Abstract
Sonic Hedgehog (Shh) has been shown to regulate wound healing in various tissues. Despite its known function in tissue regeneration, the role of Shh secreted from the gastric epithelium during tissue repair in the stomach remains unknown. Here we tested the hypothesis that Shh secreted from the acid-secreting parietal cell is a fundamental circulating factor that drives gastric repair. A mouse model expressing a parietal cell-specific deletion of Shh (PC-ShhKO) was generated using animals bearing loxP sites flanking exon 2 of the Shh gene (Shhflx/flx) and mice expressing a Cre transgene under the control of the H+,K+-ATPase β-subunit promoter. Shhflx/flx, the H+,K+-ATPase β-subunit promoter, and C57BL/6 mice served as controls. Ulcers were induced via acetic acid injury. At 1, 2, 3, 4, 5, and 7 days after the ulcer induction, gastric tissue and blood samples were collected. Parabiosis experiments were used to establish the effect of circulating Shh on ulcer repair. Control mice exhibited an increased expression of Shh in the gastric tissue and plasma that correlated with the repair of injury within 7 days after surgery. PC-ShhKO mice showed a loss of ulcer repair and reduced Shh tissue and plasma concentrations. In a parabiosis experiment whereby a control mouse was paired with a PC-ShhKO littermate and both animals subjected to gastric injury, a significant increase in the circulating Shh was measured in both parabionts. Elevated circulating Shh concentrations correlated with the repair of gastric ulcers in the PC-ShhKO parabionts. Therefore, the acid-secreting parietal cell within the stomach acts as an endocrine source of Shh during repair.
The most common diseases of the stomach are atrophic gastritis and peptic ulcer disease (1, 2). Risk factors for peptic ulcer disease include the overuse of nonsteroidal antiinflammatory drugs, Helicobacter pylori infection, smoking, and increasing age (3, 4). This is a significant problem, given that there is a steady increase in the prevalence of H pylori infection with age from 20% at age 20 years to more than 50% at age 70 years in developed countries (5). Complications that accompany ulcers include reduced vitamin B12 absorption, iron-deficiency anemia, and a higher frequency of osteoporosis (1). Even though 14.5 million individuals worldwide are afflicted with peptic ulcer disease (6), the ulcer healing process is not clearly understood. Wound healing is typically divided into three phases: 1) inflammation, 2) proliferation, and 3) remodeling. In the stomach, these phases are regulated by growth factors such as epidermal growth factor (EGF), platelet-derived growth factor, TGF-β, and vascular endothelial growth factor (VEGF). These growth factors are hormone-like molecules that interact with specific cell surface receptors to control the process of tissue repair (7).
Sonic Hedgehog (Shh), first identified for its role in embryonic patterning during development (8), has been implicated in tissue repair in the heart (9), lung (10), bone (11), cornea (12), and most recently stomach (13, 14). Shh, primarily secreted from gastric parietal cells (15, 16), regulates epithelial cell differentiation and function (17). Our recent studies show that Shh may also regulate the immune response, in particular macrophage recruitment, during the process of gastric ulcer healing (14). Using a mouse model expressing a tamoxifen-inducible, parietal cell-specific deletion of Shh, we reported delayed gastric repair in response to acetic acid induced injury that correlated with reduced macrophage infiltration (14). Secreted growth factors such TGF-β, EGF, and VEGF are known to contribute to the repair. However, it remains unclear whether Shh is a fundamental secreted factor that drives gastric repair.
The parietal cells are not only a known source of secreted acid and intrinsic factor within the human stomach but also these oxyntic cells play a potent endocrine role. Early studies demonstrate that the gastric parietal cells produce and secrete a substantial amount of growth factors that include heparin-binding EGF, amphiregulin (18), and regenerating islet-derived 1 (19) but also hormones including estrogen (20) and T (21). Given that the gastric parietal cells are also a major source of Shh (15, 16), here we tested the hypothesis that Shh secreted from the parietal cell is a fundamental circulating factor that drives gastric repair. The parabiotic model used herein consisted of a control mouse expressing parietal cell-secreted Shh conjoined to a mouse expressing a parietal cell-specific deletion of Shh (PC-ShhKO). After stable chimerization, acetic acid injury was induced to the stomachs of either the control and PC-ShhKO parabionts or the PC-ShhKO parabiont alone. We demonstrated that Shh secreted from the gastric epithelium, in particular the parietal cells, is a fundamental circulating factor that drives repair within the stomach.
Materials and Methods
Mice
Mice (both males and females) expressing a PC-ShhKO were generated as previously described by our laboratory (17). Briefly, PC-ShhKO mice were generated using transgenic animals bearing loxP sites flanking exon 2 of the Shh gene (Shhflx/flx) (kindly donated by Dr J. A. Whitsett, Department of Pediatrics, University of Cincinnati Children's Hospital Medical Center with permission from Dr A. P. McMahon, Harvard University, Boston, Massachusetts) and mice expressing a Cre transgene under the control of the H+,K+-ATPase β-subunit promoter (HKCre; kindly donated by Dr J. Gordon, Washington University, St Louis, Missouri). HKCre, Shhflox/flox, and C57BL/6 mice were used as controls. Heterozygote B6;129-Ptch1tm1Mps/J mice, expressing lacZ in a pattern mimicking endogenous Patched 1 (Ptch1) gene expression, were purchased from The Jackson Laboratory (stock number 003081). All animal studies were approved by the University of Cincinnati Institutional Animal Care and Use Committee that maintains an American Association of Assessment and Accreditation of Laboratory Animal Care facility.
Acetic acid gastric injury
Ulcers were induced based on a published protocol (22). Briefly, gastric ulcer induction was performed on mice anesthetized with isoflurane. A midline abdominal laparotomy was performed to expose the stomach. Acetic acid (100%) was applied to the serosal surface of the stomach for 25 seconds using a capillary tube (0.5 mm diameter). The muscle and skin abdominal incisions were sutured in two separate layers. Mice were euthanized and samples collected for analysis on days 1, 2, 3, 4, 5, and 7 after the ulcer induction.
Parabiosis
Parabiotic surgery was performed to establish a common circulation between control (HKCre or Shhflx/flx) and PC-ShhKO mice. Mice of the same sex were surgically joined through a modification of a previously published protocol (23). Mice were housed together for 2 weeks prior to the surgery. General anesthesia via isoflurane was administered to each mouse. After surgical preparation, preemptive analgesics were administered (buprenorphine, 0.05–2.0 mg/kg, ip), and matching skin incisions were made in each mouse from the forelimb to the hind leg. Skin was detached from the sc fascia and lightly irrigated with saline. Dorsal skin was then attached to the adjacent mouse via wound clips. To join the peritonea of the mice, small incisions were cut below the diaphragm of each mouse. The four edges of the peritoneal wall incision were surgically sutured to the four edges of the adjacent mouse to form a connected tunnel between the abdominal cavities of the mice. The ventral skin of one mouse was then attached with wound clips to the ventral skin of the adjacent mouse. Sutures were used to close and secure the skin near the forelimb and hind leg of the mice. All animals were visually assessed, weighed daily, and administered analgesics when needed. Subcutaneous fluids, peanut butter, and high-fat or breeder chow was administered daily to support recovery and assist with weight maintenance. Veterinary staff were consulted daily to monitor complications related to the surgery.
Seven days after the parabiotic surgery, ulcers were induced in both control and PC-ShhKO parabionts or in PC-ShhKO parabionts alone. Mice were monitored for 7 days after the ulcer induction and samples collected. To confirm the establishment of a joined circulation, 200 μL of 0.5% Evans blue dye in Hanks' balanced salt solution was injected into the tail vein of a control parabiont. One hour after injection, mice were euthanized and blood was collected from both parabionts through a cardiac puncture. Blood sera were separated by centrifugation, sera were then diluted to 1:100, and the absorbance was determined at 620 nm (24).
Blood collection
Blood was collected via cardiac puncture in tubes coated with EDTA and K2 (Greiner Bio-One North America Inc). After collection, serum was separated by centrifugation for 15 minutes at 3000 rpm at 4°C.
Tissue collection and histology
The stomach was cut along the greater curvature, opened, washed in PBS, and the ulcerated area measured. Tissue spanning the ulcerated area was collected and fixed in Carnoy's fixative overnight. Longitudinal sections of the stomach were paraffin embedded and 4 μm sections stained with hematoxylin and eosin (H&E) for histological evaluation.
