Abstract
Previously we showed that bisphenol A (BPA), an environmental estrogenic endocrine disruptor, rapidly altered Ca2+ handling and promoted arrhythmias in female rat hearts. The underlying molecular mechanism was not known. Here we examined the cardiac-specific signaling mechanism mediating the rapid impact of low-dose BPA in female rat ventricular myocytes. We showed that protein kinase A (PKA) and Ca2+/CaM-dependent protein kinase II (CAMKII) signaling pathways are the two major pathways activated by BPA. Exposure to 1 nM BPA rapidly increased production of cAMP and rapidly but transiently increased the phosphorylation of the ryanodine receptors by PKA but not by CAMKII. BPA also rapidly increased the phosphorylation of phospholamban (PLN), a key regulator protein of sarcoplasmic reticulum Ca2+ reuptake, by CAMKII but not PKA. The increase in CAMKII phosphorylation of PLN was mediated by phospholipase C and inositol trisphosphate receptor-mediated Ca2+ release, likely from the endoplasmic reticulum Ca2+ storage. These two pathways are likely localized, impacting only their respective target proteins. The rapid impacts of BPA on ryanodine receptors and PLN phosphorylation were mediated by estrogen receptor-β but not estrogen receptor-α. BPA's rapid signaling in cardiac myocytes did not involve activation of ERK1/2. Functional analysis showed that PKA but not CAMKII activation contributed to BPA-induced sarcoplasmic reticulum Ca2+ leak, and both PKA and CAMKII were necessary contributors to the stimulatory effect of BPA on arrhythmogenesis. These results provide mechanistic insight into BPA's rapid proarrhythmic actions in female cardiac myocytes and contribute to the assessment of the consequence and potential cardiac toxicity of BPA exposure.
Bisphenol A (BPA; Chemical Abstracts Service 80-05-7) is a high-production volume chemical with more than 2 million metric tons production per year. BPA is used mainly in the manufacturing of polycarbonate plastics and epoxy resins. It is used extensively in consumer products such as food containers, beverage and food can lining, dental sealants, thermal paper, and water pipes. There is widespread and well-documented human exposure to BPA from food, drinking water/beverage, dermal exposure, and inhalation of household dust. BPA has been detected in the urine or blood at low nanograms per milliliter concentrations from more than 90% of individuals examined in various sampled populations (1–5).
BPA is an estrogenic endocrine disruptor in a broad sense. It exerts its biological influences through a range of actions, such as acting as a selective estrogen receptor modulator and impacting estrogen receptor (ER)-mediated gene transcription, activation of nongenomic rapid signaling pathways, and disruption of thyroid hormone function (6, 7). Experimental and/or epidemiological studies suggest that BPA exposure may have potential negative impacts including cancer; obesity; diabetes; and disorders of the reproductive, neuroendocrine, and immune systems (8, 9). Recent evidence suggests that the cardiovascular system is another potential target of BPA's adverse influence. BPA exposure has been shown to be associated with cardiovascular diseases including coronary artery disease and peripheral arterial disease (10–13). Previously we demonstrated that low doses of BPA, through an ERβ-mediated mechanism, rapidly promotes arrhythmogenic-triggered activities in cardiac myocytes from female rat hearts and promotes the development of arrhythmias in female rat hearts under pathophysiological conditions including stress and ischemic injury (14–16). We showed that the cellular mechanism of the proarrhythmic actions of BPA in the female heart involved rapid alteration of myocyte Ca2+ handling, particularly an increase in sarcoplasmic reticulum (SR) Ca2+ release/leak and SR Ca2+ reuptake (14).
Limited understanding of the molecular mechanisms through which BPA acts is identified as one of the major knowledge gaps in our understanding of the consequences of BPA exposure (6). The goal of this study is to elucidate the signaling mechanisms underlying the rapid impact of BPA on myocyte Ca2+ handling and arrhythmogenesis. Ca2+ handling is at the core of heart physiology, linking electrical excitation and mechanical contraction. Key steps in Ca2+ handling include Ca2+ influx through the L-type Ca2+ channel, release of Ca2+ from the SR through the ryanodine receptors (RyR), and Ca2+ removal from the cytosol achieved by Ca2+ reuptake into the SR through sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), and to a much lesser degree by Ca2+ extrusion through the Na+/Ca2+ exchanger (17). RyR is the major intracellular Ca2+ release channel localized on the SR in cardiac myocytes. RyR-mediated Ca2+ release is critical for normal cardiac physiology (17), and aberrant RyR release is a key player in arrhythmogenesis (18). Phospholamban (PLN), through its inhibition of SERCA, is the regulator of SR Ca2+ reuptake. In this study, we investigate the signaling cascade by which low-dose BPA impacts Ca2+ handling, with a focus on BPA's rapid influence on the two key Ca2+ handling proteins, RyR and PLN.
Materials and Methods
Reagents
All reagents and solvents used were of the highest purity available. All aqueous solutions were prepared using BPA-free water (18 MΩ; < 6 parts per billion total oxidizable organics; Millipore A10 system). BPA, CAS 80/-05-7, was from TCI America, lot 111909 (ground by Battelle), and was provided by the Division of the National Toxicology Program at the National Institutes of Health/National Institute of Environmental Health Sciences. Dimethyl sulfoxide (Chromasolv Plus, HPLC ≥ 99.7%; batch number 00451HE) and H89 dihydrochloride hydrate (HPLC ≥ 98%; CAS 130964-39/-5, batch number 12K1137) were from Sigma-Aldrich. Protein kinase A (PKA) inhibitor 14–22 amide, cell-permeable, myristoylated (PKI; HPLC ≥ 95%; batch number D00123663), autocamtide-2 related inhibitory peptide, myristoylated (AIP; HPLC ≥ 95%; batch number D00121501), and 2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine) (KN93; HPLC ≥ 95%; CAS 139298-40-1, batch number D00118007) were from EMD Millipore Chemicals. Methyl-piperidino-pyrazole,1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP), CAS 289726-02-9, 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), CAS 805239-56-9, and (−)-xestospongin C, CAS 88903-69-9, were from Tocris Bioscience. A direct cAMP ELISA kit (for intracellular cAMP quantification, batch number 06281202) was from Enzo Life Science. Other chemicals were from Sigma-Aldrich unless otherwise stated.
Animals
All animal procedures were performed in accordance with protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee and recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Adult female Sprague Dawley rats (200–250 g; Charles River) were used as nonsurviving sources of ventricular myocytes. Animals were maintained on a 14-hour light, 10-hour dark light cycle in standard polycarbonate caging with Sani-chip bedding (Irradiated Aspen Sani-chip; P. J. Murphy Forest Products Corp) to eliminate possible corn-based mycoestrogen exposure. All rats were fed ad libitum Teklad diet 2020 (Harlan Laboratories Inc), which lacks soybean meal, alfalfa, or animal products that may introduce uncontrolled levels of estrogenic compounds. Sterile drinking water was generated by a dedicated water purification system (Millipore Rios 16 with ELIX UV/Progard 2), which reduces oxidizable organics to less than 1% of source levels. Drinking water was dispensed from glass water bottles.
