Abstract
It is now firmly established that TSH may influence the physiology and patho-physiology of bone by activating osteoblasts and inhibiting osteoclast activity resulting in relative osteoprotection. Whether this influence is directly exerted by pituitary-derived TSH in vivo is less certain, because we have previously reported that the suppression of pituitary TSH does not remove such protection. Here, we have characterized the functional relevance of a novel form of the TSH-β subunit, designated TSH-βv, known to be produced by murine bone marrow cells. We found that fresh bone marrow-derived macrophages (MØs) preferentially produced TSH-βv and, when cocultured with CHO cells engineered to overexpress the full-length TSH receptor, were able to generate the production of intracellular cAMP; a phenomenon not seen in control CHO cells, such results confirmed the bioactivity of the TSH variant. Furthermore, cocultures of MØs and osteoblasts were shown to enhance osteoblastogenesis, and this phenomenon was markedly reduced by antibody to TSH-β, suggesting direct interaction between MØs and osteoblasts as observed under the electron microscope. These data suggest a new paradigm of local modulation of bone biology by a MØ-derived TSH-like molecule and raise the question of the relative contribution of local vs pituitary-derived TSH in osteoprotection.
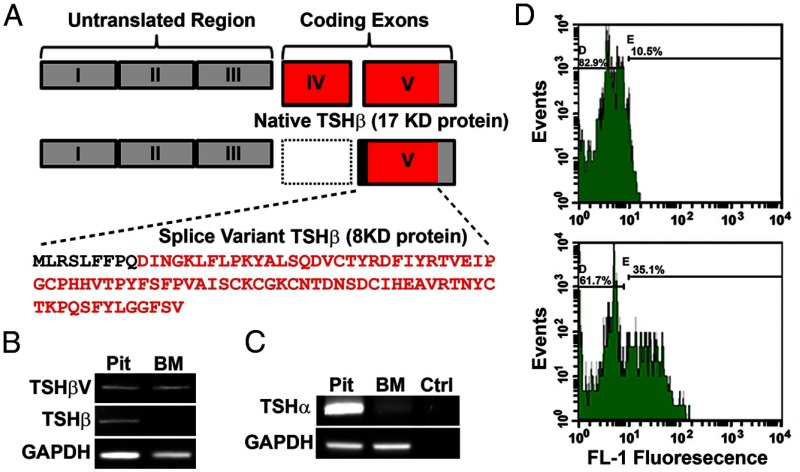
We have shown that pituitary TSH is osteoprotective in vitro and in vivo by activating osteoblasts and inhibiting osteoclasts (1). Pituitary TSH is a 28- to 30-kDa glycoprotein hormone consisting of a common α-subunit and a unique β-subunit, the latter being responsible for hormone specificity. The mouse TSH-β gene consists of 5 exons, with the coding region located in portions of exons 4 and 5. Alternative exon splicing of mouse TSH-β has been described in exons 1, 2, and 3; all of which are outside the TSH-β coding region. However, murine studies have subsequently shown that osteoprotective activity associated with the TSH receptor (TSHR) is retained in vivo even when pituitary TSH is suppressed by excessive thyroid hormone (2). Such data indicated that either the TSHR itself is able to provide the protection in the absence of TSH ligand or a local TSHR stimulator must be available to maintain TSHR signaling. This means that an extrapituitary source of TSH may be responsible for osteoprotection. In fact, possible extrapituitary sources of TSH have been known for over 20 years (3–5). Hence, parallel to the pituitary-thyroid circuit, there may be additional TSH-related circuits that may be functioning in extrathyoidal sites, including within the immune system as evidenced by the ability of immune cells to produce TSH (5, 6). Of direct relevance here is that it has been shown that bone marrow (BM) cells produce a novel TSH-β splice variant (TSH-βv), which is missing exon 4, 1 of the 2 coding exons (Figure 1A) (6).
Figure 1.
BM cells produce TSH-βv. (A) A schematic comparison of native TSH-β and novel TSH-βv. Of note is a missing exon IV in the splice variant resulting in a smaller peptide of 8 vs 17 kDa for the full-length peptide. The sequence of TSH-βv is also shown with the intronic region in bold. (B) The result of native TSH-β and novel TSH-βv PCR amplimer generation in C57/BL murine pituitary and BM samples. The PCR product size of 470 bp was exclusively found in the pituitary sample and corresponds to that of native TSH-β and contrasts with the PCR product size for the TSH-βv of 300 bp, which was found in both the pituitary and BM. (C) PCR amplification of the TSH-α gene was only positive in the pituitary sample. (D) BM cells were flushed from the femur and the red blood cells lysed and stained for TSH followed by FACS analysis. These figures depict TSH protein expression in fixed BM cells detected with anti-TSH-β (bottom) or control IgG (top) with a green fluorescent protein-labeled second antibody. GAPDH, glyceraldehydes-3-phosphate dehydrogenase. Ctrl, control; FL-1, fluorescence intensity; Pit, pituitary.
