Abstract
LH receptor (LHR) expression in the ovary is regulated by the RNA binding protein, (LHR mRNA binding protein [LRBP]), which has been identified as being mevalonate kinase. This study examined the role of microRNA miR-122 in LRBP-mediated LHR mRNA expression. Real-time PCR analysis of ovaries from pregnant mare serum gonadotropin/human chorionic gonadotropin (hCG)-primed female rats treated with hCG to down-regulate LHR expression showed that an increase in miR-122 expression preceded LHR mRNA down-regulation. The expression of miR-122 and its regulation was confirmed using fluorescent in situ hybridization of the frozen ovary sections using 5′-fluorescein isothiocyanate-labeled miR-122 locked nucleic acid probe. The increased expression of miR-122 preceded increased expression of LRBP mRNA and protein, and these increases were followed by LHR mRNA down-regulation. Inhibition of protein kinase A (PKA) and ERK1/2 signaling pathways by H89 and UO126, respectively, attenuated the hCG-mediated up-regulation of miR-122 levels. This was also confirmed in vitro using human granulosa cells. These results suggest the possibility that hCG-mediated miR-122 expression is mediated by the activation of cAMP/PKA/ERK signaling pathways. Inhibition of miR-122 by injection of the locked nucleic acid-conjugated antagomir of miR-122 abrogated the hCG-mediated increases in LRBP protein expression. Because it has been previously shown that miR-122 regulates sterol regulatory element-binding proteins (SREBPs) and SREBPs, in turn, regulate LRBP expression, the role of SREBPs in miR-122-mediated increase in LRBP expression was then examined. The levels of active forms of both SREBP-1a and SREBP-2 were increased in response to hCG treatment, and the stimulatory effect was sustained up to 4 hours. Taken together, our results suggest that hCG-induced down-regulation of LHR mRNA expression is mediated by activation of cAMP/PKA/ERK pathways to increase miR-122 expression, which then increases LRBP expression through the activation of SREBPs.
Expression of LH receptor (LHR) in the cycling ovary shows significant changes during different phases of the ovarian cycle. Receptor expression is up-regulated during the follicular phase and transiently down-regulated during the preovulatory LH surge, followed by full recovery by the midluteal phase but decreasing again with the regression of the corpus luteum (1–7). Further studies, using rodent and human ovaries, showed that these fluctuations in LHR expression, at least in part, involve a posttranscriptional mechanism mediated by an RNA binding protein (4, 6–11). LHR mRNA binding protein (LRBP) binds to the coding region of LHR mRNA and induces translational suppression causing LHR mRNA degradation (9, 12, 13). LRBP was then characterized as being mevalonate kinase (MVK) (10). Expression of LRBP showed a reciprocal relationship with LHR mRNA levels and LRBP caused accelerated degradation of LHR mRNA (4). We further demonstrated that LH/human chorionic gonadotropin (hCG)-mediated activation of cAMP/protein kinase A (PKA) and ERK pathways is required for increasing the expression of LRBP (14).
The present studies examined the in vivo regulation of LRBP expression in the ovary. Specifically, we determined the role of miRNA-122 (miR-122) in LRBP expression during ligand-induced down-regulation of LHR mRNA. Previous studies have shown that inhibition of miR-122, which is expressed in liver and a variety of other tissues, inhibits the expression of MVK/LRBP in mouse liver (15). Because MVK functions as LHR mRNA binding protein (LRBP) (6, 7), we hypothesized that miR-122 could potentially regulate LRBP expression and thus serve as a regulator of LHR mRNA expression during LH/hCG-induced down-regulation in the ovary. In the present studies, we examined the localization of miR-122 in rat ovaries using in situ hybridization technique and analyzed the changes in its expression during LHR down-regulation and the effect of suppressing miR-122 on LRBP expression during LH/hCG-induced down-regulation using a specific antagomir. We also have examined the signaling pathways involved in LH/hCG-mediated increase in miR-122 expression.
Materials and Methods
Materials
Pregnant mare serum gonadotropin was purchased from Calbiochem. Highly purified human chorionic gonadotropin (hCG; CR 127) was purchased from Dr A. F. Parlow (National Hormone and Peptide Program, Torrance, California). EDTA-free protease inhibitor mixture tablets and RNAse inhibitor were purchased from Roche Applied Science and Promega Corp., respectively. Real-time PCR primers for miR-122 and U6 small nuclear RNA (TaqMan Assay-on-Demand Gene Expression Product) as well as Taqman miRNA (micro-RNA) reverse transcription kits were from Applied Biosystems. Because LRBP was identified as MVK, anti-N-terminal MVK IgG was raised against the first 15 N-terminal amino acids of MVK (MLSEVLLVSAPGKVI), and this antibody is referred to as the LRBP antibody in the text. sterol regulatory element binding protein (SREBP)-1a mouse monoclonal antibody (IgG-2A4) was raised against amino acids 301–407 of human SREBP-1a (American Type Culture Collection). Antibodies against β-tubulin and SREBP-2 were from Sigma and Santa Cruz Biotechnology, respectively. The Super Signal West Femto chemiluminescence kit and antirabbit/antimouse IgG conjugated to horseradish peroxidase were obtained from Pierce Chemical Co. BCA reagent was purchased from GE Healthcare Life Sciences. ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole, mirVana miRNA isolation kit (Ambion), and tyramide signal amplification kit containing horseradish peroxidase goat antirabbit IgG and Alexa Fluor 488 tyramide were from Invitrogen. FITC (fluorescein isothiocyanate)-labeled miR-122 locked nucleic acid (LNA) probe and LNA-conjugated miR-122 antagomir were from Exiqon.