Isolation and culture of ventricular myocytes
Ventricular myocytes from female rat hearts were enzymatically dissociated using Langendorff perfusion as previously described (14). Isolated myocytes were suspended in 1.0 mM Ca2+-Tyrode solution for immediate experiments. For Western blotting, myocytes were cultured in a Medium-199 based solution (Gibco; catalog number 31100-035, with Earle's salts and L-glutamine, without NaHCO3, lot number 561846; prepared using BPA free water), plated on laminin (Sigma-Aldrich; CAS 114956-81-9, various lots)-coated polystyrene culture dishes at a density of approximately 5 × 104 cells/cm2. Each rat heart yielded about 2 × 106 myocytes. Myocytes were allowed to adhere and stabilize in an incubator at 37°C for 4 hours prior to treatment and collection.
Western blotting
Isolated female ventricular myocytes were treated with BPA, with or without indicated inhibitors, for the indicated length of time, washed with cold PBS solution three times, and then collected into 1 mL cold PBS solution. Each treatment group typically contained about 3 × 105 myocytes. Collected myocytes were centrifuged and the pellets snap frozen in liquid nitrogen. Proteins were extracted with 1× cell lysis buffer (Cell Signaling Technology) supplemented with complete protease inhibitor cocktail (Roche Applied Science), and phosphatase inhibitor cocktail sets I and II (EMD Millipore Chemicals). Equal amounts of protein samples from each treatment group were separated by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). The membrane was then blocked with 5% nonfat milk in PBS-0.1% Tween 20 and incubated with the indicated primary antibodies and secondary antibodies.
An ECL Western blotting analysis system (GE Healthcare) was used for developing the membrane. AlphaEaseFC software (Alpha Innotech) was used to determine the band intensities. The densitometric values of the various treatment groups were normalized to control and were expressed as fold changes. Antibodies used in this study were as follows: RYR2 (Affinity BioReagents); pSer2808-RYR2, pSer2814-RYR2, pSer16-PLN, pThr17-PLN, PLN (Badrilla); phospho-P44/42 MAPK (ERK1/2, Thr202/Tyr204) and P44/42 MAPK (ERK1/2) (Cell Signaling Technology); and horseradish peroxidase-conjugated antimouse and antirabbit secondary antibodies (Cell Signaling Technology).
Myocyte intracellular cAMP measurement
Cultured ventricular myocytes were subjected to the indicated treatments for 5 minutes at 37°C. Each treatment group typically contained about 3 × 105 myocytes. The treatment was terminated by aspirating the medium and adding 0.1 M HCl. Intracellular cAMP of myocytes was determined using the Direct cAMP ELISA kit (Enzo Life Sciences), following the manufacturer's instructions. OD data were obtained at 405 nm. The concentration of cAMP in each sample was determined based on the cAMP standard curve (four parameter sigmoidal logistic nonlinear regression). Values of treatment groups were normalized to control and expressed as fold changes.
Analysis of myocyte contraction, triggered activity, and Ca2+ spark
Analysis of the myocyte contraction, after-contraction, and Ca2+ spark was performed as previously described (14). Briefly, myocytes were excited with field stimulation (Grass S48 stimulator; Grass Instruments) with 2 milliseconds 1.5× threshold pulses at a rate of 0.5 Hz. Steady-state myocyte shortening was examined using a video-edge detector (Crescent Electronics). After-contraction was measured with stimulation of 2 milliseconds 1.5× threshold pulses at a rate of 2 Hz for 8 seconds and recorded for more 15 seconds. Data were sampled through an Axon Digidata 1322A board using the PCLAMP 9 software (both by Molecular Devices). To measure then Ca2+ spark, isolated ventricular myocytes were loaded with fluo-4 acetoxymethyl ester (5 μM; Molecular Probes) and imaged with a Zeiss LSM 710 inverted confocal microscope with an excitation wavelength of 488 nm. Signals were measured with line-scan imaging at 3.07-millisecond intervals, with each line comprising 512 pixels spaced at 0.056 mm. Ca2+ spark frequency was calculated as the count of sparks divided by the length of scanning line, divided by time of scanning, and expressed as 1:100 μm/sec, which indicates the average number of Ca2+ sparks per spatial distance per unit time. Image processing and data analysis were performed as previously described (14).
Methods of minimizing risk of bias
For those experiments that involved human sampling and thus had a potential risk of bias, utmost care was taken to minimize sampling bias. These experiments included the measurement of BPA effects on myocyte fractional shortening, incidence of triggered activities, and Ca2+ spark frequency. For these measurements, results were verified by at least one blind experiment in which the experimenter was blinded of the types of treatment (eg, control, BPA, or BPA plus kinase blockers). For Ca2+ spark measurement, blind experiments were performed to confirm the lack of effect of control treatment.
Statistical analysis
All experiments were independently repeated using myocytes isolated from at least three rat hearts. Statistical analysis was conducted using one-way ANOVA, with differences between treatment groups assessed using a multiple comparison post hoc test. Frequency of events (eg, percentage of myocyte with triggered activities) was analyzed using a χ2 test. The minimal level of statistical significance for differences in values is considered to be P < .05. Data were analyzed with SigmaPlot 11.0 (Systat Software Inc) and expressed as average ± SEM.
Results
Rapid effects of BPA in female rat cardiac myocytes
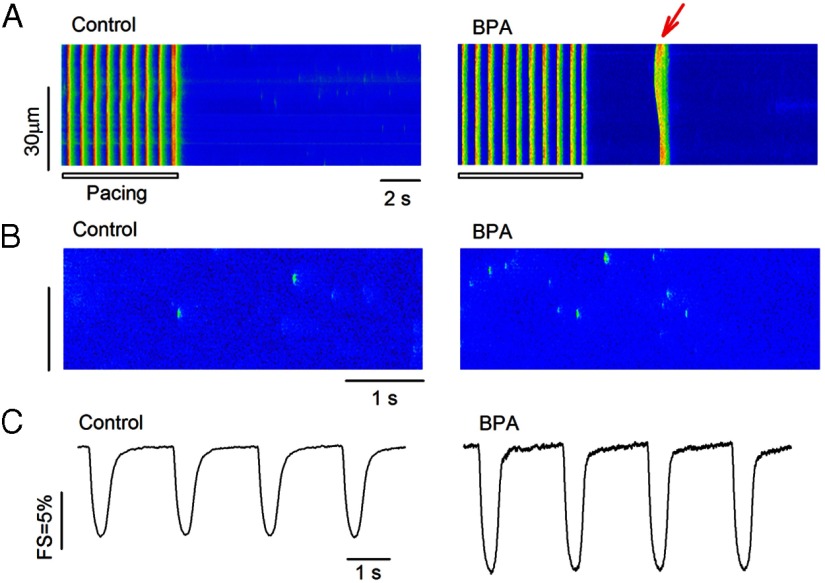
Previously we demonstrated that BPA has female-specific rapid actions in rodent ventricular myocytes (14, 15). The rapid effects (2–7 min) of 1 nM BPA in female rat cardiac myocytes were examined in the current study. Nanomolar concentration was chosen because of its relevance to human BPA exposure levels (1, 2). BPA rapidly increased spontaneous excitation after repeated pacing (Figure 1A); these aberrant excitation events are known as triggered activities and are well understood as a key arrhythmogenic mechanism in the heart (17). The proarrhythmic effect of BPA is mediated by rapid alteration of myocyte Ca2+ handling, particularly induction of SR Ca2+ release/leak (14). As shown in Figure 1B, BPA rapidly increased the frequency of Ca2+ sparks in quiescent myocytes, which represent spontaneous release of Ca2+ from the SR through RyRs. Rapid BPA exposure also markedly increased contractility of female cardiac myocytes (Figure 1C).