β-Galactosidase (X-gal) staining for paraffin-embedded tissue sections
X-gal staining of stomach sections was performed according to the manufacturer's protocol (Sigma Aldrich). Briefly, tissue was fixed in 4% paraformaldehyde/PBS for 15 minutes before staining with X-gal solution for 24 hours at 37°C in the dark. Sections were rinsed, postfixed in 10% formalin overnight at 4°C, and paraffin embedded.
Immunofluorescence
After deparaffinization, antigen retrieval was performed by heating the slides for 10 minutes at 100°C in 0.01 M sodium citrate buffer (antigen unmasking solution; Vector Laboratories). Sections were then blocked using either 20% normal donkey or 20% normal rabbit serum. Sections were immunostained with a 1:100 dilution of goat anti-Shh (Santa Cruz Biotechnology), 1:50 goat anti-Patched (Santa Cruz Biotechnology), 1:20 mouse anti-TGFα (Abcam), or mouse anti-α-smooth muscle actin (Abcam) overnight at 4°C followed by a 1-hour incubation with a 1:100 dilution of antigoat Alexa Fluor 488 or antimouse Alexa Fluor 633 secondary antibodies (Invitrogen). Coverslips were mounted onto slides with Prolong Gold antifade reagent mounting medium (Molecular Probes) and analyzed with a Zeiss LSM510 META confocal microscope.
Immunoprecipitation and Western blot analysis
Plasma was immunoprecipitated using anti-Shh 5E1 antibody (2 μg) at 4°C for 16 hours. Protein A/G agarose beads (20 μL; Santa Cruz Biotechnology) were added and samples incubated at 4°C for 16 hours. Immunoprecipitates were then washed three times using PBS and resuspended in 40 μL Laemmli loading buffer containing β-mercaptoethanol (Bio-Rad Laboratories) before loading onto 4%-20% Tris-glycine gradient gels. The gels were run at 80 V, 3.5 hours, protein transferred to nitrocellulose membranes at 105 V, 1–2 hours, and then blocked for 1 hour at room temperature using KPL detector block (Kirkegaard & Perry Laboratories, Inc). Membranes were incubated overnight at 4°C with a 1:200 dilution of anti-Shh antibody (N-19; Santa Cruz Biotechnology) or antidispatched antibody (Abcam) followed by a 1-hour incubation with 1:100 dilution of AlexaFluor antigoat 680 or antirabbit 680 antibody (Invitrogen). Membranes were imaged using a scanning densitometer (Odyssey Infrared Imaging Software System).
Enzyme-linked immunosorbent assay
Shh concentrations in gastric tissues were measured using an ELISA kit according to the manufacturer's instructions (RayBio Tech; catalog number ELM-ShhN-001). Tissue was removed as described above and homogenized in a 300-μL solution of lysis buffer and protease inhibitor cocktail (Roche). Samples were centrifuged for 30 minutes at 16 000 rpm at 4°C. Supernatant was collected, diluted 1:500, and Shh concentrations measured according to the manufacturer's protocol. The concentration of circulating Shh in blood was measured using an ELISA kit (RayBio Tech; catalog number ELH-ShhN-001C). Plasma was separated from collected blood by centrifugation at 3000 rpm for 15 minutes at 4°C. Samples were prepared and measured according to the manufacturer's protocol.
Cytokine [macrophage inflammatory protein (MIP)-1α, MIP-2, IL-6, VEGF, IL-1β, TGFβ] concentrations in the sample supernatants were determined by ELISA using Milliplex multiplex kits (Millipore) according to the manufacturer's protocol. Briefly, in a 96-well multiscreen filter plate, 25 μL sample in duplicate was incubated with 25 μL antibody coated beads overnight at 4°C on a plate shaker. Plates were then washed two times on a vacuum apparatus, and 25 μL of secondary antibody was added and incubated at room temperature for 1 hour while shaking. Finally, 25 μL of streptavidin-R-phycoerythrin was added directly to the secondary antibody and incubated for 30 minutes at room temperature with shaking. Plates were then washed two more times and 100 μL of sheath fluid was added. Plates were shaken for 5 minutes and then read using luminex technology on the Bio-Plex (Bio-Rad Laboratories). Concentrations were calculated from standard curves using recombinant proteins and expressed in picograms per milliliter. The cytokine analysis was conducted by the Cytokine and Mediator Measurement Core Laboratory.
Laser capture microdissection (LCM)
Tissue was collected from control mice after ulcer induction and embedded in Tissue Tek optimal cutting temperature compound. Frozen 8-μm sections were stained according to the manufacturer's protocol using the HistoGene LCM frozen section staining kit (Applied Biosystems; catalog number KIT0401). LCM was used to determine the localization of Shh expression in specific regions of the ulcerated tissue. Intact epithelium, ulcer margin, and granulation tissue were collected using the Molecular Devices/Arcturus VERITAS laser-capture microdissection system (model 704). Briefly, the UV cutting laser was used to trace around the region of interest, and an infrared laser (setting 90 mW and pulse 300 μsec) was used to capture cells.
Quantitative RT-PCR (qRT-PCR)
Tissue collected from the LCM was used to isolate RNA. The Arcturus PicoPure RNA isolation kit and protocol were followed for all steps (Applied Biosystems; catalog number KIT0204). RNA was reverse transcribed to cDNA using the high-capacity cDNA reverse transcription kit and protocol (Applied Biosystems; catalog number 4368814). The synthesized cDNA was amplified for hypoxanthine-guanine phosphoribosyl transferase (Applied Biosystems; Mm00446968_m1) and Shh (Applied Biosystems; Mm00436528_m1) using TaqMan preamp master mix (Applied Biosystems, catalog number 4384266) following the recommended protocol.
Microosmotic pumps
Recombinant mouse Shh (Shh N terminus; R&D Systems; catalog number 461-SH-025/CF) was reconstituted in PBS without calcium and magnesium to a concentration of 1500 ng/mL. Alzet microosmotic pumps (Durect Corp; model 1007D) were filled with PBS or recombinant mouse Shh following the manufacturer's protocol. Immediately after the acetic acid ulcer induction, the microosmotic pumps were surgically implanted into the abdominal cavity above the right leg of PC-ShhKO mice. Mice were analyzed 7 days after the surgical procedure. Mice infused with Shh received approximately 200 ng of the recombinant Shh over the time course.
Immunoneutralization of Shh
Immediately after acetic acid-induced injury, control mice were tail vein injected with 20 μg of either Shh antibody (R&D Systems; MAB4641) or IgG control antibody (R&D Systems; 6–001-F) in 100 μL of sterile PBS. Mice were given tail vein injections of antibodies daily for 6 days. Seven days after the injury, the mice were euthanized, blood was collected, and ulcer size measured. Plasma from control mice receiving anti-Shh antibody or IgG control was then used for a bioassay using C3H10T1/2 cells.
C3H10T1/2 cell culture and treatments
The murine Hedgehog-responsive pluripotent mesenchymal cell line C3H10T1/2 (purchased from American Type Culture Collection) was maintained in culture using DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (25). For Gli1 expression, the C3H10T1/2 cells were cultured in 12-well plates and treated with 1% of plasma collected from the control mice receiving anti-Shh antibody or IgG. After 2 days incubation, the RNA was collected and the Gli1 expression measured as described for qRT-PCR.
Statistical analysis
The results were tested for significance by either a one-way ANOVA or unpaired t test using commercially available software (GraphPad Prism; GraphPad Software). A value of P < .05 was considered significant.