Animals and tissues
Superovulation was induced in 23-day-old female Sprague Dawley rats by sc injection of 50 IU of pregnant mare serum gonadotropin followed by 25 IU of hCG 56 hours later. Five days after inducing superovulation, the animals were treated again with a single dose of hCG to down-regulate LHR mRNA expression. The ovaries were then collected 0 and 30 minutes and 1, 2, 3, 4, 5, 6, and 12 hours after hCG injection and were frozen in liquid nitrogen until further use. To block PKA and ERK1/2, the superovulated rats were treated sc with H-89 (100 mg/kg body weight) and UO126 (10 mg/kg body weight) 1 hour before the second hCG treatment. To inhibit miR-122, the rats were injected with an LNA-conjugated miR-122 antagomir, directly into the bursa of the ovary, on day 4. The rats were allowed to recover for 24 hours before hCG treatment and the ovaries were collected after 6 hours. All animal experimentation described in the submitted manuscript was conducted in accordance with the accepted standards of humane animal care, as outlined in the Ethical Guidelines of University of Michigan.
Cell isolation and culture
Human granulosa cells were isolated from the ovarian follicular aspirates obtained from women undergoing oocyte retrieval for in vitro fertilization (IVF) at the University of Michigan Health System and IVF Michigan Laboratory. The protocol for isolating granulosa cells has been described in detail previously (7). In brief, the isolated granulosa cells were plated onto 35-mm dishes, at a density of approximately 1 × 106 viable cells, in McCoy's 5A medium supplemented with 10% fetal bovine serum, 1.65 mg/L glutamine, nonessential amino acids, penicillin (100 U/mL), streptomycin (100 μg/mL), and fungizone (0.25 μg/mL). Media were replaced after 24 hours, and incubations were continued for an additional 2–3 days. These day 3 to day 4 cultured cells were then treated with the necessary reagents and were used for total RNA isolation followed by real-time PCR.
Real-Time PCR analysis
Total RNAs/miRNAs were reverse transcribed and subjected to real-time PCR quantitation as per the manufacturer's instructions (Applied Biosystems). The percent change in gene expression was calculated using the ΔΔCt method with U6 small nuclearRNA as the internal control, as described previously (14).
Western blot analysis
For this, tissue homogenates (S10 fractions) in radioimmune precipitation assay buffer were subjected to 10% SDS-PAGE under reducing conditions followed by Western blot analysis as previously described (4). The presence of immune complexes was detected by chemiluminescence.
Fluorescent in situ hybridization (FISH) of the ovaries
LNA-based in situ detection of miR-122 was performed in rat ovaries as described previously (16) with slight modifications. Briefly, frozen sections of the control and hCG (1 hour) treated ovaries were fixed in 4% paraformaldehyde and incubated in prehybridization mix (diethylpyrocarbonate water containing 50% formamide, 500 μg/μL yeast tRNA, and 1× Denhardt's solution) followed by 5′-FITC labeled miR-122 LNA probe (2.5 pmol/60 μL prehybridization mix) at 65°C for 4 hours. Tissue sections were washed in 0.1× SSC (3 × 10 minutes) and then in 2× SSC (1 × 5 minutes). The sections were then incubated with 3% (vol/vol) H2O2 followed by further washings in PBS (3× for 3 minutes). Tyramide signal amplification kit (Invitrogen: T20922) was used to amplify the signal, as per the manufacturer's instructions. Briefly, the sections were incubated with the blocking solution (provided with the kit) and then with the anti-FITC/horseradish peroxidase antibody (1:400 in blocking solution) for 30 minutes at room temperature. FITC-tyramide/amplification buffer mix was added to each section and incubated for 10 minutes in the dark. After washings (3 × 5 minutes with PBS) in the dark, sections were mounted using ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole, observed using a confocal photomicroscope (Olympus FluoView FV500) and photographed.
Statistical analysis