Within the BM, macrophages (MØs), in particular, secrete a variety of cytokines and growth factors (7) that may contribute to osteoblast proliferation and survival (8). More recently, MØs were shown to be intercalated within bone (called “osteomacs”), and several lines of evidence support a relationship between such cells and bone cell function (9).
Previous studies from this, and other, laboratories have established a role for TSH in bone cell regulation, but such studies have not delineated whether centrally or locally derived TSH is responsible in vivo for this influence on bone physiology. Here, we sought to determine whether the novel TSH-βv, produced by BM cells (6), has the potential to enhance osteoblastogenesis and act in an osteoprotective manner as demonstrated for pituitary TSH.
Materials and Methods
Mice and MØs
All mice used in this study were from a C57/BL6 mixed background and a mixture of males and females. These mice were born, bred, and maintained in our colony room, at the Mount Sinai School of Medicine. All procedures were in accordance with the Internal Animal Care and Use Committee at Mount Sinai School of Medicine. Fresh BM was flushed and plated in 10-cm dishes for 9 consecutive days for the derivation of BM-derived MØs (BMDMØs). BM cells were treated from the first day of culture with macrophage colony stimulating factor (MCSF)-1 (10 ng/mL) and renewed every 2 days when the media were refreshed.
Standard RT-PCRs
Total RNA was isolated from cells using TRIzol reagent (Invitrogen Corp), and chromosomal DNA was removed in accordance with manufacturer's instructions. The RNA concentration was determined on the basis of absorbance at 260 nm, and its purity was evaluated by the ratio of absorbance at 260:280 nm (>1.9). RNAs were kept frozen at −70°C until analyzed. After digestion of genomic DNA by treatment with Ambion's TURBO DNA-free deoxyribonuclease I (Ambion, Inc), total RNA (1 μg) was reverse transcribed into cDNA with random hexamers using Advantage RT for PCR kit (CLONTECH Laboratories, Inc). RT-PCRs were performed with TITANIUM Taq polymerase (CLONTECH Laboratories, Inc). Cycling conditions were as follows: 94°C for 1 minute, followed by 30 cycles of amplification (94°C denaturation for 0.5 minutes; annealing for 1 minute, annealing temperature dependent on primers; 72°C elongation for 2 minutes), with a final incubation at 72°C for 7 minutes. The amplified PCR products were separated on a 2% agarose gels. Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org, details the amplimers used.
Quantitative real-time PCR (qRT-PCR)
The qRT-PCRs were performed using an Applied Biosystems StepOne Plus Real-Time PCR system (Applied Biosystems) and a series of well-characterized primers (Supplemental Table 1). The reactions were established with Power SYBR Green master mix (Applied Biosystems), 0.4-μL (2μM) sense/antisense gene-specific primers, 2-μL cDNA, and diethylpyrocarbonate-treated water to a final volume of 20 μL. The PCR mix was denatured at 95°C for 60 seconds before the first PCR cycle. The thermal cycle profile was: denaturizing for 30 seconds at 95°C, annealing for 30 seconds at 57°–60°C (dependent on primers), and extension for 60 seconds at 72°C. A total of 40 PCR cycles was used. PCR efficiency, uniformity, and linear dynamic range of each qRT-PCR assay was assessed by the construction of standard curves using DNA standards. An average threshold cycle from triple assays was used for further calculation. For each target gene, the relative gene expression was normalized to that of the glyceraldehyde-3-phosphate dehydrogenase housekeeping gene using Applied Biosystems Step One Plus Real-Time PCR systems software. Data presented (mean) are from 3 independent experiments, in which all sample sets were analyzed in triplicate.
Flow cytometry and cell sorting
Fresh BM cells were fractionated into CD-11b+ and CD-11b− cells and then further subfractionated into 4 populations of monocytes (MOs), MØ, neutrophils, and lymphocytes as described in Ref. 10. For cell staining, BM cells were flushed and stained for F480+, CD-11c and CD14 surface antigens (E-Biosciences), and anti-TSH-β (Santa Cruz Biotechnology, Inc). Also, BMDMØ and control Raw cells were cultured as described above and stained for fluorescence activated cell sorting (FACS) analysis.
Generation of cAMP
BM was extracted from C57/129 mixed background mice and then plated and cultured at a density of 2 × 105 cell/96-well plate with 10-ng/mL MCSF for 6 consecutive days. On day 6, CHO-TSHR cells and nontransfected CHO cells (11) were plated on top of the differentiated MØ cells at a density of 3 × 104 cells/96-well pates. Cells were allowed to make contact for 48 hours, then lysed, and intracellular cAMP levels measured by EIA (Amersham cAMP Biotrak EIA System, GE Healthcare Bio-Sciences Corp).
Computer modeling
Homology modeling of TSH-β and its splice variant were carried out using Modeler version 9v7 (12), taking the FSH crystal structure (PDB ID, 1FL7) as the template (13). A fast molecular dynamics minimization of both structures was performed as part of the Modeler routine. The chain alignment of the TSH-β conserved region with FSH was taken as discussed in the reported work (14), and the structures were further validated in the VERIFY3D program (15). Docking of TSH-β and its splice variant was then carried out using the HEX server (http://hexserver.loria.fr/) with the crystal structure of the TSHR ectodomain (ECD) (16). We compared and validated the HEX server residue contact results for full-length TSH docking with the TSHR ECD with those of previously published computational results (17, 18).