Figure 1.
Rapid impact of BPA in female rat ventricular myocytes. A, Example confocal images showing Ca2+ transients in myocytes elicited by pacing under control (left panel) and upon acute exposure to 1 nM BPA (right panel). Arrow indicates spontaneous Ca2+ after-transient (ie, triggered activity) after pacing (n = 6 hearts). The control group had 49 myocytes, with three myocytes showing triggered activities. The BPA group had 52 myocytes, with 16 myocytes showing triggered activities (P < .01). Statistical analysis of the frequency of the triggered activities was performed using the χ2 test. B, Representative confocal images showing Ca2+ sparks in myocytes under control (left panel) and upon acute exposure to 1 nM BPA (right panel) (n = 3 hearts). Ca2+ spark frequency was 1.55 ± 0.18 (per 100 μm/sec) for control and 2.75 ± 0.19 (per 100 μm/sec) for the BPA treatment group (n = 12 myocytes for each group; P < .05). C, Representative contraction traces from myocytes under control (left panel) and upon acute exposure to 1 nM BPA (right panel; n = 4 hearts). FS was 8.3% ± 0.14% for control (n = 49) and 14.1% ± 0.21% for the BPA treatment group (n = 44 myocytes; P < .05). For B and C, statistical analysis was performed using a Student's t test. Data were expressed as average ± SEM.
Mechanism of BPA's rapid effect: role of the cAMP/PKA signaling pathway
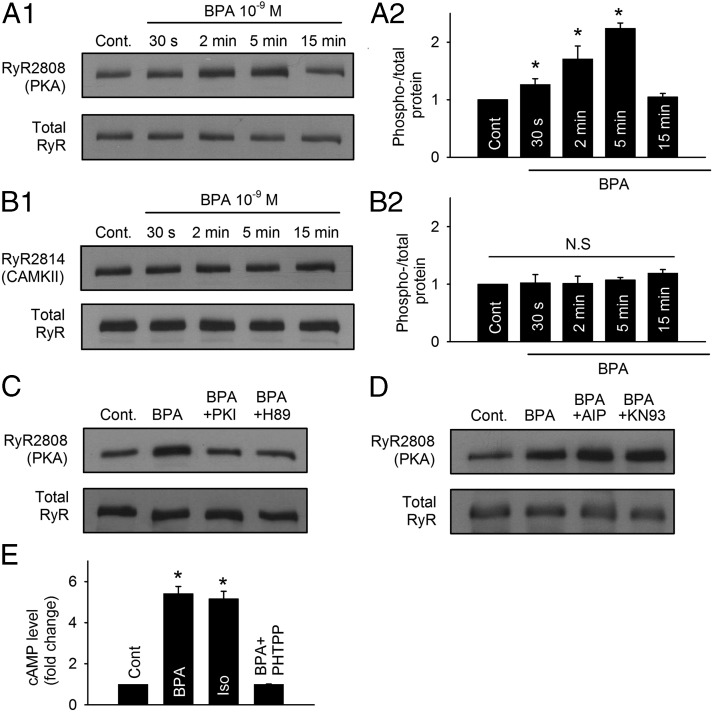
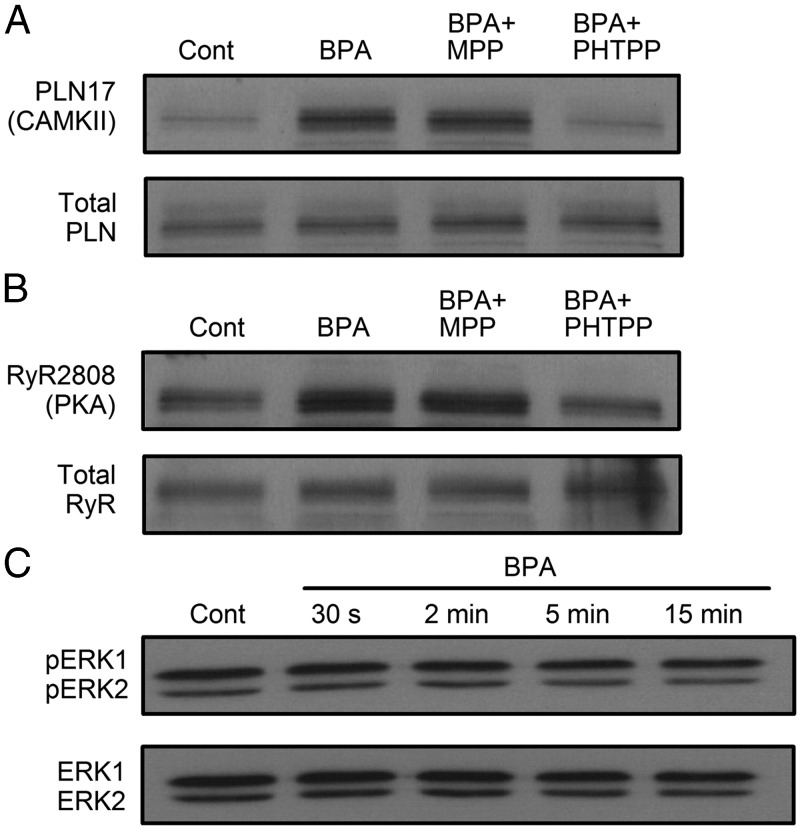
RyR is the SR Ca2+ release channel in cardiac myocytes. The activity of RyR is subjected to regulation by phosphorylation, either by PKA at serine 2808 or by the Ca2+/CaM-dependent protein kinase II (CAMKII) at serine 2814. Phosphorylation of RyR at either site increases the opening probability of RyR by decreasing the threshold of its Ca2+ sensor (19). The effect of BPA on the phosphorylation status of RyR was examined by Western blotting in ventricular myocytes from female rats. Exposure to 1 nM BPA rapidly and transiently increased phosphorylation at the serine 2808 PKA site (Figure 2A1). The effect was readily detectable at 30 seconds of exposure and peaked at 5 minutes; at peak, the phospho/total-RyR level was more than 2-fold of control (2.24- ± 1.07-fold, P < .05; Fig. 2A2). The phosphorylation level returned to baseline at 15 minutes of exposure. By contrast, BPA (1 nM) did not change the phosphorylation status of RyR at the CAMKII site (serine 2814) within 15 minute of exposure (Fig. 2, B1 and B2). The role of PKA in mediating the rapid increase of RyR phosphorylation was further examined using two unrelated PKA inhibitors, PKI and H89. Both PKI and H89 abolished the RyR phosphorylation at PKA site induced by BPA exposure (measured at 5 min; Figure 2C). By comparison, blockade of CAMKII using two unrelated inhibitors, AIP and KN93, did not affect RyR serine 2808 phosphorylation upon BPA exposure (Figure 2D). These results demonstrate that BPA rapidly alters the phosphorylation level of RyR through PKA but not CAMKII.