Statistical analysis was carried out using one-way ANOVA followed by the Tukey multiple comparison test. Values were considered statistically significant for P < .05. Each experiment was repeated at least 3 times with similar results. Blots and autoradiograms shown are representative of a minimum of 3 experiments.
Results
LH/hCG increases miR-122 expression
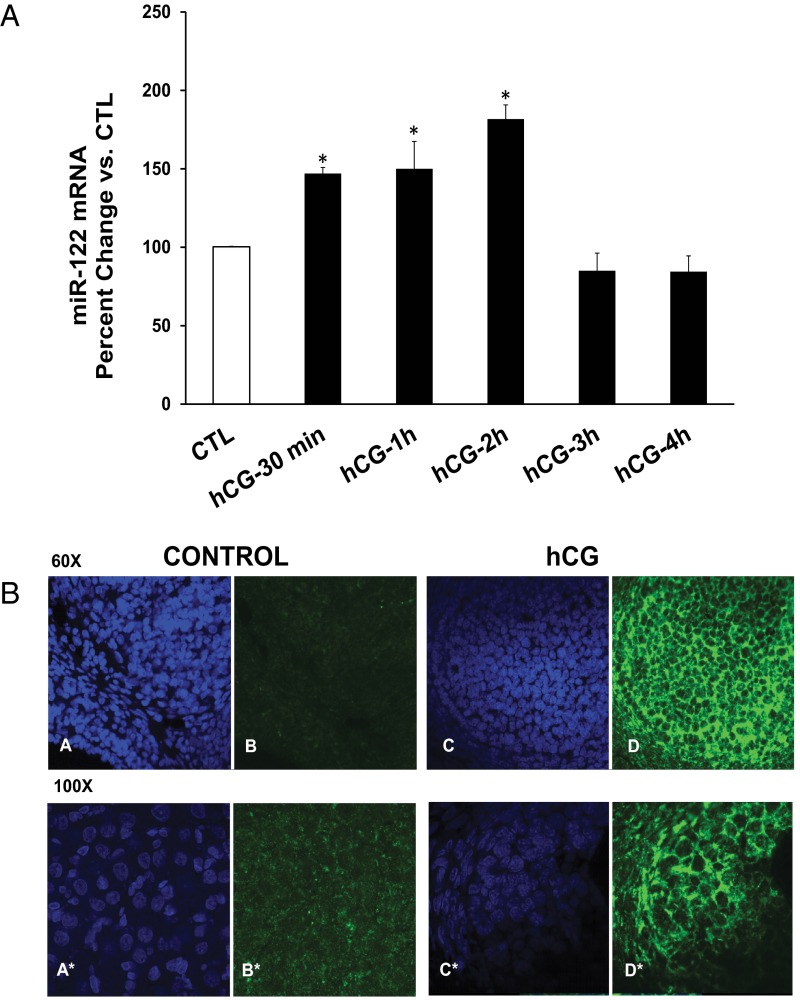
In order to determine a possible role for miR-122 in LRBP and LHR regulation, we examined the changes in the levels of miR-122 during LHR down-regulation using real-time PCR. As shown in Figure 1A, there was a significant increase in the expression of miR-122 immediately after hCG treatment. The increase was evident initially at 30 minutes (147 ± 4% vs control [CTL], P < .05) post-hCG and peaked around 1–2 hours (hCG-1h, 150 ± 17% vs CTL; hCG-2h, 181 ± 9% vs CTL, P < .05) but started to decline thereafter. The expression of miR-122 in the ovary and its up-regulation in response to hCG was further confirmed by fluorescence in situ hybridization (FISH) of frozen (5 μm) sections of the normal and hCG-treated (1 hour) rat ovaries, using 5′-FITC-labeled miR-122 LNA probe as described in Materials and Methods. The sections were then analyzed using confocal microscopy (Figure 1B). The results showed that whereas there was low or no expression of miR-122 in the CTL ovaries, its expression showed an increase in response to hCG treatment.
Figure 1.
Analysis of miR-122 expression in the ovaries using real-time PCR and fluorescent in situ hybridization analysis. A, Superovulated rats were injected with hCG on day 5. Ovaries were collected 0 and 30 minutes and 1, 2, 3, and 4 hours later. Total RNA was isolated from the ovaries and the levels of miR-122 and U6 snRNA were determined by real-time PCR using predesigned primers and probes as described in Materials and Methods. The graphs represent changes in miR-122 levels normalized to U6 small nuclear RNA and shown as percent change vs CTL. Error bars represent mean ±SE. *, P < .05, n = 3. B, Fluorescent in situ hybridization analysis of CTL and hCG-treated (1 hour) frozen rat ovary sections were conducted using FITC-labeled miR-122 LNA probe as described in detail in Materials and Methods. Each panel shows 4′,6-diamidino-2-phenylindole staining (A, A*, C & C*; blue fluorescence) on the left and FITC (miR-122) staining (B, B*, D & D*, green fluorescence) on the right. Top and bottom panels show ×60 and ×100 magnifications, respectively. Experiment was repeated 4 times with same results.
Expressions of miR-122, LRBP, and LHR mRNA follow a temporal relationship during hCG-induced down-regulation
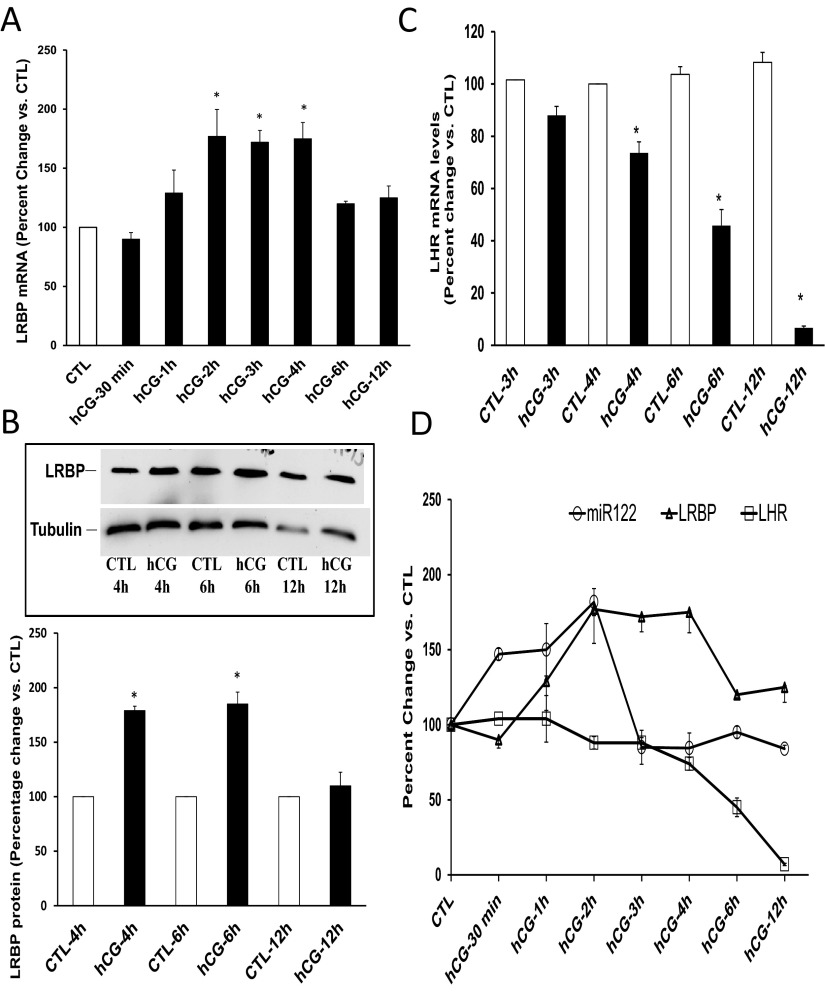
Experiments were then carried out to determine the temporal relationship between miR-122 expression and ligand-induced down-regulation of LHR mRNA. A close relationship between the timeframe of expressions of miR-122, LRBP, and LHR mRNA was observed. miR-122 expression showed an increase immediately after hCG treatment. As shown in Figure 1A, the increase was initially seen at 30 minutes post hCG and remained steady until 2 hours. This was followed by a rapid surge in LRBP mRNA levels. The levels of LRBP mRNA (Figure 2A) increased at 2 hours after hCG treatment (177 ± 22% vs CTL, P < .05) and remained high until 4 hours (hCG-3h, 172 ± 10%; and hCG-4h, 175 ± 13% vs CTL, P < .05). This was followed by an increase in the protein levels of LRBP (Figure 2B). The increase in the protein expression of LRBP was seen at 4 hours (179 ± 4% vs CTL, P < .05) and sustained until 6 hours (180 ± 0.55% vs CTL, P < .05) of hCG treatment. LHR mRNA levels started to decline at 4 hours post hCG, and a minimum decrease was attained by 12 hours (Figure 2C) (The values are expressed as percent change vs CTL: hCG-4h, 73.6 ± 4.5; hCG-6h, 45.8 ± 6.2; and hCG-12h, 6.6 ± 0.72, P < .05). As shown in Figure 2, B and D, the increase in the expression of miR-122 was followed by that of LRBP mRNA and protein, resulting in LHR mRNA down-regulation. This temporal relationship suggested a role for miR-122 in mediating an increase in LRBP levels causing LHR mRNA down-regulation.