Mass spectrometry for identification of TSH-βv
To identify TSH-βv secretion from BMDMØ, we used matrix-assisted laser desorption/ionization-mass spectrometry analyses. BMDMØs were cultured with 10-ng/mL MCSF for a total of 9 consecutive days with media refreshed every 2–3 days. During the first 7 days, cultures were treated with media containing 10% fetal bovine serum, then for the remaining 2 days, the cultures were treated with media containing no serum. Supernatant was then collected on day 9 and analyzed after precipitation with ammonium sulfate and dissolved in 50% acetonitrile. Plain DMEM was subjected to the same conditions, and the precipitation was used as the control.
Immunoprecipitation of TSH-βv
To extract total cellular protein, lysis buffer containing 50mM Tris-HCl, 0.5% Triton X-100, 2mM EDTA, 150mM NaCl (pH 7.5), and 1mM phenylmethylsulfonyl fluoride was added to cells and gently scrapped, then centrifuged. Protein concentration in the extracts was estimated using the Lowry technique; 400 μg of protein were then precipitated with 2.5 μg/mL of anti-TSH antibody (Sc7831) by incubating at 4°C overnight in radioimmunoprecipitation assay buffer containing protease inhibitors. The complex was then captured with protein G agarose beads and reduced with 5× sample buffer containing 100mM dithiothreitol at 100°C for 5 minutes. The samples were then electrophoresed on a 4–18% gradient sodium dodecyl sulfate-polyacrylamide gel and then electrotransfered to polyvinylidene fluoride. After blocking the polyvinylidene fluoride membrane with 5% BSA, the membrane was further incubated with anti-TSH-β (no. 1) (1:10 000) and probed using antimouse IgG conjugated with peroxidase (Santa Cruz Biotechnology, Inc). The signals were detected using enhanced chemiluminescent.
Calvaria-derived and femur-derived osteoblast differentiation and enrichment
Mouse pups less than 5 days old were euthanized and their calvaria collagenase digested through a series of sequential digestions. The first 2 digests were discarded, and the subsequent digests were pooled and plated. Femurs from adult mice were similarly collagenase digested as described (19). These cells were then treated with osteoblast differentiation medium containing 10mM/mL β-glycerophosphate,1mM/mL dexamethasone, and 50-μg/mL ascorbic acid, with and without anti-TSH-β for an additional 10 consecutive days. For these experiments, we used a high potency rabbit antibody (no. 1) to a specific TSH-β sequence (CKLFLPKYALSQDVCTYRDF) prepared for us by Hong Kong GenicBio Co, which inhibits TSH-induced cAMP generation in CHO-TSHR cells and immunoprecipitates bovine TSH.
Immunostaining and confocal imaging
BMDMØ and Raw cells were plated in glass dishes at 5 × 105, and upon 70%–80% confluency, the cells were washed with 1× PBS, then fixed in 4% paraformaldehyde for 10 minutes and blocked with 5% BSA in PBS for 30 minutes followed by F480+ staining. For intracellular TSH staining, the fixed cells were permeabilized with 90% ice-cold methanol for 30 minutes followed by blocking for 30 minutes and stained with anti-TSH-β (no. Sc-7813; Santa Cruz Biotechnology, Inc) along with F480+ surface antigen for an additional hour. Stained cells were visualized under a Zeiss LSM-700 confocal microscope.
Statistical analyses
Differences resulting from treatments were assessed by Student's t test. All differences were considered statistically significant with P < .05. All data are expressed as mean ± SEM.
Results
Identification of a TSH-βv in MØs
RT-PCR analysis of full-length TSH-β was positive with mouse pituitary cDNA but negative with murine BM cells (Figure 1B). In contrast, RT-PCR for the TSH-βv was positive with both pituitary cDNA and cDNA prepared from mouse BM cells as evidenced by the expected 300-bp amplified fragment (Figure 1B). The identity of this fragment was confirmed by sequence analysis. BM cultures were negative for TSHRα gene transcription (Figure 1C). FACS analysis of fixed BM cells using a specific polyclonal antibody to full-length TSH indicated the presence of TSH protein in more than 25% of cells (Figure 1D). BM cells were then fractionated into CD-11b+ and CD-11b− and the TSH-βv appeared to be mostly produced by the CD-11b+ cells as assessed by PCR (Figure 2A). The CD-11b+ BM cell population was then sorted by FACS into neutrophils (Gr-1+ CD-115−), MOs (Gr-1+ CD-115+), lymphocytes (CD-115+), and MØs (Gr-1+ F4/80+ CD-115−) (10). These 4 cell populations were then subjected to RT-PCR, and we found that the TSH-βv was primarily transcribed in the MØ myeloid lineage (Figure 2B). When fixed and stained with anti-TSH and subjected to FACS analysis, approximately 11% of the MØ cells were TSH positive (Figure 2C). Direct histomorphometric analysis of anti-TSH binding to the cultured MØ is illustrated in Figure 3.