Figure 2.
Rapid impact of BPA on RyR via the cAMP/PKA pathway in female rat myocytes. A1 and A2, Representative immunoblot and quantification of RyR serine 2808 (PKA site) phosphorylation and total RyR under control and upon exposure to 1 nM BPA for indicated time points (n = 5 hearts). cont, control. B1 and B2, Representative immunoblot and quantification of RyR serine 2814 (CAMKII site) phosphorylation and total RyR under control and upon exposure to 1 nM BPA for indicated time points (n = 4 hearts). C, Immunoblot of RyR serine 2808 (PKA site) phosphorylation and total RyR expression under control, BPA, BPA + 1 μM PKI, and BPA + 1 μM H89 treatment for 5 minutes (n = 3 hearts). D, Immunoblot of RyR serine 2808 (PKA site) phosphorylation and total RyR expression under control, BPA, BPA + 1 μM AIP, and BPA + 1 μM KN93 treatment for 5 minutes. E, ELISA quantification of intracellular cAMP from myocytes under control and treated with BPA, 0.1 μM isoproterenol (Iso), and BPA + 5 μM PHTPP for 5 minutes (n = 3 hearts). All values were normalized to control. *, P < .05 vs control; N.S, not significant (P > .5).
Converted from ATP by adenylyl cyclase (AC), cAMP is the upstream activator of PKA. To further probe the BPA-activated PKA signaling pathway, intracellular cAMP level upon BPA exposure was measured by an ELISA in isolated female ventricular myocytes. Compared with control, BPA (1 nM) exposure for 5 minutes markedly increased the intracellular cAMP level in the myocytes by more than 5-fold (5.42- ± 0.34-fold, P < .05; Figure 2E). Indeed, the effect was comparable with that elicited by full β-adrenergic receptor stimulation with isoproterenol (0.1 μM, 5 min). PHTPP, an ERβ selective blocker, completely abolished BPA's effect on cAMP production, consistent with the previously reported dominating role of ERβ in mediating the rapid effects of BPA in female myocytes (15).
Mechanism of BPA's rapid effect: role of the CAMKII signaling pathway
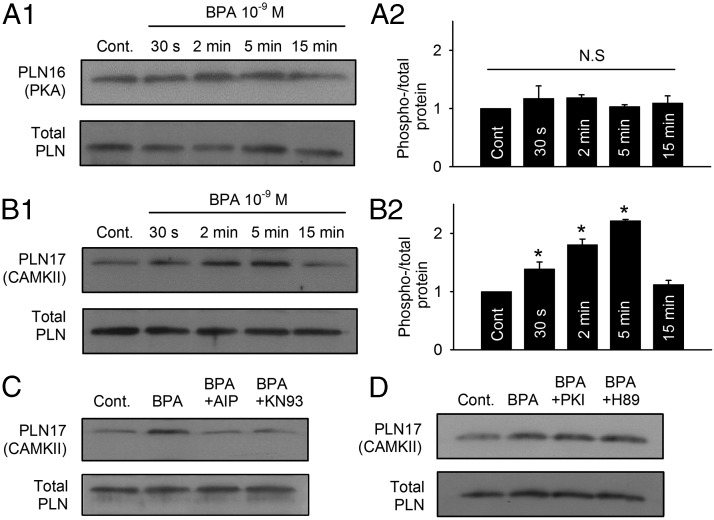
Through its inhibition of SERCA, PLN is the central regulator of SR Ca2+ reuptake. Similar to RyR, PLN can also be phosphorylated by both PKA and CAMKII, at serine 16 and threonine 17, respectively. Phosphorylation of PLN releases its inhibition on SERCA, thereby increasing Ca2+ reuptake into the SR (20). In contrast to the phosphorylation pattern of RyR elicited by BPA, exposure to 1 nM BPA did not change the phosphorylation level of PLN at the PKA site (Figure 3, A1 and A2). Instead, PLN phosphorylation at the CAMKII site (threonine 17) rapidly increased within 30 seconds of BPA exposure and peaked at 5 minutes (Figure 3B1); at peak, the average phospho/total PLN values at the CAMKII site was more than 2-fold of the control level (2.22- ± 1.03-fold, P < .05; Figure 3B2). The increase in PLN phosphorylation level was transient and returned to baseline level after 15 minute of exposure. Two CAMKII inhibitors, AIP and KN93, abolished the BPA-induced PLN phosphorylation at CAMKII site (measured at 5 min; Figure 3C). PKA inhibitors PKI and H89 did not affect PLN phosphorylation by CAMKII upon BPA exposure (Figure 3D). These results demonstrate that BPA rapidly alters the phosphorylation status of PLN through CAMKII but not PKA.
Figure 3.
Rapid impact of BPA on PLN phosphorylation by CAMKII in female rat myocytes. A1 and A2, Representative immunoblot and quantification of PLN serine 16 (PKA site) phosphorylation and total PLN under control and upon exposure to 1 nM BPA for indicated time points (n = 3 hearts). cont, control. B1 and B2, Representative immunoblot and quantification of PLN threonine 17 (CAMKII site) phosphorylation and total PLN under control and upon exposure to 1 nM M BPA for indicated time points (n = 4 hearts). C, Immunoblot of PLN threonine 17 (CAMKII site) phosphorylation and total PLN under control, BPA, BPA + 1 μM AIP, and BPA + 1 μM KN93 treatment for 5 minutes (n = 4 hearts). D, Immunoblot of PLN threonine 17 (CAMKII site) phosphorylation and total PLN under control, BPA, BPA + 1 μM PKI, and BPA + 1 μM H89 treatment for 5 minutes. All values were normalized to control. *, P < .05 vs control; N.S, not significant (P > .5).