Figure 2.
Temporal relationship of miR-122, LRBP, and LHR mRNA expressions in the ovary following hCG treatment. Superovulated rats were injected with hCG on day 5 and ovaries were collected 0 and 30 minutes and 1, 2, 3, 4, 6, and 12 hours later. Equal amounts of RNA from the CTL or hCG-treated ovaries were subjected to real-time PCR using predesigned primers and probes for rat LRBP or LHR mRNA (A and C) as described in Materials and Methods. The graphs represent ratio of LRBP or LHR mRNA levels normalized to 18S RNA and are shown as percent change vs CTL. Error bars represent mean ± SE. *, P < .05; n = 4. Equal amounts of protein from the CTL or hCG-treated S10 fractions were subjected to Western blot analysis using LRBP antibody (B). The membranes were stripped and reprobed for tubulin (bottom lane). The blots shown are representative of 3 independent experiments. The graphs represent LRBP levels normalized to tubulin and are shown as percent change vs control. Error bars represent mean ± SE. *, P < .05; n = 4. The graphic representation of the relationship between the time course of miR-122, LRBP, and LHR mRNA expressions after hCG treatment is shown in panel D.
cAMP/PKA pathway and ERK pathways are responsible for hCG-mediated miR-122 expression
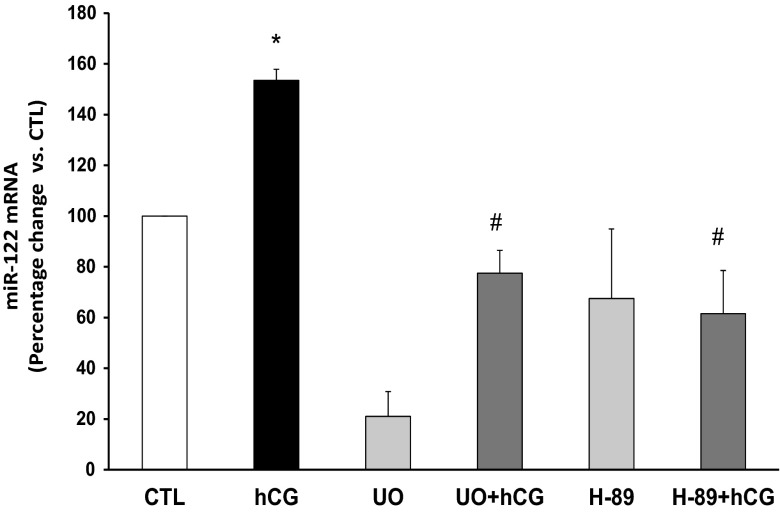
To examine the downstream signaling pathways involved in hCG-mediated increases in miR-122 levels, superovulated rats were treated with inhibitors of PKA and ERK pathways before inducing down-regulation by hCG. This was based on our previous studies showing that cAMP/PKA and ERK pathways are responsible for the hCG-mediated increases in LRBP expression and LHR mRNA down-regulation (3, 14). The results in Figure 3 showed that inhibition of PKA using H-89 attenuated the hCG-mediated increase in miR-122 levels (percent change, hCG-154 ± 4.3 vs CTL; H-89+hCG-61.5 ± 17 vs CTL, P < .05; Figure 3). Inhibition of ERK1/2 using UO126 also abrogated hCG-mediated miR-122 increase (percent change, hCG-154 ± 4.3 vs CTL; UO+hCG-77.5 ± 8.9 vs CTL, P < .05). We have previously shown that the cAMP/PKA pathway plays an integral role in the induction of LRBP expression and its binding to LHR mRNA during hCG-mediated LHR mRNA down-regulation. Taken together, our present results suggest that the activation of cAMP/PKA and ERK1/2 pathways in response to hCG treatment sets in motion a series of events resulting in the increase in miR-122 levels culminating in LHR mRNA down-regulation.
Figure 3.
Inhibition of PKA and ERK1/2 inhibits hCG-induced increases in miR-122 expression in the rat ovaries. Superovulated rats were injected with H-89 (100 mg/kg body weight) or UO126 (10 mg/kg body weight) 1 hour before hCG injection on day 5 and ovaries were collected 1 hour later. Total RNA was isolated from the ovaries, and the levels of miR-122 and U6 small nuclear RNA (CTL) were determined by real-time PCR using predesigned primers and probes as described in Materials and Methods. The graphs represent changes in miR-122 levels normalized to U6 small nuclear RNA and shown as percent change vs CTL. Error bars represent mean ± SE. *, P < .05; n = 3.
