquired immune deficiency syndrome. Hum Immunol. 1993;38:30–41 [DOI] [PubMed] [Google Scholar]
- 34. Muchmore EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol. 1998;107:187–198 [DOI] [PubMed] [Google Scholar]
- 35. Buchlis G, Odorizzi P, Soto PC, et al. Enhanced T cell function in a mouse model of human glycosylation. J Immunol. 2013;191:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simons ND, Wagner RS, Lorenz JG. Genetic diversity of North American captive-born gorillas (Gorilla gorilla gorilla). Ecol Evol. 2012;3:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ingbar SH. Autoregulation of the thyroid. Response to iodide excess and depletion. Mayo Clin Proc. 1972;47:814–823 [PubMed] [Google Scholar]
- 38. Chu M, Seltzer TF. Myxedema coma induced by ingestion of raw bok choy. N Engl J Med. 2010;362:1945–1946 [DOI] [PubMed] [Google Scholar]
- 39. Biassoni P, Ravera G, Bertocchi J, Schenone F, Bourdoux P. Influence of dietary habits on thyroid status of a nomadic people, the Bororo shepherds, roaming a central African region affected by severe iodine deficiency. Eur J Endocrinol. 1998;138:681–685 [DOI] [PubMed] [Google Scholar]
- 40. Tomer Y, Villanueva R. Hepatitis C and thyroid autoimmunity: is there a link? Am J Med. 2004;117:60–61 [DOI] [PubMed] [Google Scholar]
- 41. Tomer Y. Hepatitis C and interferon induced thyroiditis. J Autoimmun. 2010;34:J322–J326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Antonelli A, Ferri C, Pampana A, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10–13 [DOI] [PubMed] [Google Scholar]
- 43. Indolfi G, Stagi S, Bartolini E, et al. Thyroid function and anti-thyroid autoantibodies in untreated children with vertically acquired chronic hepatitis C virus infection. Clin Endocrinol (Oxf). 2008;68:117–121 [DOI] [PubMed] [Google Scholar]
- 44. Kading RC, Borland EM, Cranfield M, Powers AM. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater congo basin. J Wildl Dis. 2013;49:587–599 [DOI] [PubMed] [Google Scholar]
- 45. Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tamer G, Arik S, Tamer I, Coksert D. Relative vitamin D insufficiency in Hashimoto's thyroiditis. Thyroid. 2011;21:891–896 [DOI] [PubMed] [Google Scholar]
- 47. Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol. 2012;167:43–48 [DOI] [PubMed] [Google Scholar]
- 48. Kaack B, Walker L, Brizzee KR, Wolf RH. Comparative normal levels of serum triiodothyronine and thyroxine in nonhuman primates. Lab Anim Sci. 1979;29:191–194 [PubMed] [Google Scholar]
- 49. Lair S, Crawshaw GJ, Mehren KG, Perrone MA. Evaluation of a human immunometric assay for the determination of thyroid-stimulating hormone in nonhuman primates. J Zoo Wildl Med. 2000;31:267–268 [DOI] [PubMed] [Google Scholar]
- 50. Behringer V, Borchers C, Deschner T, Mostl E, Selzer D, Hohmann G. Measurements of salivary alpha amylase and salivary cortisol in hominoid primates reveal within-species consistency and between-species differences. PLoS One. 2013;8:e60773. [DOI] [PMC free article] [PubMed] [Google Scholar]