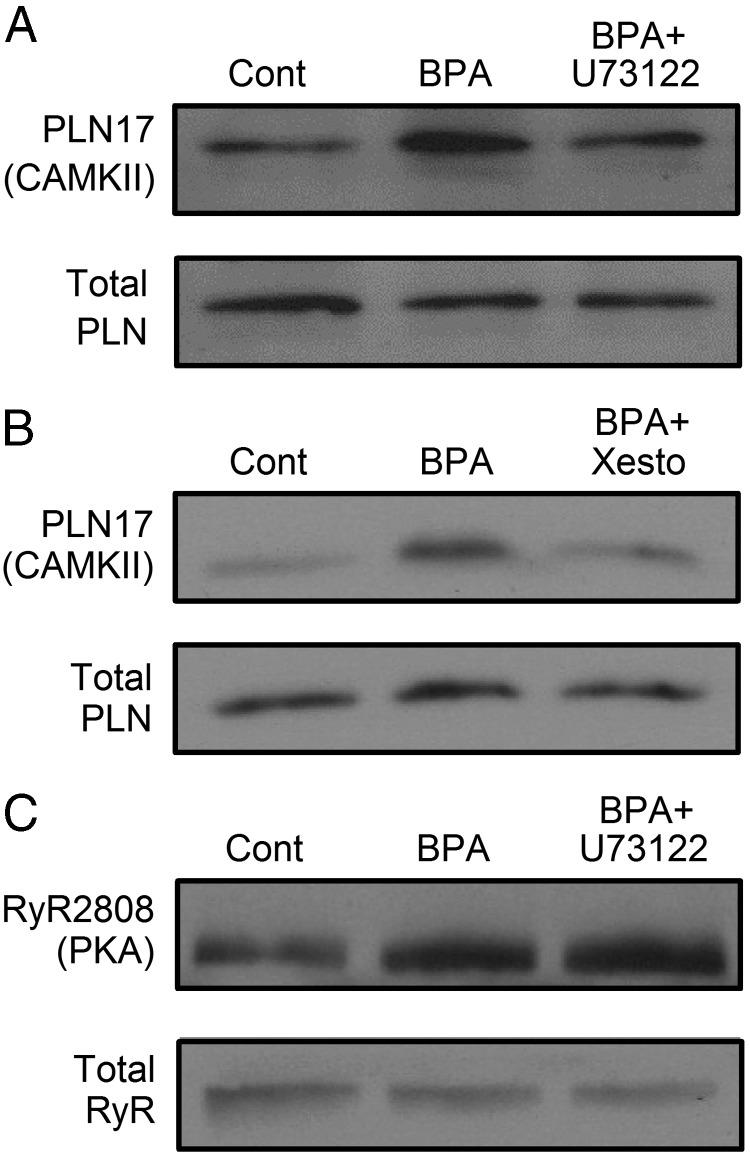
The roles of phospholipase C (PLC) and inositol trisphosphate receptor (IP3R) in mediating PLN phosphorylation were examined. Blockade of either PLC with U73122 (Figure 4A) or IP3R with xestospongin C (Figure 4B) completely abolished the increase in CAMKII phosphorylation of PLN induced by BPA. These results suggest that the upstream elements in the BPA-activated CAMKII signaling pathway involve PLC activation, inositol trisphosphate production, and IP3R-mediated Ca2+ release, likely from the endoplasmic reticulum Ca2+ storage.
Figure 4.
Role of PLC/IP3R in BPA rapid actions in female rat ventricular myocytes. A, Immunoblot of PLN threonine 17 (CAMKII site) phosphorylation and total PLN under control, BPA, and BPA + 1 μM U73122 treatment for 5 minutes. cont, control. B, Immunoblot of PLN threonine 17 phosphorylation and total PLN under control, BPA, and BPA + 5 μM xestospongin C treatment for 5 minutes. C, Immunoblot of RyR serine 2808 (PKA site) phosphorylation and total RyR under control, BPA, and BPA + 1 μM U73122 treatment for 5 minutes. The same results have been repeated three times for all experiments. BPA = 1 nM for all experiments. Xesto, xestospongin C.
Blockade of PLC with U73122 did not affect BPA-induced RyR phosphorylation by PKA (Figure 4C), suggesting that in cardiac myocytes, the PLC signaling pathway does not cross-activate the cAMP/PKA pathway.
Mechanism of BPA's rapid effect: roles of ERs and ERK1/2 activation
Previously we demonstrated that in female rat myocytes, the rapid effects of BPA on arrhythmogenesis and myocyte mechanics were mediated by ERβ signaling (15). The roles of ERα and ERβ in mediating BPA's rapid alteration of RyR and PLN phosphorylation status were examined (Figure 5, A and B). The BPA-induced increases in PLN phosphorylation at the CAMKII site and RyR phosphorylation at the PKA site were both completely blocked by the ERβ blocker PHTPP but not by the ERα blocker MPP.
Figure 5.
Role of ERs and ERK1/2 in BPA rapid actions in female rat ventricular myocytes. A and B, Immunoblot of PLN threonine 17 and RyR serine 2808 phosphorylation, respectively, and total PLN and RyR under control, BPA, BPA + 1 μM MPP, and BPA + 5 μM PHTPP treatment for 5 minutes. cont, control. C, Immunoblot of ERK1/2 (p42/p44) phosphorylation and total ERK1/2 under control and upon acute exposure to 1 nM BPA for indicated time points. The same results have been repeated three times for all experiments.
ERK MAPK signaling is a key step in the rapid signaling of BPA in a number of cells types (6). The rapid effect of BPA on ERK1/2 phosphorylation was examined in female ventricular myocytes. Within 15 minutes of BPA exposure, there was no detectable change in ERK1/2 phosphorylation (Figure 5C), suggesting that the MAPK pathway is not involved in BPA's rapid actions in female rat ventricular myocytes.
Roles of PKA and CAMKII: analysis of functional end points
SR Ca2+ release/leak
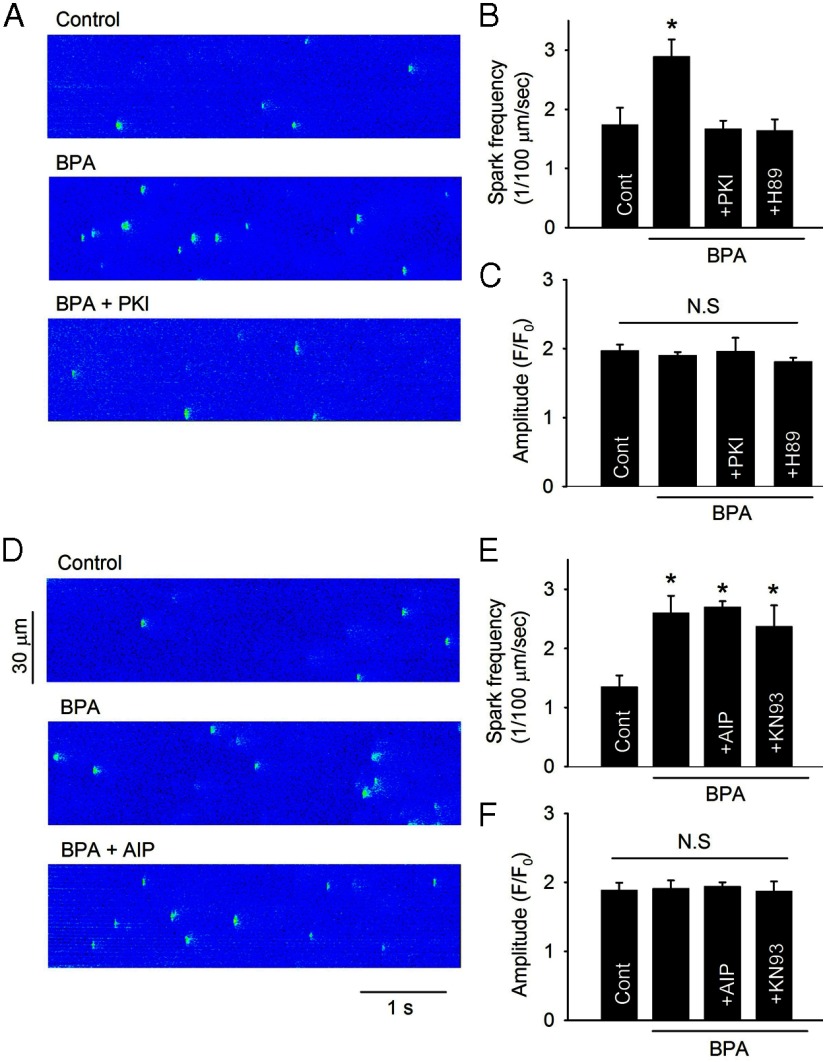
The increase in spontaneous SR Ca2+ release (ie, SR Ca2+ leak) plays a central role in the proarrhythmic effects of BPA (14). Consistent with the RyR immunoblotting results, inhibition of PKA by PKI or H89 completely blocked the increase in spontaneous SR Ca2+ release induced by BPA, measured as Ca2+ spark frequency (Figure 6, A and B). On the other hand, inhibition of CAMKII by either AIP or KN93 did not affect BPA-induced Ca2+ spark increase (Figure 6, D and E). No changes were observed in Ca2+ spark peak amplitude (Figure 6, C and F) or spatial/temporal properties (data not shown) under any of the treatments. These results suggest that PKA phosphorylation of RyR is responsible for the increase in SR Ca2+ release upon BPA exposure.