Figure 2.
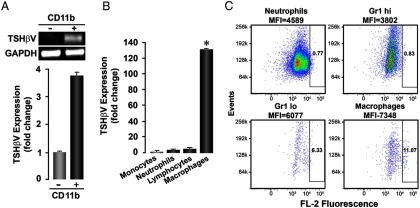
TSH-βv is produced by MØs. (A) Upper panel, fresh BM cells from C57/BL6 mice were subfractioned into the myeloid and the lymphoid lineage by FACS sorting and then PCR amplified for the novel TSH-βv. As depicted, the CD-11b+ cells of the myeloid origin predominantly transcribed the novel TSH-βv by conventional PCR analysis of cDNA. (A) Lower panel, real-time PCR analysis confirmed that the CD-11b+ cells were the primary source of the variant. (B) Further subfractionation of CD-11b+ cells into MØs, lymphocytes, neutrophils, and MØs for the purpose of identifying the cell responsible for producing the novel TSH-βv revealed that, by real-time PCR, the MØ population was the primary source. (C) Fresh BM cells were isolated, FACS sorted into the same 4 fractions, and then immunostained for TSH-β with anti-TSH (Santa Cruz Biotechnology, Inc), showing that 11% of the MØs expressed the variant protein.
Figure 3.
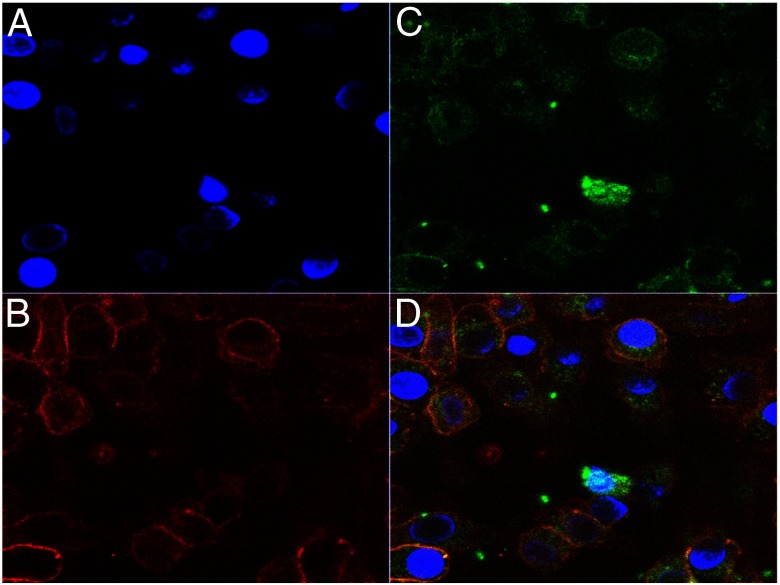
Intracellular staining for TSH-βv. BMDMØs were cultured from fresh BM for 7 days with MCSF (10 ng/mL) and their nuclei labeled with 4′,6′-diamidino-2-phenylindole (DAPI) (A) and stained for F480+ surface antigen (B) and intracellular TSH-βv with anti-TSH (C). The overlay (D) illustrates that MØs contain intracellular TSH-βv (×630).
Potential interaction of TSH-βv and TSHR
We have shown previously that calvaria-derived osteoblasts and ES cell-derived osteoblasts and osteoclasts express the TSHR, so the potential secretion of a local TSH-like splice variant may have an important influence on bone physiology. To examine whether the TSH-βv was capable of activating the TSHR, we first modeled its interaction with the known TSHR ECD structure (Figure 4, A and B). The results showed that both TSH-β and its splice variant had favorable binding conformations with the leucine-rich concave region of the TSHR ECD. However, although modeling the native TSH-β subunit produced the expected conformation (data not shown), modeling the variant produced a distinctly different structure, but it retained its interaction with the concave surface of the leucine-rich repeat region of the TSHR ECD, and its binding to residues mirrored 4 of the residues binding to the native subunit: ARG(38-TSHR)-PHE(86-splice), ARG(80-TSHR)-TYR(87-splice), ARG(112-TSHR)-ASN(12-splice), and LYS(209-TSHR)-PHE(7-splice). These data suggested that the variant subunit exhibited the potential for TSHR signaling.
Figure 4.