Results
PC-ShhKO mice exhibit impaired wound healing in response to acetic acid-induced injury
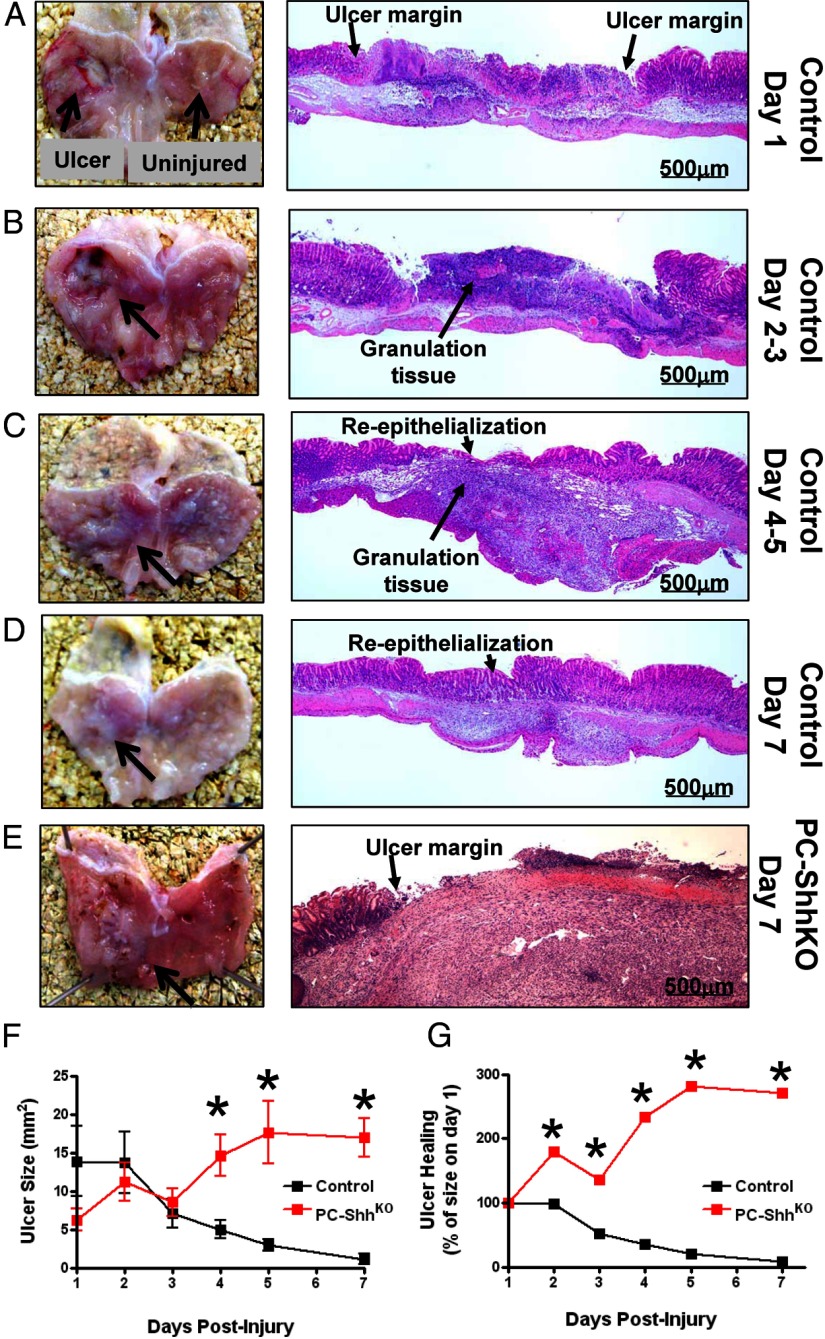
To assess the role of gastric epithelial Shh in gastric tissue repair, ulcers were created in control and PC-ShhKO mice via acetic acid-induced injury. Gross morphology and histology of gastric tissue at day 1 after the injury demonstrated ablation of the gastric epithelium and obvious ulcer margins (Figure 1A). At days 2 and 3 after the injury, we observed the formation of granulation tissue that correlated with an obvious gastric ulcer (Figure 1B). Between days 4 and 7 after the injury, there was a reduction in the gastric ulcer visible by gross morphology that correlated with the reepithelialization of the mucosa (Figure 1, C and D). In contrast, gross morphology and histology of the PC-ShhKO mice revealed a prominent ulcerated area without reepithelialization 7 days after the injury (Figure 1E). Measurement of the ulcerated area demonstrated that the control mice had significantly reduced ulcer size by day 7, whereas the PC-ShhKO mice showed a significant increase in ulcer size (Figure 1, F and G).
Figure 1.
Gross morphological and histological changes in control and PC-ShhKO mice in response to acetic acid-induced injury within the stomach. Gross morphology and H&E stains of control stomachs collected on days 1 (A), 2–3 (B), 4–5 (C), and 7 (D) postulcer induction depicting sites of ulcerated tissue and uninjured tissue used in experimental procedures. E, Gross morphology and H&E stain of PC-ShhKO stomach collected 7 days after the ulcer induction. F and G, Ulcer sizes for control and PC-ShhKO mice 1, 2, 3, 4, 5, and 7 days after the ulcer induction demonstrating disrupted ulcer repair in PC-ShhKO compared with complete healing in control mice (n = 5–17 mice per time point). *, P < .05 compared with control ulcer size.
Masson's trichrome stain was performed to identify fibrotic tissue in control and PC-ShhKO mice 7 days after ulcer induction (Supplemental Figure 1, A and B, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The blue indicated collagen, connective tissue, or fibrotic tissue. In the PC-ShhKO histology, the ulcerated tissue was almost entirely blue, indicating fibrotic tissue (Supplemental Figure 1B) when compared with the trichrome-stained control stomach section (Supplemental Figure 1A). Collectively, these data suggest that loss of gastric epithelial Shh results in the impairment of gastric tissue repair.
Expression of Shh in gastric tissue is induced during wound healing
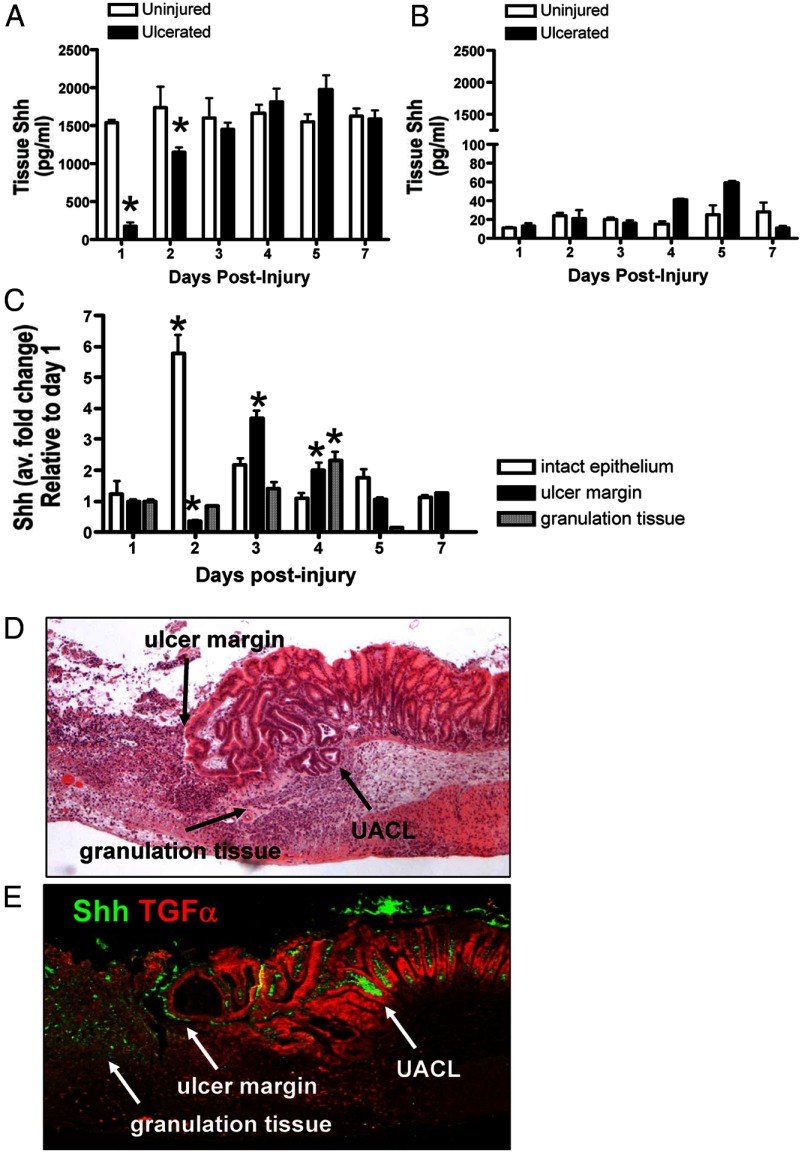
After injury, Shh concentrations in the gastric tissues collected from control and PC-ShhKO mice were measured by ELISA. In control mice, 1 day after the acetic acid-induced injury, Shh was significantly decreased in the ulcerated tissue when compared with the adjacent uninjured tissue (Figure 2A). The concentration of Shh steadily increased after the initial decline in the injured tissue, suggesting that the ulcer damage initially ablated the parietal cells, which are the primary source of gastric Shh. The concentration of Shh was significantly lower in tissue collected from PC-ShhKO ulcers compared with control (Figure 2B). Induction of Shh was not observed in the ulcerated tissue of PC-ShhKO mice.
Figure 2.