LH/hCG-mediated increase in the expression of miR-122 in human granulosa cells is mediated by ERK1/2
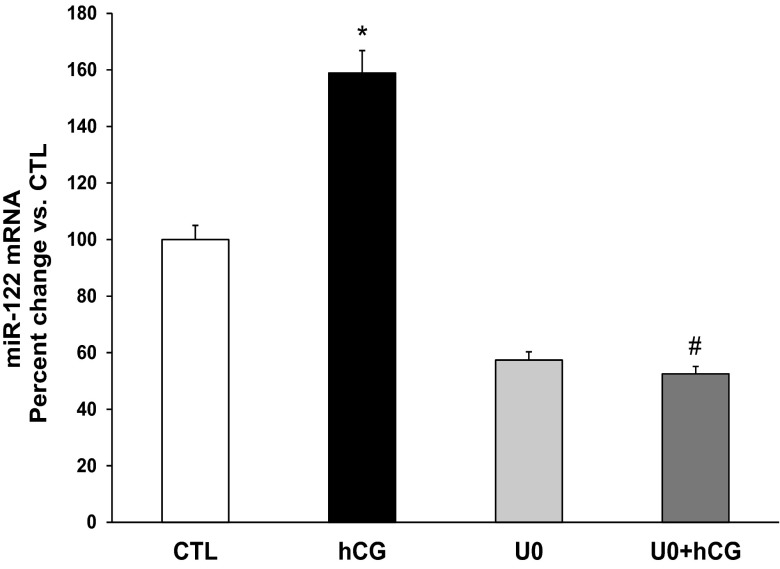
The increase in miR-122 levels during LHR mRNA down-regulation was further confirmed in human granulosa cells under in vitro conditions. In the granulosa cells isolated from follicular aspirates for IVF, LHR is down-regulated on the day of collection because subjects had received high doses of hCG to induce ovulation prior to the retrieval (7). We have shown that these cultured granulosa cells do recover from down-regulation upon incubation with serum-containing media for 48 hours (7). Following recovery from down-regulation, LHR mRNA expression in these cells can again be down-regulated by treatment with hCG in vitro causing loss of LHR mRNA transcripts with corresponding increases in LRBP levels and the binding activity of LRBP to LHR mRNA (6, 7, 14). We have followed the same treatment paradigm in this study. There was a significant increase in the expression of miR-122 at 1 hour following hCG treatment (percent change: hCG, 159 ± 4.5 vs CTL, P < .05; Figure 4). This increase was completely abrogated when cells were pretreated with UO126 1 hour before hCG stimulation (percent change: UO+hCG, 52.5 ± 2.1 vs CTL respectively, P < .05 vs hCG). This further supported the role of ERK1/2 in hCG-mediated increase of miR-122 expression.
Figure 4.
Inhibition of PKA and ERK1/2 inhibits hCG-induced increases in miR-122 expression in human granulosa cells. Day 4 granulosa cells were serum starved, treated with hCG (10 IU/mL) with or without the ERK1/2 inhibitor UO126 (10 μM; 1-hour pretreatment) for a total of 1 hour and were processed for RNA isolation as described in Materials and Methods. RNAs were reverse transcribed, and the resulting cDNAs were subjected to real-time PCR quantitation using specific primers and probes for miR-122 and U6 small nuclear RNA. The graphs represent changes in miR-122 levels normalized to U6 snRNA and shown as percent change vs CTL. Error bars represent mean ± SE. *, P < .05 vs CTL; #, P < .05 vs hCG; n = 4.
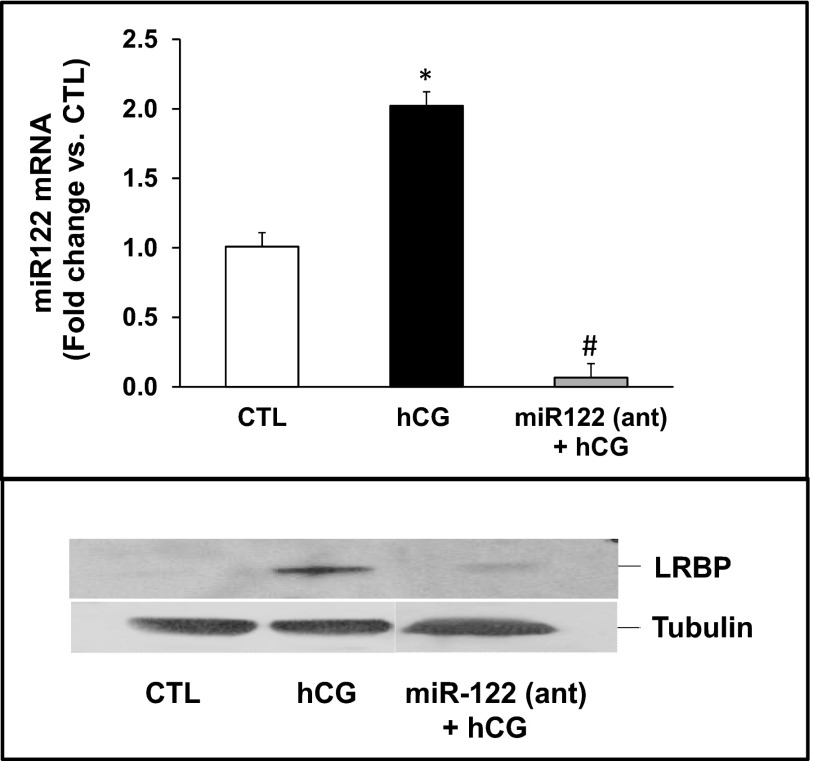
Inhibition of miR-122 abrogated hCG-induced LRBP protein expression during LHR down-regulation
The role of miR-122 as an upstream regulator of LRBP was then tested by inhibiting miR-122 expression using an antagomir followed by determining the changes in hCG-induced LRBP expression. miR-122 was inhibited by injecting superovulated rats with an antagomir of miR-122 24 hours before treating with hCG. miR-122 expression was completely abolished by treatment with antagomir, as demonstrated by the real-time PCR results shown in the upper panel of Figure 5. Furthermore, the increase in LRBP protein levels seen in response to hCG was abrogated in response to miR-122 antagomir treatment (Figure 5, lower panel), confirming that the increase in LRBP seen during ligand-induced down-regulation of LHR mRNA is mediated by miR-122.