Figure 6.
PKA but not CAMKII mediates BPA-induced SR Ca2+ leak in female rat ventricular myocytes. A and D, Effect of PKA blockade with PKI and CAMKII blockade with AIP, respectively, on BPA-induced increase in diastolic Ca2+ sparks. B and E, Average Ca2+ spark frequency. cont, control. C and F, Average Ca2+ spark amplitude under indicated treatments. Data are averages of total 128 sparks from 10 myocytes for B and C and 206 sparks from 22 myocytes for E and F (n = 6 hearts). BPA = 1 nM for all experiments; PKI, H89, AIP, and KN93 concentration = 1 μM. *, P < .05 vs control. N.S, not significant (P > .5).
Arrhythmogenic triggered activity
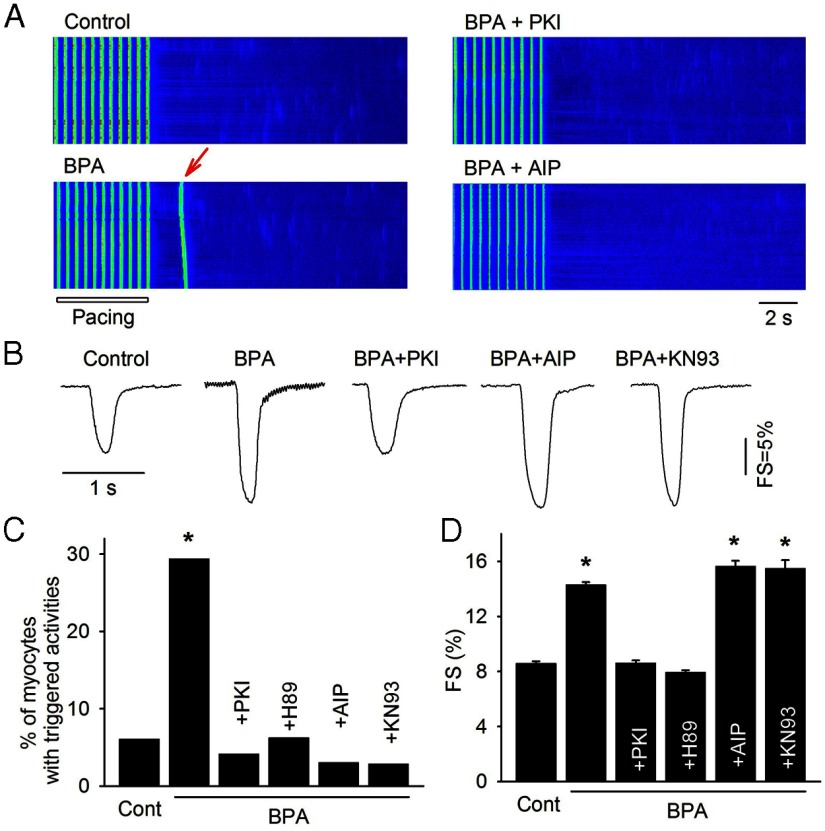
BPA rapidly increased the percentage of myocytes with triggered activities, measured as spontaneous Ca2+ transients after pacing (6% in control vs 29% in BPA, P < .05; Figure 7, A and C). Such proarrhythmic action was abolished by PKA inhibitors PKI or H89 or by CAMKII blockers AIP or KN93 (Figure 7, A and C). These results demonstrate that activations of both PKA and CAMKII are necessary contributors to the proarrhythmic action of BPA in female cardiac myocytes.
Figure 7.
Role of PKA and CAMKII in mediating BPA's effects on triggered activity and myocyte contractility. A, Example confocal images of Ca2+ transients in myocytes elicited by pacing under control, BPA, BPA + PKI, and BPA + AIP. Arrow indicates spontaneous Ca2+ after-transient (ie, triggered activity) after pacing. B, Representative contraction traces in myocytes under control, BPA, and BPA in the presence of PKI, AIP, or KN93. Quantifications for A and B are shown in C and D, respectively. C, Percentages of myocytes with triggered activities under various conditions (n = 33–49 myocytes from 3 hearts). A χ2 test was used to analyze the frequency of triggered activities among treatment groups. D, Average FS of myocytes under various conditions (n = 21–55 myocytes from four hearts). Statistical analysis was performed using one-way ANOVA and a multiple comparison post hoc test. Minimal level of statistical significance for differences in values was considered to be P < .05. *, P < .05 vs control.
Myocyte contractility
In previous studies, we used myocyte contractility as an index to gauge the impact of BPA on myocyte Ca2+ handling. BPA rapidly enhanced contractility of female ventricular myocytes [fractional shortening (FS) = 8.6% in control vs 14.3% in BPA, P < .05; Figure 7, B and D[, and the stimulatory effect was blocked by PKA inhibitors PKI or H89. Interestingly, CAMKII blockers AIP or KN93 did not affect BPA's effect on myocyte contractility (Figure 7, B and D). These results suggest that PKA signaling and the increase in SR Ca2+ release is primarily responsible for the stimulatory effect of BPA on myocyte contraction.