Changes in Shh gastric tissue concentrations and gene expression. Changes in Shh tissue concentrations in gastric tissue collected from the stomachs of control (A) and PC-ShhKO (B) mice 1, 2, 3, 4, 5, and 7 days after acetic acid-induced injury. Data are expressed as the mean ± SEM with three to five mice per time point. *, P < .05 compared with uninjured gastric tissue. C, qRT-PCR was performed using RNA collected from intact epithelium, ulcer margin, and granulation tissue using LCM. Data are expressed as the mean ± SEM relative to day 1 with three to five mice per time point. *, P < .05 compared with day 1. D, H&E of control mouse stomach collected 5 days after acetic acid-induced injury showing a clear ulcer margin and infiltrating immune cells forming the granulation tissue. E, Immunofluorescence staining using an anti-Shh and anti-TGFα antibodies of a stomach section collected from a control mouse 5 days after acetic acid-induced injury. Shh expression (green) was observed at the ulcer margin and granulation tissue. Shh expression (green) also colocalized with TGFα-positive (red) UACL.
Tissue from the uninjured or intact epithelium taken from an area beyond the ulcer margin that contained normal glandular epithelial architecture, ulcer margin, and granulation tissue at days 1–7 after the injury was collected by LCM from regions indicated in Supplemental Figure 1C. Compared with Shh expression levels measured 1 day after the injury, there was a significant reduction in gene expression at the ulcer margin at day 2 (Figure 2C). By days 3 and 4, Shh was reexpressed at the ulcer margin at levels significantly greater than those detected at day 1 (Figure 2C). Interestingly, there was a significant induction in the Shh gene expression within the uninjured or intact epithelium within 2 days of injury (Figure 2C). A significant induction in Shh gene expression was also detected within the granulation tissue 4 days after the injury (Figure 2C).
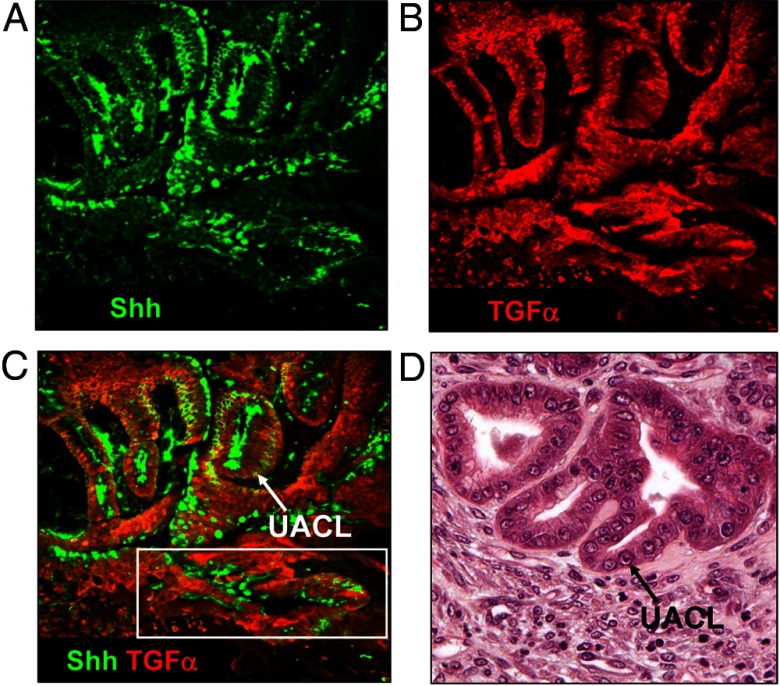
The ulceration of the epithelium along the gastrointestinal (GI) tract induces the development of a novel cell lineage from GI stem cells known as the ulcer-associated cell lineage (UACL). The lineage produces neutral mucin, shows a unique lectin-binding profile, is nonproliferative, and contains and secretes abundant amounts of EGF and TGFα (26–28). Shh localization was confirmed by immunofluorescence staining using antibodies specific for Shh and TGFα (Figure 2, D and E). Compared with the H&E, strong Shh expression was detected at both the ulcer margin and within the granulation tissue within the healing zone of the injured site (Figure 2E). A higher magnification of the H&E stain clearly showed the presence of the UACL below the regenerating glands at the ulcer margin and within the granulation tissue (Figure 3D), which were also positive for Shh and TGFα expression (Figure 3, A–C). Immunostaining using antibodies specific for Shh and α-smooth muscle actin revealed that there was no expression of Shh within the muscle of the mucosa (Supplemental Figure 2). Collectively, these data indicate that after gastric injury, the Shh reexpression occurs in the control mice that coincided with the ulcer repair.
Figure 3.
Expression of Shh within the UACL. Immunofluorescence staining using an anti-Shh and anti-TGFα antibodies of stomach sections collected from a control mouse 5 days after an acetic acid-induced injury. A, Shh expression (green) colocalized with TGFα-positive (red) UACL at the ulcer margin and within the granulation tissue (B). Merged image is shown in C. D, H&E demonstrating the presence of the ulcer-associated cell lineage at the base of regenerating glands at the ulcer margin and within the granulation tissue.
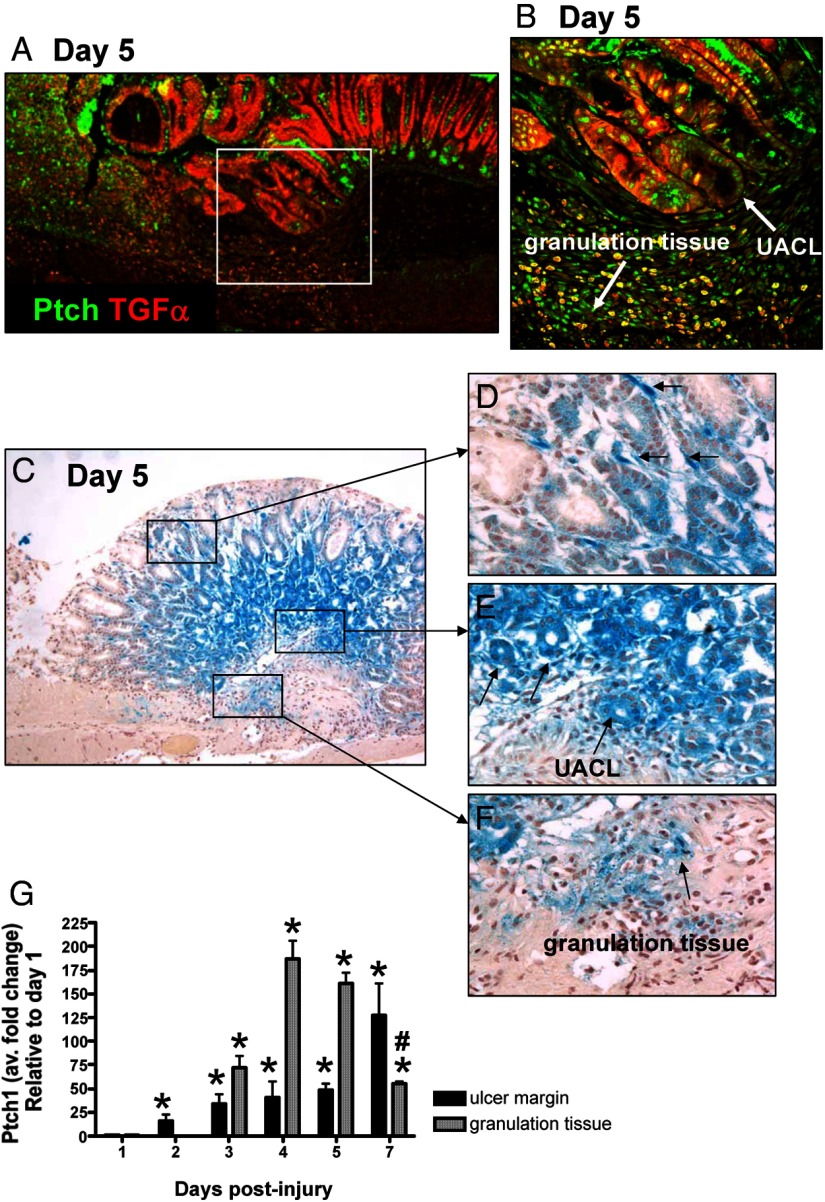
Shh induces transcription of target genes by opposing the repressive activity of its receptor Ptch (29, 30). To investigate receptor expression during healing, localization of Ptch in the ulcerated tissue was assessed by immunofluorescence and qRT-PCR (Figure 4). Based on immunostaining, Ptch was predominantly expressed within the granulation tissue, composed of infiltrating immune cells and fibroblasts, and along the ulcer margin specifically within the UACL (Figure 4, A and B). We confirmed the expression of Ptch within the healing zone using the heterozygote B6;129-Ptch1tm1Mps/J mice that expressed lacZ in a pattern mimicking endogenous Ptch1 gene expression (Figure 4, C and D). Consistent with the immunofluorescence staining in Figure 4, A and B, Ptch was strongly expressed within the epithelium at the ulcer margin (Figure 4E). In addition, infiltrating Ptch-positive cells were identified within the granulation tissue (Figure 4F). We also noted Ptch-positive cells within the mesenchyme (Figure 4D). The expression of Ptch correlated with wound healing as demonstrated by qRT-PCR using RNA isolated from LCM granulation tissue and tissue collected from the ulcer margin (Figure 4G). From these data, we demonstrated that there was a significant increase in Ptch expression within the ulcer margin within 2 days after the injury. A transient increase in Ptch expression was recorded within the granulation tissue during the 7-day healing process (Figure 4G).