Figure 5.
Inhibition of miR-122 abrogated hCG-induced increases in LRBP expression. LNA-conjugated miR-122 antagomir was injected into the bursa of the ovaries of superovulated rats on day 4 followed by hCG on day 5. Ovaries were collected 6 hours later. Equal amounts of protein from the CTL, hCG-treated, or antagomir + hCG-treated ovaries were subjected to real-time PCR using predesigned primers and probes for miR-122 (upper panel) as described in Materials and Methods. The graphs represent ratio of miR-122 levels normalized to U6 small nuclear RNA and are shown as percent change vs CTL. Error bars represent mean ± SE. Equal amounts of protein from the CTL, hCG-treated, or antagomir + hCG-treated S10 fractions were subjected to Western blot analysis using LRBP antibody (lower panel, top lane). The membranes were stripped and reprobed for tubulin (lower panel, bottom lane).
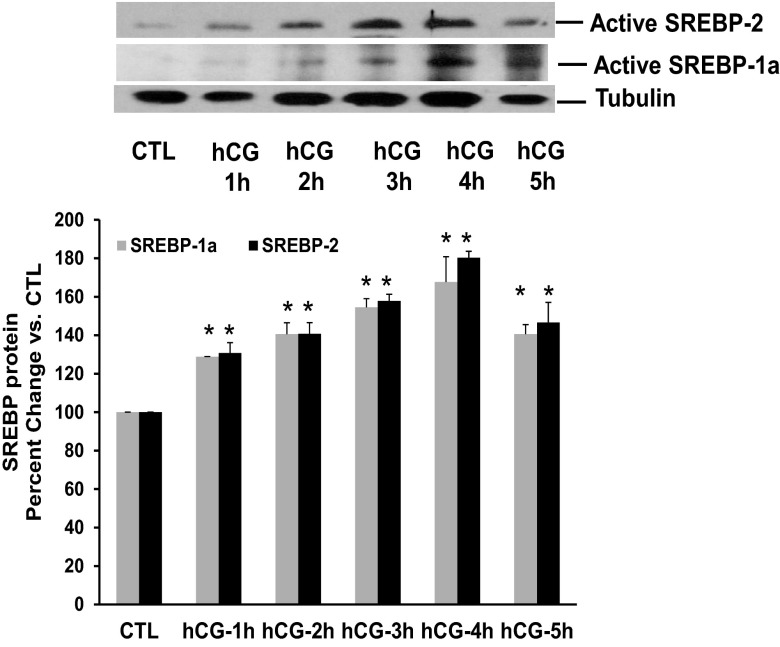
Time course of hCG-mediated SREBP-1a and SREBP-2 activation suggests a role for SREBPs in miR-122-mediated LRBP induction
The mechanism by which miR-122 increases LRBP levels was then investigated by examining the temporal relationship between the levels of SREBP-1a/SREBP-2, miR-122, and LRBP expression. SREBPs exist in active (65–68 kDa) and inactive (125 kDa) forms. We have previously reported that the antibodies recognize both the precursor and active forms of SREBP-2 and SREBP-1a (17). Autoradiograms of the bands corresponding to the processed (active) forms of SREBP-2 and SREBP-1A are presented in Figure 6. A linear increase in the expression of the active form of SREBP-2 (68 kDa) was observed starting at 1 hour of hCG stimulation and sustained to 4 hours (Figure 6, upper panel). The levels were decreased by 5 hours. Quantification of the bands seen in Western blot images of active SREBP-2 yielded the following results: percentage vs CTL; hCG-1h, 128.9 ± 0.07*; hCG-2h, 140 ± 5.8*; hCG-3h, 154.5 ± 4.5*; hCG-4h, 167.6 ± 13.1*; hCG-5h, 140.6 ± 4.9*; *, P < .05; n = 3. The same trend was also observed for SREBP-1a (Figure 6, upper panel, middle lane). The intensity of the active form of SREBP-1a increased at 1 hour post hCG and showed a linear increase until 4 hours, after which it started to decline. Taken together, the data show that the increase in miR-122 expression starts at 30 minutes after hCG treatment (as shown in Figure 1) and reaches a maximum around 1–2 hours. The activation of SREBPs starts at 1 hour after hCG treatment reaching a maximum by 4 hours (Figure 6). This is followed by increase in the protein expression of LRBP starting at 4 hours and reaching maximum by 6 hours (Figure 2B). These results established a relationship between the expressions of miR-122, SREBP activation, and LRBP, indicating a role for SREBP activation in miR-122-mediated increase in LRBP expression.
Figure 6.
Activation of SREBP-1a and SREBP-2 during hCG-induced LHR mRNA down-regulation. Superovulated rats were injected with hCG on day 5, and ovaries were collected 1, 2, 3, 4, and 5 hours later. S10 fractions were prepared using radioimmune immunoprecipitation assay buffer. Equal amounts of protein from the CTL or hCG-treated S10 fractions were subjected to Western blot analysis using SREBP-2 antibody. The blots were stripped and reprobed first with SREBP-1a and then with tubulin. The blots shown are representative of 3 independent experiments. The graphs represent SREBP levels normalized to tubulin and are shown as percent change vs control. Error bars represent mean ± SE. *, P < .05; n = 3.