Discussion
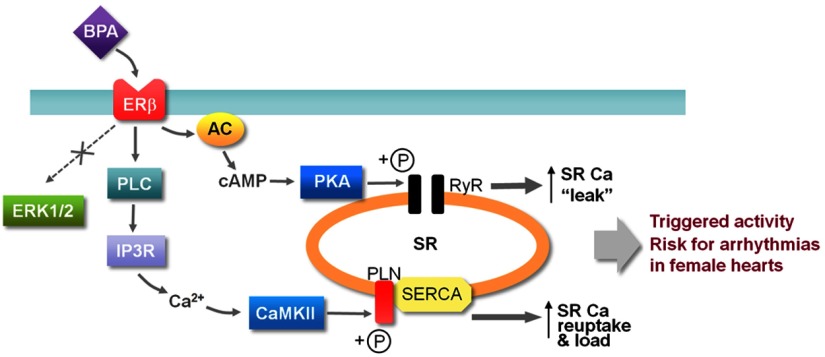
Previously we showed that BPA rapidly promoted cardiac arrhythmias in female adult rat hearts via alteration of Ca2+ handling (14). In the present study, we elucidated the cardiac-specific signaling mechanism underlying BPA's rapid actions. We show that in female rat cardiac myocytes, the PKA and CAMKII signaling pathways are the two major pathways that mediate the rapid impact of BPA on cardiac Ca2+ handling and arrhythmogenesis (Figure 8). Thus, increased production of cAMP, likely a result of activation of AC, leads to PKA activation and phosphorylation of RyR; rapid increase in RyR phosphorylation increases RyR opening probabilities and SR Ca2+ leak. BPA also rapidly leads to the activation of PLC and production of inositol 1,4,5-trisphosphate (IP3). The resulting IP3R-mediated Ca2+ release, likely from the endoplasmic reticulum Ca2+ storage, activates CAMKII and leads to increased PLN phosphorylation, which releases the inhibition of PLN on SERCA and increases SR Ca2+ reuptake. Both pathways contribute to the BPA-induced triggered activities in cardiac myocytes and increase the risk for arrhythmias in the female heart (Figure 8). The activations of both pathways are rapid but transient and are both mediated by ERβ signaling. The prominent role of ERβ in mediating the rapid signaling of BPA is consistent with our previous results using an ERβ knockout model (15). These pathways are likely localized and not global, impacting only their respective protein targets. ERK1/2, which are commonly implicated in the signaling cascade of BPA, are not involved in the rapid actions of BPA in the heart.
Figure 8.
Schematic illustration of the rapid signaling cascade of BPA in female rat ventricular myocytes.
Remarkably, BPA rapidly increased the cAMP level in female myocytes to a level comparable with that induced by full β-adrenergic activation. BPA activation of cAMP/PKA signaling has been described in several types of tissues, including neurons, placenta, and several cancer cell lines (21–23). Downstream of cAMP production, PKA activation rapidly increased the phosphorylation of RyR but not PLN. This is distinct from the downstream effects of β-adrenergic stimulation, which elicits PKA phosphorylation of both RyR and PLN (24). RyR phosphorylation is a key regulatory mechanism of SR Ca2+ release in cardiac myocytes and also plays an important role in arrhythmogenesis. In heart failure, RyR becomes PKA hyperphosphorylated due to remodeling of the RyR macromolecular complex as well as loss of phosphatase activities (25). These hyperphosphorylated RyR channels result in defective RyR function, SR Ca2+ leakage, and the development of life-threatening ventricular arrhythmias (25). These findings in pathological conditions support the notion that BPA-induced PKA phosphorylation of RyR may play a detrimental role in cardiac arrhythmias. Indeed, we show that blockade of either RyR opening (14) or PKA inhibition (Figure 7) abolishes BPA-induced triggered activities.
Modulation of intracellular Ca2+ by BPA has been reported in several types of cells including hippocampal neurons, renal tubular cells, islet of Langerhans, and prostate cancer cells (26–31). Although there seems to be a spontaneous link between intracellular Ca2+ elevation and activation of CAMKII, a Ca2+-responding kinase, our study is the first to directly demonstrate a role of the CAMKII pathway in mediating BPA's rapid actions. In the heart, Ca2+ handling is a highly coordinated process fine-tuned by several Ca2+-dependent mechanisms, one of which is CAMKII. Under hyper-β-adrenergic stimulation, CAMKII is activated by elevated intracellular Ca2+ and phosphorylates several downstream target Ca2+ handling proteins to regulate myocyte functions (17). CAMKII's phosphorylation of PLN increases SERCA Ca2+ reuptake and cytosolic Ca2+ removal, enabling faster relaxation of the myocytes and diastolic filling of the heart under fast heart rate (32). Under pathological conditions, CAMKII activation favors myocardial dysfunction and electrical instability of the heart. Animal experiments linked several forms of arrhythmias to CAMKII activation, including atrial fibrillation, sinus node dysfunction, ventricular tachyarrhythmias, and inherited tachyarrhythmias (33). In disease settings such as heart failure, increased CAMKII expression disturbs normal cytosolic Ca2+ homeostasis by hyperphosphorylating a group of Ca2+ handling proteins, leading to triggered activities and potentially cardiac arrhythmias (34). Consistent with the role of CAMKII in arrhythmogenesis under disease conditions, we show that BPA-induced CAMKII activation is an essential contributor to the development of triggered activities. However, CAMKII-only phosphorylation of PLN has not been previously described in the heart and its exact influence on cardiac function is not known. The consequence of such a unique impact of BPA on Ca2+ handling remains to be elucidated.
It is worth noting that BPA activates CAMKII via a PLC-dependent mechanism, which is distinct from that described under physiological/pathophysiological conditions. Under hyper-β-adrenergic stimulation, CAMKII activation is secondary to PKA activation and is due to elevated intracellular Ca2+ produced by increased SR Ca2+ release and increased Ca2+ influx through the L-type channel (35). By contrast, we found that BPA-induced CAMKII activation is independent of the cAMP/PKA pathway. Blockade of PKA with PKI and H89, although abolishing PKA phosphorylation of RyR, did not affect CAMKII phosphorylation of PLN. Instead, PLC or IP3R blockers completely abolished CAMKII activation as measured by PLN phosphorylation, suggesting that in contrast to adrenergic stimulation, the source of Ca2+ for CAMKII activation under BPA exposure is from the endoplasmic reticulum through IP3R and is induced by PLC activation and IP3 production.
Similarly, the BPA-activated cAMP/PKA pathway in cardiac myocytes is separate from, and not cross-activated by, the PLC pathway. It has been shown that PKA is downstream of the AC/cAMP signaling cascade initiated by Gαs-coupled membrane ER (36), and this appears to be the case in our study. In hypothalamic neurons PKA can also be activated by Gαq-coupled membrane ER through PLC production of diacylglycerol, activation of protein kinase C (PKC), PKC phosphorylation/activation of AC, and the subsequent production of cAMP (37, 38). However, such PLC-PKC-AC signaling cascade does not play a significant role in BPA-induced PKA activation in cardiac myocytes; we show that PLC blocker, although abolishing CAMKII activation, did not affect PKA activation upon BPA exposure (Figure 4).
Interestingly, although both RyR and PLN have PKA and CAMKII phosphorylation sites, upon BPA exposure, PKA and CAMKII preferably phosphorylate RyR and PLN, respectively. Such target protein-specific actions are likely due to localized/compartmentalized signaling. Indeed, both kinases have been reported to have target-specific phosphorylation in the cardiac myocytes (32, 39). Thus, PKA can interact with A-kinase-anchoring proteins, a type of intracellular scaffolding protein, to control the signal transduction of cAMP both temporally and spatially (39). It has been shown that RyR can also bind to the A-kinase-anchoring protein complex and be phosphorylated by PKA on the cardiomyocyte nucleus envelope (40). CAMKII can also colocalize with phosphatases and target proteins to regulate transcription factors and gene expression in myocytes (32). BPA likely activates such localized and/or compartmentalized signaling pathways to elicit its impact on Ca2+ handling in cardiac myocytes. Our data also suggest that BPA rapid signaling in cardiac myocytes is complex and likely involves a number of additional regulators and cofactors, which remain to be elucidated.