Figure 4.
Expression of Ptch within the healing zone of the injured stomach. A, Immunofluorescence staining using an anti-Ptch and anti-TGFα antibodies of stomach sections collected from a control mouse 5 days after acetic acid-induced injury. Ptch expression (green) colocalized with TGFα-positive (red) UACL at the ulcer margin and within the granulation tissue. Higher magnification is shown in B. C, β-Galactosidase activity in the gastric mucosa of control mice 5 days after the acetic acid-induce injury using the heterozygote B61(29-Ptch1tm1Mps/J Ptch reporter mice). X-gal staining demonstrating Ptch1 expression shown within the mesenchyme (D), at the ulcer margin (E) and within the granulation tissue (F). G, qRT-PCR was performed using RNA collected from the ulcer margin and granulation tissue using LCM. Data are expressed as the mean ± SEM relative to day 1 with three to four mice per time point. *, P < .05 compared with day 1; #, P < .05 compared with days 4 and 5.
Circulating Shh derived from the gastric parietal cell promotes wound repair
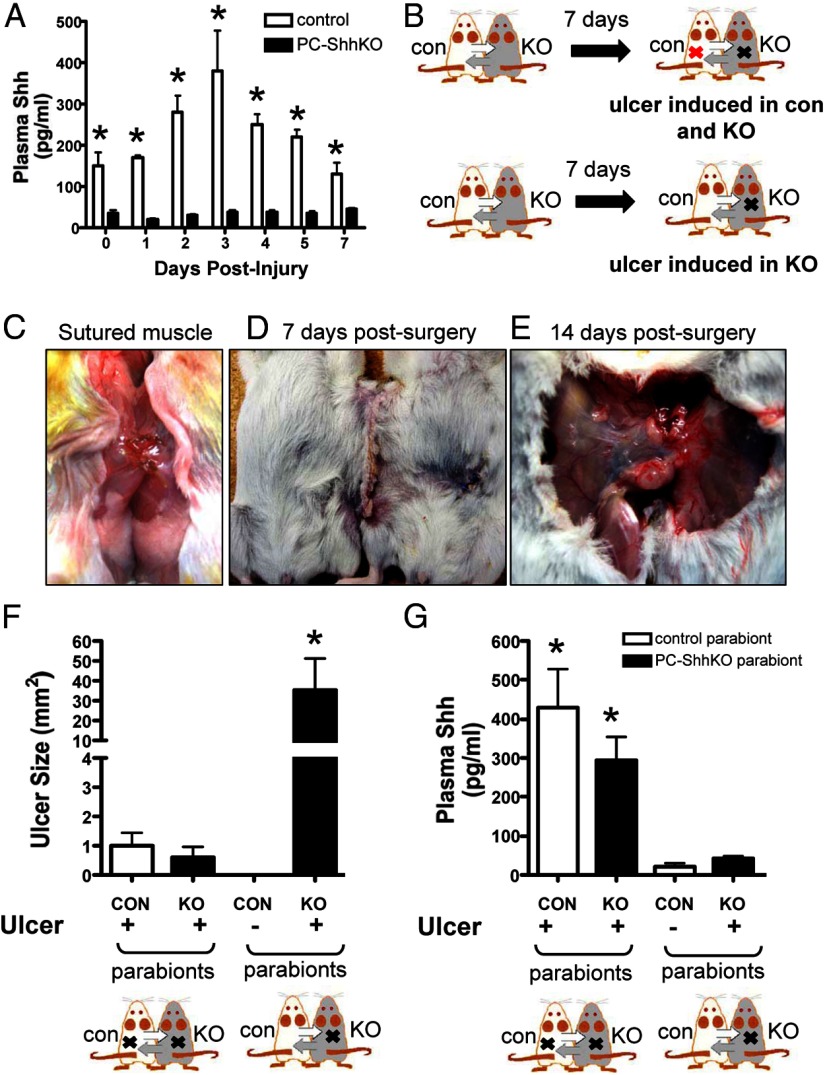
After ulceration, gastric epithelial Shh is secreted into circulation. Levels of Shh in the sera were elevated days 2, 3, 4, and 5 after the injury in control mice (Figure 5A). No increases in circulating Shh were seen in PC-ShhKO mice that received gastric injury (Figure 5A). Data presented in Figure 5A implicated a role of circulating Shh in ulcer repair. Thus, to establish the role for secreted Shh in gastric ulcer repair, a parabiosis model was used. In this model a control mouse was conjoined to a PC-ShhKO mouse to establish a joined circulation. After being paired for 7 days, ulcers were induced in either both of the parabionts or in the PC-ShhKO parabiont alone (Figure 5, B and C–E). Ulcers were measured 7 days after the injury. A shared circulation between the parabiotic animals was confirmed by Evans blue dye (Supplemental Figure 3A). Absorbance readings of plasma collected from both parabionts was comparable between the Evans blue-injected animal (control) and the uninjected PC-ShhKO mouse (Supplemental Figure 4A). Analysis of ulcer size showed that induction of ulcers in both parabionts resulted in ulcer sizes comparable with unpaired control 7 days after the injury (Figure 5F). Figure 5F was indicative that the ulcer healed in both the control and PC-ShhKO parabionts. In marked contrast, when the PC-ShhKO parabiont alone received an ulcer, the size was significantly larger than that of the control and PC-ShhKO parabionts that both received ulcers (Figure 5F).
Figure 5.
Changes in circulating Shh concentrations and ulcer sizes in control and PC-ShhKO parabionts. A, Changes in circulating Shh concentrations in blood collected from control and PC-ShhKO mice at 0, 1, 2, 3, 4, 5, and 7 days after acetic acid-induced injury. Data are expressed as the mean ± SEM with three to five mice per time point. *, P < .05 compared with control day 0. B, Design of the parabiosis experiments using control (CON) and PC-ShhKO (KO) parabionts. Surgery was performed to pair control and PC-ShhKO mice to establish a common circulation. An incision was made to expose the abdominal cavity wall and skin was pulled away from the muscle layer. C, A small incision was made below the diaphragm of each mouse, and the muscle layer of the two abdominal cavities were sutured together to form an opening between the abdominal cavities of each mouse. D, Wound clips were used to attach the skin between the two mice. E, Abdominal cavities were fused 14 days after parabiosis surgery. F, Ulcer size 7 days after the injury in control (CON) and PC-ShhKO (KO) parabionts in which both control and PC-ShhKO mice received an ulcer or parabionts in which only PC-ShhKO mice received ulcers. G, Shh concentration in plasma collected from control (CON) and PC-ShhKO (KO) parabionts. Data are expressed as the mean ± SEM with three to five pairs. *, P < .05 compared with parabionts with ulcers in both control and PC-ShhKO mice.
The concentration of Shh in circulation in parabiosis mice was examined on day 7 after ulcer injury using an ELISA (Figure 5G). Parabionts in which both control and PC-ShhKO mice received ulcers exhibited high levels of circulating Shh in the blood. Parabionts in which the PC-ShhKO mouse alone received an ulcer had significantly decreased levels of circulating Shh (Figure 5G). The circulating levels of Shh in the parabionts in which the PC-ShhKO mice alone received an ulcer were significantly lower compared with those levels measured in the circulation of control mice 7 days after the injury (Figure 5A). Therefore, we performed an experiment whereby control and PC-ShhKO mice were paired but did not receive gastric injury and Shh concentrations were measured in the circulation (Supplemental Figure 3B). In the uninjured parabionts, the concentration of Shh in circulation was comparable with levels of Shh in parabionts in which an ulcer was induced in the PC-ShhKO mouse alone 7 days after the injury.
The dispatched gene is required for long-range Hedgehog signaling and is essential for all Hedgehog patterning activities examined in the early mouse embryo (31, 32). These studies show that secreted Shh is often associated with dispatched. To determine the form of circulating Shh, we immunoprecipitated Shh protein from plasma collected from control mice 3 days after the injury. Western blot analysis showed the presence of the 19-kDa Shh protein coimmunoprecipitated with dispatched (Supplemental Figure 4).