Discussion
It is now well recognized that miRNAs are important regulators of gene expression (18–20). In the majority of instances, they bind to the 3′-untranslated region of protein-coding mRNA transcripts to prevent translation, and in some cases, lead to mRNA degradation (21, 22). Here we sought to examine whether the translational suppression and degradation of LHR mRNA under conditions that mimic preovulatory LH surge is regulated by miRNAs. To examine this, we first determined the temporal relationship between miR-122 and LRBP expression during down-regulation of LHR mRNA expression. We tested the notion that if miR-122 plays a role in LRBP expression, a comparison of the time course would show a sequential change in miR-122, followed by LRBP and finally LHR mRNA. Our results supported this idea. LHR mRNA undergoes dynamic regulation during the normal ovarian cycle (1–7). We have shown that, in response to treatment with a high concentration of LH/hCG, LHR mRNA begins to undergo down-regulation by 4 hours reaching a maximum by 24 hours. At later time points, LHR mRNA levels begin to rise, reaching normal levels by 48 hours. Time course of LRBP mRNA expression shows an increase at 2 hours and reaching a maximum level at 4 hours but declining thereafter. Thus, it appears that once the down-regulation machinery is set in motion, LRBP levels start to decline and reach normal levels. In agreement with this trend, the initial surge seen in the levels of miR-122 begins to decline after 2 hours of hCG stimulation. Thus miR-122 does not cause direct translational suppression or degradation of LHR mRNA but indirectly regulates its levels by influencing the expression of an RNA-binding protein. This is consistent with other reports in the literature, in which miRNAs have been shown to act by regulating the expression of RNA-binding proteins and vice versa (23–25).
Although miRNAs are recognized to down-regulate gene expression by inhibiting translation or inducing target mRNA degradation, there are several examples in the literature in which miRNAs also up-regulate gene expression through other mechanisms (26–28). Recent reports show that only 30%–40% of mRNAs are up-regulated after miRNA inhibitions that contain predicted miRNA sites (15, 29). Similarly, a large fraction of genes that contain a predicted miRNA site do not display detectable down-regulation after transfection of the miRNA (18, 19, 30, 31). The changes in expression seen after these transfections are thought to be mediated by impaired endogenous miRNA targeting through saturation of RNA induced silencing complex (32) and, most importantly, by secondary effects (33). Here we show that miR-122 increases LRBP expression indirectly, probably by targeting some of its repressors (34). Such indirect miRNA-mediated regulation has been reported to occur in several systems (35–37).
Previous reports from our laboratory have shown that cAMP/PKA pathway activated by hCG stimulation in rat ovaries or human granulosa cells plays a crucial role in increasing LRBP expression (3, 14). The ERK pathway, downstream of cAMP/PKA, is also instrumental in hCG-mediated LRBP induction in human granulosa cells (14). Both cAMP/PKA and ERK1/2 pathways have been shown to regulate several miRNAs (38–41). In particular, one study showed that in PC12 cells, luteolin-induced cAMP response element-binding protein activation, miR-132 expression and neurite outgrowth were inhibited by adenylate cyclase, PKA, and ERK 1/2 inhibitors (41). Our results showing that inhibition of both PKA and ERK 1/2 pathways inhibits miR-122 induction in response to hCG stimulation in the ovary are consistent with these reports. Thus it is logical to conclude that LH/hCG mediates its regulatory effects on LHR mRNA at least partially through miR-122, causing induction of LRBP. The decline in LRBP expression in response to miR-122 antagomir treatment supports this conclusion.
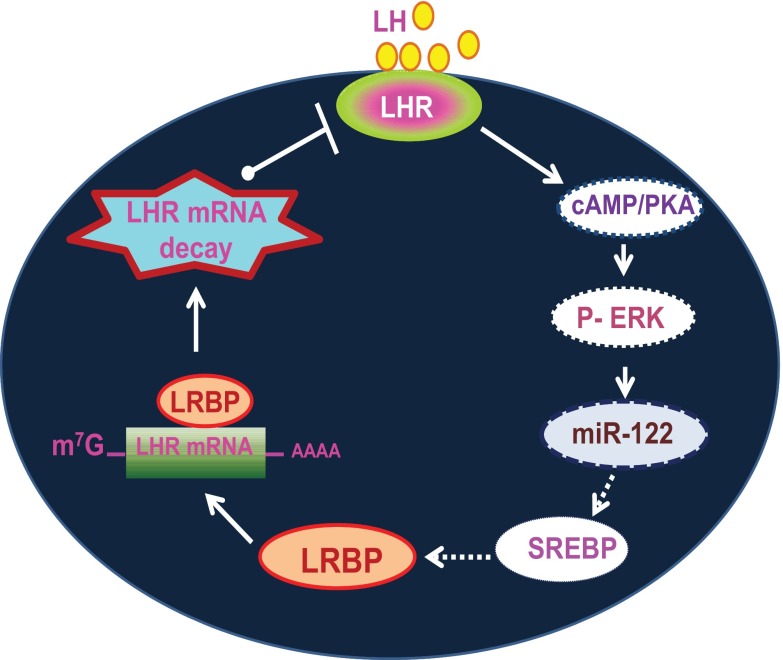
Previous studies have shown that miR-122 can regulate the levels of SREBP-2, a transcription factor that is involved in the regulation of lipid and cholesterol biosynthesis. We therefore examined whether similar mechanisms are involved in hCG-mediated increase in miR-122 expression. Our previous studies demonstrated that LH/hCG activates SREBP-1a in the ovary and that this is followed by an increase in the expression of LRBP (17). On the basis of these results, we examined the role of SREBPs in miR-122-mediated activation of LRBP in the context of ligand-mediated LHR mRNA down-regulation. Of the 3 transcription factors belonging to the SREBP family, SREBP-2 and SREBP-1a regulate transcription of cholesterol-related genes (42, 43). Based on our results, we provide a model (Figure 7) for the miR-122-mediated posttranscriptional mechanism to regulate LHR mRNA expression during ligand-induced down-regulation of LHR. Our results show that LH/hCG induces the activation of both SREBP-1a and SREBP-2, which then induces the expression of LRBP. It has also been shown that miR-122 inhibition by antisense oligonucleotides in mice resulted in reduced cholesterol synthesis (44). The time course of hCG-mediated SREBP activation in the whole ovaries also strongly supports the possibility that it is downstream of hCG-induced cAMP/PKA/ERK/miR-122 pathways and upstream of LRBP induction. This is followed by the activation of SREBP-1a and SREBP-2, which then leads to an increase in the expression of LRBP. LRBP binds to LHR mRNA leading to the translational suppression and degradation of the mRNA, as illustrated in Figure 7.