We showed that ERK1/2 phosphorylation was not altered within 15 minutes of BPA exposure in female myocytes. BPA rapid signaling network has been shown to involve activation of ERK1/2 in various tissues and cell lines including placenta, cerebellum granule cells, human testicular cancer cells, breast cancer cells, and immune cells (6, 21, 23, 41, 42) but not in pituitary cells or human seminoma cells (6, 22). In cancer cells, activation of ERK1/2 signaling is associated with cancer cell proliferation, migration, and invasion (22, 41). Although ERK1/2 activation upon longer-term BPA exposure has not been tested in our study and remains a possibility, the lack of detectable activation within 15 minutes of exposure indicates that ERK1/2 does not play a role in the rapid impact of BPA on cardiac Ca2+ handling and arrhythmogenesis, events that occur within minutes upon BPA exposure. Our results further support the notion of tissue/cell-specific actions of BPA.
In this study, we used rodent hearts as the experimental model. There is common physiology and pathophysiology in the hearts of large animals (eg, human and dog) and small animals (eg, rat and mouse). However, due to the different demands imposed by body size, there are distinct cardiac physiological properties and disease mechanisms in large animals that are not shared in rodents. The effect of BPA on human hearts and the underlying mechanisms are not currently known and is a topic of significant importance for understanding the potential impact of BPA on human cardiac health.
In summary, we elucidated the cardiac-specific signaling cascade of BPA, and provided mechanistic insight into BPA's rapid impact on myocyte Ca2+ handling and arrhythmogenesis in female rat cardiac myocytes. BPA rapidly activates two parallel signaling pathways, the cAMP/PKA pathway and the PLC/ IP3/Ca2+/CAMKII pathway, which selectively impact two key Ca2+ handling proteins, RyR and PLN. These actions underlie the stimulatory effect of BPA on triggered activities and arrhythmia in the female heart. These results contribute to the assessment of the consequence and potential cardiac toxicity of BPA exposure.
Acknowledgments
This work was supported by National Institute of Health Grant R01-ES017262 (to H.-S.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- adenylyl cyclase
- AIP
- autocamtide-2 related inhibitory peptide, myristoylated
- BPA
- bisphenol A
- CAMKII
- CaM-dependent protein kinase II
- ER
- estrogen receptor
- FS
- fractional shortening
- IP3
- inositol trisphosphate
- IP3R
- inositol trisphosphate receptor
- KN93
- 2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine)
- MPP
- methyl-piperidino-pyrazole,1,3-bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- PHTPP
- 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- PKA
- protein kinase A
- PKC
- protein kinase C
- PKI
- protein kinase inhibitor
- PLC
- phospholipase C
- PLN
- phospholamban
- RyR
- ryanodine receptor
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- SR
- sarcoplasmic reticulum.
References
- 1. Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ye X, Pierik FH, Mackenbach JP, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, The Netherlands: the Generation R study. Environ Res. 2008;108:260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2011;21:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoekstra EJ, Simoneau C. Release of bisphenol A from polycarbonate: a review. Crit Rev Food Sci Nutr. 2013;53:386–402 [DOI] [PubMed] [Google Scholar]
- 6. Wetherill YB, Akingbemi BT, Kanno J, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198 [DOI] [PubMed] [Google Scholar]
- 7. Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69 [DOI] [PubMed] [Google Scholar]
- 8. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Melzer D, Osborne NJ, Henley WE, et al. Urinary bisphenol a concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–1490 [DOI] [PubMed] [Google Scholar]
- 11. Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5(1):e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310 [DOI] [PubMed] [Google Scholar]
- 13. Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120:1297–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang H-S. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. 2011;6:e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Belcher SM, Chen Y, Yan S, Wang H-S. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17β-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153:712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yan S, Song W, Chen Y, Hong K, Rubinstein J, Wang H-S. Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia-reperfusion in female rat hearts. Food Chem Toxicol. 2013;56C:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bers DM. Ca2+ cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49 [DOI] [PubMed] [Google Scholar]
- 18. Betzenhauser MJ, Marks AR. Ryanodine receptor channelopathies. Pflugers Arch. 2010;460:467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Petegem F. Ryanodine receptors: structure and function. J Biol Chem. 2012;287:31624–31632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouskine A, Nebout M, Mograbi B, Brucker-Davis F, Roger C, Fenichel P. Estrogens promote human testicular germ cell cancer through a membrane-mediated activation of extracellular regulated kinase and protein kinase A. Endocrinology. 2008;149:565–573 [DOI] [PubMed] [Google Scholar]
- 22. Bouskine A, Nebout M, Brucker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect. 2009;117:1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang H, Tan WJ, Wang CC, Leung LK. Bisphenol A induces corticotropin-releasing hormone expression in the placental cells JEG-3. Reprod Toxicol. 2012;34:317–322 [DOI] [PubMed] [Google Scholar]
- 24. Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205 [DOI] [PubMed] [Google Scholar]
- 25. Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the Ca2+ release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376 [DOI] [PubMed] [Google Scholar]
- 26. Alonso-Magdalena P, Laribi O, Ropero AB, et al. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic α-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113:969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanabe N, Kimoto T, Kawato S. Rapid Ca2+ signaling induced by bisphenol A in cultured rat hippocampal neurons. Neuro Endocrinol Lett. 2006;27:97–104 [PubMed] [Google Scholar]
- 28. Lee S, Suk K, Kim IK, et al. Signaling pathways of bisphenol A-induced apoptosis in hippocampal neuronal cells: role of calcium-induced reactive oxygen species, mitogen-activated protein kinases, and nuclear factor-κB. J Neurosci Res. 2008;86:2932–2942 [DOI] [PubMed] [Google Scholar]
- 29. Kuo CC, Huang JK, Chou CT, et al. Effect of bisphenol A on Ca2+ fluxes and viability in Madin-Darby canine renal tubular cells. Drug Chem Toxicol. 2011;34:454–461 [DOI] [PubMed] [Google Scholar]
- 30. Soriano S, Alonso-Magdalena P, Garcia-Arevalo M, et al. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One. 2012;7:e31109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Derouiche S, Warnier M, Mariot P, et al. Bisphenol A stimulates human prostate cancer cell migration remodelling of Ca2+ signalling. Springerplus. 2013;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640 [DOI] [PubMed] [Google Scholar]
- 33. Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rokita AG, Anderson ME. New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII). Circulation. 2012;126:2125–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grimm M, Brown JH. β-Adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2010;48:322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levin ER. Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1425–R1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelly MJ, Ronnekleiv OK. Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature. Front Neuroendocrinol. 2012;33:376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qiu J, Bosch MA, Tobias SC, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McConnachie G, Langeberg LK, Scott JD. AKAP signaling complexes: getting to the heart of the matter. Trends Mol Med. 2006;12:317–323 [DOI] [PubMed] [Google Scholar]
- 40. Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci. 2001;114:3167–3176 [DOI] [PubMed] [Google Scholar]
- 41. Ptak A, Gregoraszczuk EL. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol Lett. 2012;210:332–337 [DOI] [PubMed] [Google Scholar]
- 42. Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal-regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406 [DOI] [PubMed] [Google Scholar]