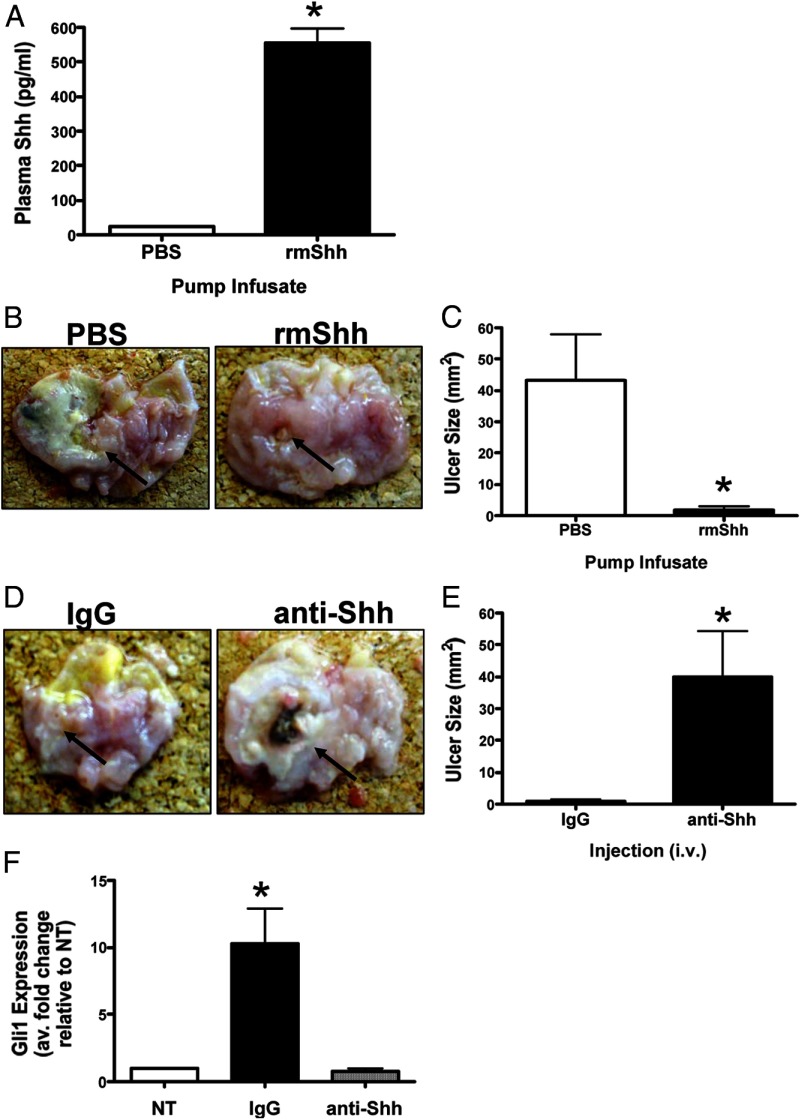
Collectively these data demonstrated that circulating Shh was secreted from the gastric parietal cell of control mice and contributed to the repair in the PC-ShhKO parabionts. To further test the role of circulating Shh in gastric repair, we next exogenously infused Shh into the PC-ShhKO mice via microosmotic pumps for 7 days after the gastric injury (Figure 6A). PC-ShhKO mice infused with Shh had a significantly reduced ulcer size 7 days after the injury compared with the PC-ShhKO mice infused with PBS (Figure 6, B and C). The data from the infusion of Shh into the PC-ShhKO mice together with the parabiosis experiments demonstrated that circulating Shh promotes repair of the gastric epithelium following acetic acid-induced injury.
Figure 6.
Changes in ulcer size in PC-ShhKO mice infused with exogenous Shh. A, Changes in circulating Shh concentrations in PC-ShhKO mice infused with either PBS or rmShh for 7 days after acetic acid-induced injury. B, Gross morphology of stomachs collected from PBS or rmShh infused PC-ShhKO mice. Arrows indicate ulcerated area. C, Changes in PC-ShhKO mouse ulcer size in response to either PBS or rmShh infusion for 7 days after acetic acid-induced injury. D, Gross morphology of stomachs collected from IgG or anti-Shh antibody-injected control mice. Arrows indicate ulcerated area. E, Changes in control mouse ulcer size in response to either IgG or anti-Shh antibody injections for 7 days after acetic acid-induced injury. Data are expressed as the mean ± SEM with five eight mice per group. *, P < .05 compared with PBS-infused or IgG-injected mice. F, Changes in Gli1 expression in Hedgehog-responsive cell line C3H10T1/2 in response to serum collected from either IgG- or anti-Shh antibody-injected control mice. *, P < .05 compared with no treatment (NT) (n = 5 serum samples collected from individual mice).
To further demonstrate the critical role of circulating Shh in repair, an immunoneutralization experiment was performed using control mice. Figure 6, D and E, showed that injection of an anti-Shh antibody resulted in significantly larger ulcer sizes 7 days after the injury compared with control mice injected with an IgG. We assessed the bioactivity of secreted Shh within the plasma using a bioassay with Hedgehog response cell line C3H10T1/2 (murine pluripotent mesenchymal cells) (Figure 6F). Although plasma collected from the IgG-injected controls induced a significant induction in Gli1 in cultured C3H10T1/2 cells, plasma collected from anti-Shh antibody-injected mice did not, thus demonstrating the successful immunoneutralization.
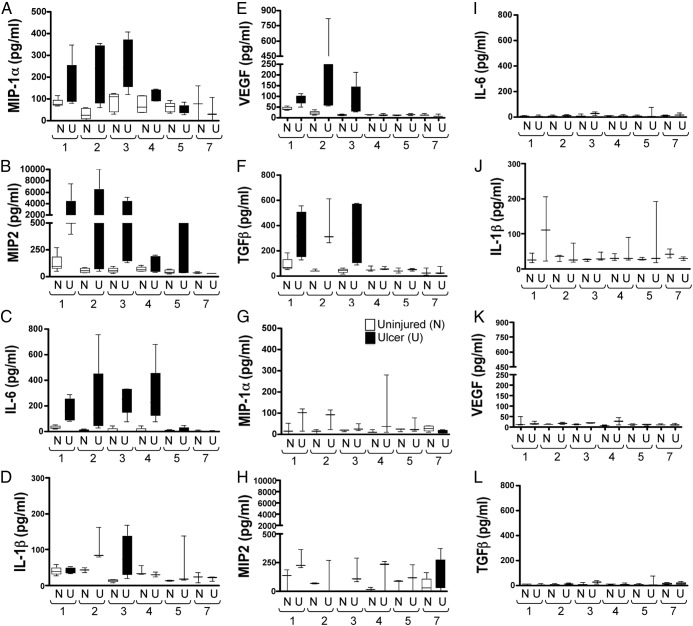
PC-ShhKO mice exhibit diminished growth factor and cytokine responses after injury
Our recent studies show that Shh may regulate the immune response during the process of ulcer healing (14). Using a Luminex-based assay, a number of growth and angiogenesis factors and cytokine concentrations associated with repair (MIP-1α, MIP-2, TGFβ, IL-6, and IL-1β) were measured in the gastric tissue of control and PC-ShhKO mice (Figure 7). In control mice, chemoattractant cytokines macrophage inflammatory protein MIP-1α and MIP-2, typically expressed at sites of inflammation in response to injury, were significantly elevated within the injured stomach in addition to cytokines IL-6 and IL-1β (Figure 7, A–D). In addition, VEGF and TGFβ concentrations were significantly elevated in the injured stomachs of control mice (Figure 7, E and F). In contrast, PC-ShhKO mice exhibited no significant increases in these growth factors and cytokines (Figure 7, G–L). These data suggests that Shh may be upstream of factors that promote tissue repair.
Figure 7.
Expression of cytokine and growth factors in ulcerated stomachs of controls and PC-ShhKO mice. Expression of MIP-1α, MIP-2, IL-6, IL-1β, VEGF, and TGFβ using a Luminex multiplex-based assay in control (A–F) and PC-ShhKO (G–L) mouse stomachs collected 1, 2, 3, 4, 5, and 7 days after ulcer induction. n = 4–8 mice/time point.