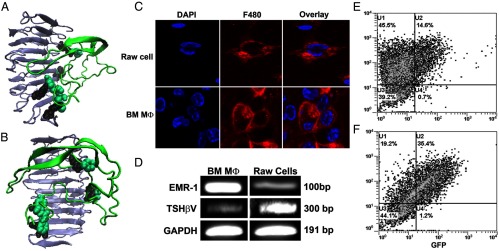
(A and B) TSH-βv binding to the TSHR ECD. Computer modeling of the TSH-βv binding with the TSHR ECD showed docking of the peptide (green) at the concave surface of the leucine-rich repeat region (gray/black). Two rotated views are illustrated. The binding affinity of the variant was comparable with that of the native TSH-β subunit (638 vs 649 μm, respectively). (C–F) MØ phenotyping. (C) Validation of MØ content in BMDMØ cultures was obtained by staining for F480+ surface antigen (red) in a similar way to RAW cells, a MØ cell line, as a positive control. (D) By conventional PCR, the EMR1 gene, a specific MØ marker, was expressed in both BMDMØ and RAW cell cultures, and both were transcribed TSH-βv. (E and F) The BMDMØs were then subjected to FACS analysis to determine their M1 (C) vs M2 (D) phenotype. Analyses showed that 14.6% of the cells were of the M1 phenotype (CD-11c+), and 35.6% of the cells were of the M2 phenotype (CD14+). GAPDH, glyceraldehydes-3-phosphate dehydrogenase; DAPI, 4′,6′-diamidino-2-phenylindole. GFP, green fluorescence protein.
Characterization of MØ cultures
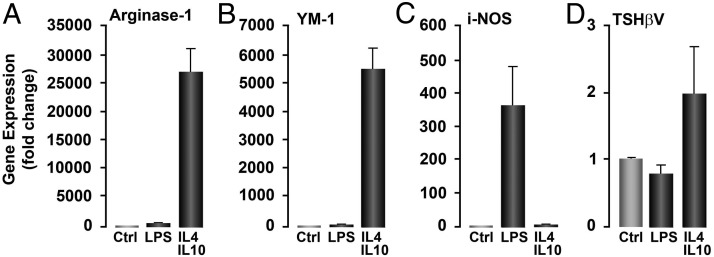
BM cells were cultured for 8–9 days in the presence of MØ colony-stimulating factor colony stimulating factor-1, leading to more than 95% BM-derived F4/80+ MΦ (Figure 4C) as shown by FACS analysis (data not shown). MØ-specific EMR-1 gene expression further confirmed their identity (Figure 4D). FACS analysis of the cultured cells stained for CD-11c (classically activated M1) and CD14 (alternatively activated M2) showed that 14% of the MØs were M1 phenotype (Figure 4E) and 35% were M2 phenotype (Figure 4F). Hence, there were 3 times as many M2 cells as M1 cells in these cultures, and this was further reflected in their directed responses to lipopolysaccharide for M1 (using inducible nitric oxide synthase expression as the readout) (Figure 5C) and IL-4 and IL-10 for M2 (using arginase-1 and Mus musculus secretory protein precursor (YM1) gene expression as the readouts) (Figure 5, A and B), where more TSH-βv gene transcription was detected in the M2-directed responses.
Figure 5.
M2 phenotype is the major source of TSH-βv transcription. BMDMØs were cultured from fresh BM for 7 days under the influence of MCSF 10 ng/mL and further inducted into M1- and M2-like cells by 24 hours of treatment with an IL-4/IL-10 (20 ng/mL each) combination and lipopolysaccharide (100 ng/mL), respectively. Cells were then extracted with TRIzol and real-time PCR amplified for M2 markers (arginase 1 and Mus musculus secretory protein precursor, YM-1) (A and B) and for M1 (i-NOS) (C). Testing for TSH-βv itself (D) revealed transcription of both phenotypes. LPS, lipopolysaccharide; Ctrl, control.
MØs produce bioactive TSH-βv
In order to evaluate the TSHR signaling potential of the TSH-βv, the serum-free culture supernatant from BMDMØs was first subjected to analysis by mass spectrometry, and fragments (shown in bold below) were identified as sequences from TSH-βv confirming its release into the medium: VCTYRDFIYRTVEIPGCPHHVTPYFSFPVAVSCKCGKCNTDNSDCIHEAVRTNYCTKPQSFYLGGFSV. Similarly, we were able to precipitate an 8-kDa fragment from BMDMØ cell lysates using rabbit ant-TSH (no. 1) (Supplemental Figure 1). The biological action of the variant was then demonstrated by cocultures of BMDMØ with CHO cells expressing the TSHR as illustrated in Figure 6A. We showed that BMDMØ could induce cAMP generation in CHO-TSHR cells but not in native CHO cells without the receptor (Figure 6B).
Figure 6.
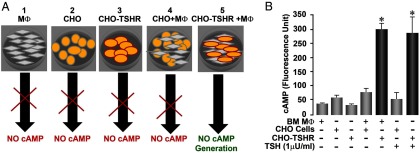
Bioactivity of TSH-βv. (A) A schematic representation of 5 experimental conditions used in studying the ability of BMDMØs to release TSH-βv and signal at the TSHR as evidenced by cAMP generation. The following controls were used: 1) MØs alone, 2) CHO cells alone, 3) CHO-TSHR cells alone, 4) CHO cells cocultured with MØs, and 5) the experiment itself consisted of CHO-TSHR cells with MØs. (B) When BMDMØs were cocultured with CHO cells transfected with the TSHR (CHO+TSHR), a cAMP response was elicited that was 5-fold greater than when MØs were cocultured with CHO cells without the TSHR. The cAMP generation in response to MØs in coculture was comparable with that of 1 mU/mL of TSH. Data are shown ±SEM of 2 replicate experiments. *P < .01.