Discussion
In the current study, we show that Shh is a fundamental secreted protein mediating gastric ulcer healing. In mice lacking parietal cell-specific Shh (PC-ShhKO), ulcer repair was impaired as indicated by the lack of reepithelialization and epithelial gland regeneration, the presence of extensive fibrotic tissue, and persistence of prominent ulcer sizes. These data were consistent with our recently published findings demonstrating that Shh is crucial for ulcer healing in a mouse model expressing a tamoxifen-inducible deletion of Shh in parietal cells (14). Moreover, a study by Kang et al (13) showed disrupted ulcer healing when mice are treated with Hedgehog signaling inhibitor cyclopamine. Here we advance our current knowledge by demonstrating that the gastric epithelium, in particular the parietal cell, serves as an endocrine source of Shh during repair.
There was a significant increase in circulating Shh expression within 2 days after the injury in control mice. Interestingly, PC-ShhKO mice exhibited approximately 80% lower circulating Shh concentrations compared with controls, regardless of injury. Such data suggest that the parietal cell is a major source of Shh that is found within the circulation. It is well established that gastric parietal cells express (33, 34) and secrete (16) Shh. In the stomach, synthesis of Shh results in an approximately 45-kDa protein that is processed via an acid-dependent and hormonally regulated (gastrin) mechanism (15). We also demonstrated that the secreted 19-kDa processed form of Shh protein coimmunoprecipitated with the dispatched gene that is required for long-range Hedgehog signaling (31, 32). In addition to secreting Shh, parietal cells are also known to secrete a number of factors regulating differentiation and function including TGFβ, Wnt, fibroblast growth factors, amphiregulin, and EGF (33, 35–38). Earlier studies in rats have even shown that parietal cells play a potent endocrine role in secreting estrogen (20) and T (21). The significant reduction in the levels of circulating Shh seen in the PC-ShhKO mice and the lack of any increases of Shh in the blood after the injury suggests that circulating Shh is secreted from the parietal cells. Our data demonstrate for the first time that the parietal cell serves as an endocrine source of Shh during repair in response to injury.
The presence of Shh in the circulation of control mice after gastric injury indicated that Shh is an important factor that modulates gastric repair. To determine the role of circulating Shh derived from gastric parietal cells, a parabiosis mouse model was used. The pairing of a control mouse with a PC-ShhKO mouse and the induction of ulcers in both mice resulted in repair in the PC-ShhKO animal. This repair was evident from the reepithelialization of the ulcerated tissue and the reduced ulcer size 7 days after the injury. Induction of ulcers in both control and PC-ShhKO parabionts resulted in increased levels of circulating Shh in both parabionts compared with levels when ulcers were induced in PC-ShhKO parabionts alone. Parabiosis mice have been previously shown to be a viable model for studying the role of key circulating factors in wound repair (39). Pietramaggiori et al (39) demonstrated that the pairing of diabetic mice with wild-type mice results in significantly improved wound healing in the diabetic parabionts when exposed to healthy peripheral circulation. Similarly, our study showed that increased circulating Shh coincided with wound healing in PC-ShhKO parabionts. The exogenous infusion of recombinant mouse Shh (rmShh) into PC-ShhKO mice also facilitated ulcer repair when compared with transgenic mice infused with PBS. Furthermore, the immunoneutralization of Shh with an anti-Shh antibody blocked repair in the injured stomachs of control mice. Collectively, this further demonstrated an important role for circulating Shh in promoting gastric ulcer repair.
From our findings we may ask the question of the mechanism by which circulating Shh facilitates repair in the stomach in response to injury. The answer to this question is evidenced by our recently published studies demonstrating that Shh signaling is a regulator of the immune response during repair (14) and bacterial infection (40). In a mouse model expressing a tamoxifen-inducible parietal cell-specific deletion of Shh, injury results in the induction of Shh expression from the surrounding epithelium that subsequently recruits macrophages to the site of injury and thus triggering repair (14). Furthermore, bone marrow chimera experiments with donor cells collected from mice that have a myeloid cell-specific deletion of the Hedgehog signal transduction protein smoothened (LysMCre/SmoKO) demonstrates that Shh signals to the macrophages to induce recruitment during the initiation of gastritis in response to H pylori infection (40). Overall, we observed a dampened immune response in the PC-ShhKO mice after the injury when compared with the controls as indicated by decreased cytokine expression levels in IL-1β, TGF β, IL-6, MIP-2, and MIP-1α. Thus, Shh secreted from the gastric epithelium is likely to facilitate repair in the stomach by regulating the immune response. In addition, it may also be that other secreted factors and cytokines, similar to those measured in Figure 7, increase in the circulation of the PC-ShhKO parabionts paired to the control mice after the wound induction. Whether one or more of these factors contribute to wound repair in the PC-ShhKO parabionts in this experiment is possible. Therefore, we cannot exclude the possibility that cytokines or factors that are known to regulate repair, other than Shh, also play a critical role in our observations.
We observed an increase in Shh expression in tissue collected from the ulcer margin. In support of the current findings, our laboratory recently reported that Shh is expressed in the ulcerated gastric tissue 2 days after the injury (14). The reexpression of Shh within the gastric epithelium was found to be independent of parietal cell-expressed Shh, and protein was localized at the ulcer margin within the regenerating epithelial glands (14). We hypothesize that Shh may also act locally to regulate epithelial proliferation and regeneration. The ulceration of the epithelium along the human GI tract induces the development of a novel cell lineage from GI stem cells known as the ulcer-associated cell lineage. This lineage initially appears as a bud from the base of gastric glands, adjacent to the ulcer, and grows locally as a tubule that eventually differentiates into newly formed epithelium (28, 41). The lineage produces neutral mucin, shows a unique lectin-binding profile, is nonproliferative, and contains and secretes abundant amounts of EGF and TGFα (26–28). Our data clearly demonstrate the strong expression of Shh within the UACL at the ulcer margin. Based on this evidence, we may propose that Shh, also released from the regenerative epithelial glands, acts to stimulate cell proliferation, regeneration, and ulcer healing but requires further investigation.
We also observed significant Shh expression within the granulation tissue of the healing zone by both qRT-PCR and immunofluorescence staining. The granulation tissue develops at the ulcer base within 48 hours after injury or ulceration (7). Granulation tissue consists of macrophages, fibroblasts, and proliferating endothelial cells that play a critical role in angoiogenesis/neovascularization, a process known to be regulated by TGFβ (7). Our data suggest that Shh may also be expressed by these cells during the process of ulcer repair. In support of this notion, it was recently shown that liver-derived macrophages produce Hedgehog ligands including Shh and Indian Hedgehog in response to Schistosomiasis mansoni (42). However, whether fibroblasts and endothelial cells express Shh within the granulation tissue of the injured stomach remains to be determined. Shh induces transcription of target genes by opposing the repressive activity of its receptor Ptch (29, 30). Importantly, Ptch expression was predominantly expressed within the granulation tissue of the stomach within the healing zone. There is strong evidence for the existence of Hedgehog-responsive cells within the granulation tissue. For example, it is clear that Ptch is expressed on T lymphocytes (43, 44) and macrophages (40, 45). Granulation tissue is a critical component of the ulcer healing process. Granulation tissue supplies connective tissue cells responsible for synthesizing the extracellular matrix for restoration of lamina propria restoration and the microvasculature (7). Our data suggest that Hedgehog signaling may play a role in these processes.
This report, to the best of our knowledge, is the first to identify a specific role for circulating Shh in facilitating gastric ulcer repair and identifies the parietal cell as an important endocrine cell during wound repair. Interestingly, recent evidence demonstrates that Shh is also detected in human plasma (46). Data presented by El-Zaatari et al, (46) demonstrate that the bladder, prostate, cervix, stomach, and liver are among the highest Shh-expressing organs in the body. Thus, Shh secreted into the circulation from tissues and organs including the stomach warrants further investigation.
Acknowledgments
We thank Dr Debra Kemper (senior staff veterinarian, Laboratory Animal Medical Services Veterinarian Services, University of Cincinnati) and Erin Bartley, RVT (senior research assistant) for their guidance and assistance with the parabiosis surgeries. We acknowledge the assistance of Jacob Turner (University of Cincinnati, undergraduate, Department of Biochemistry, University of Cincinnati) with the qRT-PCR.
This work was supported by National Institutes of Health Grant 1R01DK083402 (to Y.Z.). Work was also supported in part by the Digestive Health Center Cincinnati Children's Medical Health Center (Digestive Health Center: Bench to Bedside Research in Pediatric Digestive Disease) Grant CHTF/SUB DK078392.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EGF
- epidermal growth factor
- GI
- gastrointestinal
- H&E
- hematoxylin and eosin
- HKCre
- H+,K+-ATPase β-subunit promoter
- LCM
- laser capture microdissection
- MIP
- macrophage inflammatory protein
- PC-ShhKO
- mice expressing a parietal cell-specific deletion of Shh
- Ptch
- Patched
- qRT-PCR
- quantitative RT-PCR
- rmShh
- recombinant mouse Shh
- Shh
- Sonic Hedgehog
- UACL
- ulcer-associated cell lineage
- VEGF
- vascular endothelial growth factor
- X-gal
- β-galactosidase.
References
- 1. Grassi M, Petraccia L, Mennuni G, et al. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutr Hosp. 2011;26:659–668 [DOI] [PubMed] [Google Scholar]
- 2. Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043–1052 [DOI] [PubMed] [Google Scholar]
- 3. Pilotto A, Franceschi M, Leandro G, Di Mario F, Valerio G. The effect of Helicobacter pylori infection on NSAID-related gastroduodenal damage in the elderly. Eur J Gastroenterol Hepatol. 1997;9:951–956 [DOI] [PubMed] [Google Scholar]
- 4. Somerville K, Faulkner G, Langman M. Non-steroidal anti-inflammatory drugs and bleeding peptic ulcer. Lancet. 1986;1:462–464 [DOI] [PubMed] [Google Scholar]
- 5. Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559–578 [DOI] [PubMed] [Google Scholar]
- 6. Pleis JR, Lucas JW. Summary health statistics for US adults: National Health Interview Survey, 2007. Vital Health Stat. 2009;10:240. [PubMed] [Google Scholar]
- 7. Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50:S24–S33 [DOI] [PubMed] [Google Scholar]
- 8. Chiang C, Litingtung Y, Lee E, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413 [DOI] [PubMed] [Google Scholar]
- 9. Palladino M, Gatto I, Neri V, et al. Pleiotropic beneficial effects of sonic hedgehog gene therapy in an experimental model of peripheral limb ischemia. Mol Ther. 2011;19:658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fitch PM, Howie SE, Wallace WA. Oxidative damage and TGF-β differentially induce lung epithelial cell sonic hedgehog and tenascin-C expression: implications for the regulation of lung remodelling in idiopathic interstitial lung disease. Int J Exp Pathol. 2011;92:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levi B, James AW, Nelson ER, et al. Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts. Stem Cells. 2011;20:243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujita K, Miyamoto T, Saika S. Sonic hedgehog: its expression in a healing cornea and its role in neovascularization. Mol Vis. 2009;15:1036–1044 [PMC free article] [PubMed] [Google Scholar]
- 13. Kang DH, Han ME, Song MH, et al. The role of hedgehog signaling during gastric regeneration. J Gastroenterol. 2009;44:372–379 [DOI] [PubMed] [Google Scholar]
- 14. Xiao C, Feng R, Engevik A, et al. Sonic Hedgehog contributes to gastric mucosal restitution after injury. Lab Invest. 2013;93:96–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zavros Y, Waghray M, Tessier A, et al. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265–33274 [DOI] [PubMed] [Google Scholar]
- 16. Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+,K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol. 2008;295:G99–G111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiao C, Ogle SA, Schumacher MA, et al. Loss of parietal cell expression of sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beales IL. Gastrin and interleukin-1β stimulate growth factor secretion from cultured rabbit gastric parietal cells. Life Sci. 2004;75:2983–2995 [DOI] [PubMed] [Google Scholar]
- 19. Miyaoka Y, Kadowaki Y, Ishihara S, et al. Transgenic overexpression of Reg protein caused gastric cell proliferation and differentiation along parietal cell and chief cell lineages. Oncogene. 2004;23:3572–3579 [DOI] [PubMed] [Google Scholar]
- 20. Ueyama T, Shirasawa N, Numazawa M, et al. Gastric parietal cells: potent endocrine role in secreting estrogen as a possible regulator of gastro-hepatic axis. Endocrinology. 2002;143:3162–3170 [DOI] [PubMed] [Google Scholar]
- 21. Le Goascogne C, Sananès N, Eychenne B, Gouézou M, Baulieu EE, Robel P. Androgen biosynthesis in the stomach: expression of cytochrome P450 17 α-hydroxylase/17,20-lyase messenger ribonucleic acid and protein, and metabolism of pregnenolone and progesterone by parietal cells of the rat gastric mucosa. Endocrinology. 1995;136:1744–1752 [DOI] [PubMed] [Google Scholar]
- 22. Okabe S, Amagase K. An overview of acetic acid ulcer models—the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321–1341 [DOI] [PubMed] [Google Scholar]
- 23. Duyverman AM, Kohno M, Duda DG, Jain RK, Fukumura D. A transient parabiosis skin transplantation model in mice. Nature Protoc. 2012;7:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang Q, Oldenburg R, Otsuru S, Grand-Pierre AE, Horwitz EM, Uitto J. Parabiotic heterogenetic pairing of Abcc6−/−/Rag1−/− mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am J Pathol. 2010;176:1855–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams KP, Rayhorn P, Chi-Rosso G, et al. Functional antagonists of sonic hedgehog reveal the importance of the N terminus for activity. J Cell Sci. 1999;112:4405–4414 [DOI] [PubMed] [Google Scholar]
- 26. Wright NA, Pike CM, Elia G. Ulceration induces a novel epidermal growth factor-secreting cell lineage in human gastrointestinal mucosa. Digestion. 1990;46:125–133 [DOI] [PubMed] [Google Scholar]
- 27. Longman RJ, Douthwaite J, Sylvester PA, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47:792–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patel K, Hanby AM, Ahnen DJ, Playford RJ, Wright NA. The kinetic organization of the ulcer-associated cell lineage (UACL): delineation of a novel putative stem-cell region. Epithelial Cell Biol. 1994;3:156–160 [PubMed] [Google Scholar]
- 29. Stone DM, Hynes M, Armanini M, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nautre. 1996;384:129–134 [DOI] [PubMed] [Google Scholar]
- 30. Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312 [DOI] [PubMed] [Google Scholar]
- 31. Etheridge LA, Crawford TQ, Zhang S, Roelink H. Evidence for a role of vertebrate Disp1 in long-range Shh signaling. Development. 2010;137:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma Y, Erkner A, Gong R, et al. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63. [DOI] [PubMed] [Google Scholar]
- 33. Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–15708 [DOI] [PubMed] [Google Scholar]
- 34. Van Den Brink GR, Hardwick JC, Tytgat GN, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–328 [DOI] [PubMed] [Google Scholar]
- 35. Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology. 2007;132:1804–1819 [DOI] [PubMed] [Google Scholar]
- 36. Chen MC, Lee AT, Karnes WE, et al. Paracrine control of gastric epithelial cell growth in culture by transforming growth factor-α. Am J Physiol. 1993;264:G390–G396 [DOI] [PubMed] [Google Scholar]
- 37. Beauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ., Jr Localization of transforming growth factor a and its receptor in gastric mucosal cells. J Clin Invest. 1989;84:1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stepan V, Pausawasdi N, Ramamoorthy S, Todisco A. The Akt and MAPK signal-transduction pathways regulate growth factor actions in isolated gastric parietal cells. Gastroenterology. 2004;127:1150–1161 [DOI] [PubMed] [Google Scholar]
- 39. Pietramaggiori G, Scherer SS, Alperovich M, Chen B, Orgill DP, Wagers AJ. Improved cutaneous healing in diabetic mice exposed to healthy peripheral circulation. J Invest Dermatol. 2009;129:2265–2274 [DOI] [PubMed] [Google Scholar]
- 40. Schumacher MA, Donnelly JM, Engevik AC, et al. Gastric sonic hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology. 2012;142:1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82–85 [DOI] [PubMed] [Google Scholar]
- 42. Pereira TA, Xie G, Choi SS, et al. Macrophage-derived Hedgehog ligands promotes fibrogenic and angiogenic responses in human schistosomiasis mansoni. Liver Int. 2013;33:149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stewart GA, Hoyne G, Ahmad SA, et al. Expression of the developmental Sonic hedgehog (Shh) signalling pathway is up-regulated in chronic lung fibrosis and the Shh receptor patched 1 is present in circulating T lymphocytes. J Pathol. 2003;199:488–495 [DOI] [PubMed] [Google Scholar]
- 44. Stewart GA, Lowrey J, Wakelin SJ, et al. Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol. 2002;169:5451–5457 [DOI] [PubMed] [Google Scholar]
- 45. Dunaeva M, Voo S, van Oosterhoud C, Waltenberger J. Sonic hedgehog is a potent chemoattractant for human monocytes: diabetes mellitus inhibits Sonic hedgehog-induced monocyte chemotaxis. Basic Res Cardiol. 2010;105:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. El-Zaatari M, Daignault S, Tessier A, et al. Plasma Shh levels reduced in pancreatic cancer patients. Pancreas. 2012;41:1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]