MØs colocalize with osteoblasts
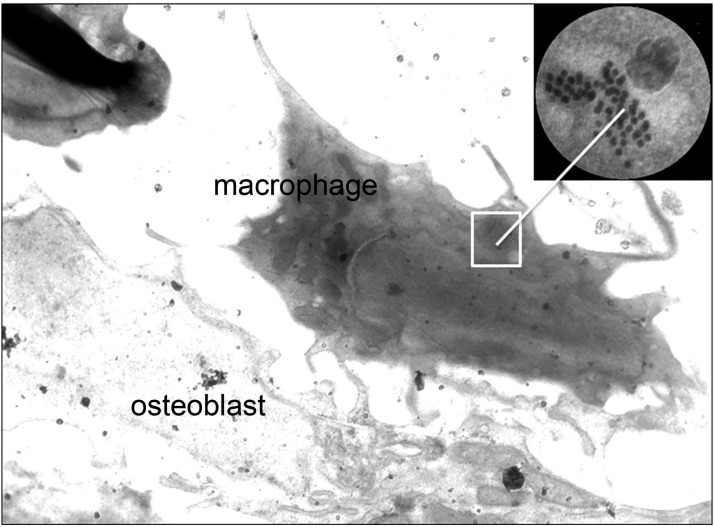
Morphometry by electron microscopy (EM) studies of mouse vertebral bone identified MΦ (as osteomacs) in close association with osteoblasts (Figure 7). Strikingly, some of these MØ had TSH-containing granules identified by anti-TSH and colloidal gold staining (Figure 7, inset), further confirming the potential for a local TSH modulatory circuit.
Figure 7.
Bone EM shows intracellular TSH-containing vesicles. EM studies revealed the interaction of MØs, containing TSH secretary vesicles, localized adjacent to osteoblasts in bone slices, suggesting paracrine regulation of bone cells by MØ-derived TSH-βv. The MØ and osteoblast in the figure are as marked, and the inset shows a magnified view of the now gold-labeled intracellular TSH-containing vesicles detected using our specific TSH-β peptide antibody (no. 1).
MØs enhance osteoblastogenesis via the production of TSH-βv
Primary mouse neonatal calvaria-derived preosteoblast cells were cultured with osteogenic factors and found to contain mostly cells that stained positive for alkaline phosphatase (ALP), demonstrating their osteoblast lineage (data not shown), but also made up of approximately 15% MØs by FACS analysis with anti-F480. These mixed osteoblast cultures also expressed the EMR1 gene by RT-PCR (as in Figure 4), again confirming the presence of MØ in the culture. When these osteoblast cultures were treated with TSH-β antibody, there was a significant down-regulation of collagen gene expression (Figure 8A).
Figure 8.
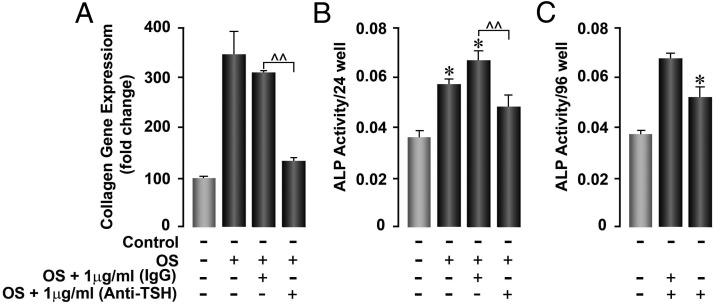
(A) To determine the influence of TSH-βv on osteoblast formation, calvarial cells were stimulated for 22 days under 4 experimental conditions as indicated. The inhibitory effect of anti-TSH on osteoblast formation was shown in comparison with control IgG by measuring collagen gene expression. Osteoblast gene expression of collagen was enhanced by osteogenic stimulation but down-regulated by anti-TSH-β antibody. At 22 days, the cultures contained 16.6% of MØs by FACS analysis with anti-F480. *P < .02; **P < .001. (B) Femoral preosteoblast-like cells were sequentially digested from bone, then cultured with osteogenic stimulation for 22 days, by which time they contained approximately 25% MØs by FACS analysis with anti-F480. Cultured cells were then treated with anti-TSH to illustrate the effect of blocking TSH-βv secreted by MØ. Significant inhibition of osteoblast formation was again observed. *P < .02; **P < .001. (C) Calvarial preosteoblast-like cells were sequentially digested from bone then cultured with osteogenic stimulation for 11 days. Cultured cells were then treated with conditioned medium from BMDMØ with osteogenic stimulatory factors and ±anti-TSH (no. 1) to illustrate the effect of blocking TSH-βv secreted by MØ. Significant stimulation of ALP activity occurred with conditioned medium and control IgG, and inhibition of this effect was seen with rabbit anti-TSH indicative of inhibition of TSH-induced osteoblastogenesis. *P < .02; **P < .04. OS, Osteogenic stimulation.
Using a similar approach, primary cells from digests of adult femurs were plated and treated with osteogenic stimulation for 7 days and were found to constitute 25% MØs by FACS, and these cells also expressed the EMR1 and TSH-βv genes (data not shown). When tested for osteoblast formation using ALP as the readout, the addition of anti-TSH-β significantly reduced ALP activity (Figure 8B).
Together, these data demonstrated that MØs enhanced osteoblast cultures and antibody to TSH reduced this effect, thus indicating that MØ may enhance osteoblast formation via their secretion of TSH-βv.
Discussion
Native pituitary TSH has physiological relevance in bone biology and pathophysiology (20–25). Studies have shown that TSH inhibits osteoclast formation (21, 25) and enhances osteoblast formation (21, 26), thus providing potential osteoprotection. However, the role of an extrapituitary, local, thyroid stimulator in bone cell modulation has not been studied. Here, we first sought to determine whether a TSH-βv was locally produced within the BM microenvironment and bone matrix and then to identify the immune cells responsible for its production. We subsequently examined the physiological significance of this TSH-βv on osteoblast differentiation.
These data show that the novel TSH-βv is indeed expressed by BM cells, consistent with previous findings (6), and that the variant is primarily produced by CD-11b+ myeloid cells. Moreover, in an attempt to identify the cells responsible for producing the TSH-βv, the CD-11b+ cells were further subfractionated into lymphocytes, MO, neutrophils, and MØ, and we showed that MØs were the predominant producer of this TSH-βv. Peptide analysis of culture supernatant, as well as intracellular staining for TSH-βv, further confirmed the production of TSH-βv by the MØ population. Here, we showed clearly that MØs enhance osteoblast formation, and these results confirm previous findings (27). MØs are known to produce an abundance of cytokines (28, 29), which can convey both destructive and reparative signals (30, 31), and TSH-βv appears to be an additional modulatory factor.
One of the paradoxes in studying MO/MØ function is that MØs can exhibit both inflammatory and reparative functions (32). Studies in mice indicate that one reason for this paradox is that MØ respond to peripheral cues and those MØ residing in regenerating tissues will provide mostly reparative functions (33). Reparative MØs have been shown to mediate tissue remodeling by digesting and ingesting fibrotic matrix, phagocytosing necrotic, and apoptotic cellular debris and generating cytokines and factors that promote successful regeneration of parenchymal cells. All these actions would enhance bone remodeling and osteoprotection.
As predicted by our modeling scenario, the TSH-βv was biologically active as evident from its ability to elicit a cAMP response only in the presence of the TSHR and by its ability to enhance osteoblastogenesis. Furthermore, we showed that MØs containing TSH-secretary granules were colocalized with osteoblasts within bone tissues, thus indicating that so called osteomacs may be the principal source of this modulatory protein. Because previous studies have shown that TSH is important in suppressing osteoclasts (20, 23) while enhancing osteoblast activity (21, 26), the presence of MØs near the osteoblast suggests that there is a paracrine loop of TSH-βv regulating their activity via their TSHR receptors (34).
In conclusion, we have shown that TSH-βv is produced by MØs within the bone and BM microenvironment and is capable of signaling to, and enhancing the formation of, local osteoblasts through paracrine communication. This local regulatory circuit is likely to be of adaptive value as a physiologically efficient modulator of bone turnover with the ability to dampen osteoclastic-generated catabolic effects. How the influence and relative importance of pituitary-derived TSH and variant compares with locally derived TSH-βv remains uncertain, but the fact that suppression of pituitary TSH fails to remove the protective action of the TSHR suggests that the local circuit is physiologically relevant.
Acknowledgments
We thank Dr Risheng Ma and Dr Xiaoming Yin for critical reading of this manuscript.
This work was supported in part by National Institutes of Health Grants DK080459 (to M.Z. and T.F.D.), DK069713 and DK052464 (to T.F.D.), and DK80490 and DK70526 (to M.Z.) and by the Veterans Administration Merit Review Program (T.F.D.).
Disclosure Summary: M.Z. is a named inventor of a pending patent application filed by Mount Sinai School of Medicine. In the event the pending patent is licensed, he would be entitled to a share of any proceeds. T.F.D. is a member of the Board of Kronus, Inc (Star, Idaho). R.B., A.K.H., L.C., M.R.A., S.A.M., R.L., A.C., A.T., M.M., L.L., and H.C.B have nothing to disclose.
Footnotes
- ALP
- alkaline phosphatase
- BM
- bone marrow
- BMDMØ
- BM-derived MØ
- ECD
- ectodomain
- EM
- electron microscopy
- FACS
- fluorescence activated cell sorting
- MCSF
- macrophage colony stimulating factor
- MO
- monocyte
- MØ
- macrophage
- qRT-PCR
- quantitative real-time PCR
- TSHR
- TSH receptor
- TSH-βv
- TSH-β splice variant.
References
- 1. Zaidi M, Davies TF, Zallone A, et al. Thyroid-stimulating hormone, thyroid hormones, and bone loss. Curr Osteoporos Rep. 2009;7:47–52 [DOI] [PubMed] [Google Scholar]
- 2. Baliram R, Sun L, Cao J, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest. 2012;122:3737–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kruger TE, Blalock JE. Cellular requirements for thyrotropin enhancement of in vitro antibody production. J Immunol. 1986;137:197–200 [PubMed] [Google Scholar]
- 4. Kruger TE, Smith LR, Harbour DV, Blalock JE. Thyrotropin: an endogenous regulator of the in vitro immune response. J Immunol. 1989;142:744–747 [PubMed] [Google Scholar]
- 5. Smith EM, Phan M, Kruger TE, Coppenhaver DH, Blalock JE. Human lymphocyte production of immunoreactive thyrotropin. Proc Natl Acad Sci USA. 1983;80:6010–6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vincent BH, Montufar-Solis D, Teng BB, Amendt BA, Schaefer J, Klein JR. Bone marrow cells produce a novel TSHβ splice variant that is upregulated in the thyroid following systemic virus infection. Genes Immun. 2009;10:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stow JL, Low PC, Offenhäuser C, Sangermani D. Cytokine secretion in macrophages and other cells: pathways and mediators. Immunobiology. 2009;214:601–612 [DOI] [PubMed] [Google Scholar]
- 8. Rifas L, Shen V, Mitchell K, Peck WA. Macrophage-derived growth factor for osteoblast-like cells and chondrocytes. Proc Natl Acad Sci USA. 1984;81:4558–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gruber HE. Bone and the immune system. Proc Soc Exp Biol Med. 1991;197:219–225 [DOI] [PubMed] [Google Scholar]
- 10. Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Latif R, Michalek K, Davies TF. Subunit interactions influence TSHR multimerization. Mol Endocrinol. 2010;24:2009–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815 [DOI] [PubMed] [Google Scholar]
- 13. Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15:378–389 [DOI] [PubMed] [Google Scholar]
- 14. Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowie JU, Lüthy R, Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991;253:164–170 [DOI] [PubMed] [Google Scholar]
- 16. Ritchie DW, Venkatraman V. Ultra-fast FFT protein docking on graphics processors. Bioinformatics. 2010;26:2398–2405 [DOI] [PubMed] [Google Scholar]
- 17. Núñez Miguel R, Sanders J, Jeffreys J, et al. Analysis of the thyrotropin receptor-thyrotropin interaction by comparative modeling. Thyroid. 2004;14:991–1011 [DOI] [PubMed] [Google Scholar]
- 18. Sanders J, Chirgadze DY, Sanders P, et al. Crystal structure of the TSH receptor in complex with a thyroid-stimulating autoantibody. Thyroid. 2007;17:395–410 [DOI] [PubMed] [Google Scholar]
- 19. Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol. 2012;816:19–29 [DOI] [PubMed] [Google Scholar]
- 20. Abe E, Sun L, Mechanick J, et al. Bone loss in thyroid disease: role of low TSH and high thyroid hormone. Ann NY Acad Sci. 2007;1116:383–391 [DOI] [PubMed] [Google Scholar]
- 21. Baliram R, Latif R, Berkowitz J, et al. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc Natl Acad Sci USA. 2011;108:16277–16282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma R, Morshed S, Latif R, Zaidi M, Davies TF. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid. 2011;21:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun L, Vukicevic S, Baliram R, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2008;105:4289–4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamoah K, Brebene A, Baliram R, et al. High-mobility group box proteins modulate tumor necrosis factor-α expression in osteoclastogenesis via a novel deoxyribonucleic acid sequence. Mol Endocrinol. 2008;22:1141–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abe E, Marians RC, Yu W, et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162 [DOI] [PubMed] [Google Scholar]
- 26. Sampath TK, Simic P, Sendak R, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res. 2007;22:849–859 [DOI] [PubMed] [Google Scholar]
- 27. Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977 [DOI] [PubMed] [Google Scholar]
- 28. Holgate ST. The role of mast cells and basophils in inflammation. Clin Exp Allergy. 2000;30(suppl 1):28–32 [DOI] [PubMed] [Google Scholar]
- 29. Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53 [DOI] [PubMed] [Google Scholar]
- 30. Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin Reprod Med. 2007;25:437–444 [DOI] [PubMed] [Google Scholar]
- 31. Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203 [DOI] [PubMed] [Google Scholar]
- 32. Kaplan J, Keogh EA. Studies on the physiology of macrophage receptors for α-macroglobulin X protease complexes. Ann NY Acad Sci. 1983;421:442–456 [DOI] [PubMed] [Google Scholar]
- 33. Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pekonen F, Weintraub BD. Thyrotropin binding to cultured lymphocytes and thyroid cells. Endocrinology. 1978;103:1668–1677 [DOI] [PubMed] [Google Scholar]