Figure 7.
Schematic model depicting the proposed signaling pathway in LH/hCG-induced miR-122-mediated LHR mRNA down-regulation. Binding of ligand to LHR induces activation of ERK1/2 through the cAMP/PKA pathway. This leads to an increase in the expression of miR-122, which causes the activation of SREBPs through its interaction with unidentified targets. This is followed by the increased expression of LRBP and its LHR mRNA binding activity, which ultimately results in LHR mRNA degradation. Direct activation is shown by solid arrows and indirect interaction/activation is shown by broken arrows.
Acknowledgments
We thank Helle Peegel and other members of the laboratory for critical reading of the manuscript and many helpful suggestions.
This work was supported by National Institutes of Health Grant R01 HD06656.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 4439
- CTL
- control
- FITC
- fluorescein isothiocyanate
- hCG
- human chorionic gonadotropin
- IVF
- in vitro fertilization
- LHR
- LH receptor
- LNA
- locked nucleic acid
- LRBP
- LHR mRNA-binding protein
- miR-122
- miRNA-122
- miRNA
- micro-RNA
- MVK
- mevalonate kinase
- PKA
- protein kinase A
- SREBP
- sterol regulatory element binding protein.
References
- 1. LaPolt PS, Oikawa M, Jia XC, Dargan C, Hsueh AJ. Gonadotropin-induced up- and down-regulation of rat ovarian LH receptor message levels during follicular growth, ovulation and luteinization. Endocrinology. 1990;126:3277–3279 [DOI] [PubMed] [Google Scholar]
- 2. Segaloff DL, Wang HY, Richards JS. Hormonal regulation of luteinizing hormone/chorionic gonadotropin receptor mRNA in rat ovarian cells during follicular development and luteinization. Mol Endocrinol. 1990;4:1856–1865 [DOI] [PubMed] [Google Scholar]
- 3. Peegel H, Randolph J, Jr, Midgley AR, Menon KMJ. In situ hybridization of luteinizing hormone/human chorionic gonadotropin receptor messenger ribonucleic acid during hormone-induced down-regulation and the subsequent recovery in rat corpus luteum. Endocrinology. 1994;135:1044–1051 [DOI] [PubMed] [Google Scholar]
- 4. Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002;277:21468–21473 [DOI] [PubMed] [Google Scholar]
- 5. Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128:388–393 [DOI] [PubMed] [Google Scholar]
- 6. Wang L, Nair AK, Menon KMJ. Ribonucleic acid binding protein-mediated regulation of luteinizing hormone receptor expression in granulosa cells: relationship to sterol metabolism. Mol Endocrinol. 2007;21:2233–2241 [DOI] [PubMed] [Google Scholar]
- 7. Nair AK, Peegel H, Menon KMJ. The role of luteinizing hormone/human chorionic gonadotropin receptor-specific mRNA binding protein in regulating receptor expression in human ovarian granulosa cells. J Clin Endocrinol Metab. 2006;91:2239–2243 [DOI] [PubMed] [Google Scholar]
- 8. Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mrna binding during receptor down-regulation. J Biol Chem. 1998;273:10658–10664 [DOI] [PubMed] [Google Scholar]
- 9. Kash JC, Menon KMJ. Sequence-specific binding of a hormonally regulated mRNA binding protein to cytidine-rich sequences in the lutropin receptor open reading frame. Biochemistry. 1999;38:16889–16897 [DOI] [PubMed] [Google Scholar]
- 10. Nair AK, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA from ovary. J Biol Chem. 2004;279:14937–14944 [DOI] [PubMed] [Google Scholar]
- 11. Menon KMJ, Nair AK, Wang L, Peegel H. Regulation of luteinizing hormone receptor mRNA expression by a specific RNA binding protein in the ovary. Mol Cell Endocrinol. 2007;260–262:109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nair AK, Menon KMJ. Regulation of luteinizing hormone receptor expression: evidence of translational suppression in vitro by a hormonally regulated mRNA-binding protein and its endogenous association with luteinizing hormone receptor mRNA in the ovary. J Biol Chem. 2005;280:42809–42816 [DOI] [PubMed] [Google Scholar]
- 13. Menon B, Peegel H, Menon KMJ. Evidence for the association of luteinizing hormone receptor mRNA-binding protein with the translating ribosomes during receptor downregulation. Biochim Biophys Acta. 2009;1793:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menon B, Franzo-Romain M, Damanpour S, Menon KMJ. Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol Endocrinol. 2011;25:282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689 [DOI] [PubMed] [Google Scholar]
- 16. Obernosterer G, Martinez J, Alenius M. Locked nucleic acid-based in situ detection of microRNAs in mouse tissue sections. Nat Protoc. 2007;2:1508–1514 [DOI] [PubMed] [Google Scholar]
- 17. Palaniappan M, Menon KMJ. Regulation of sterol regulatory element-binding transcription factor 1a by human chorionic gonadotropin and insulin in cultured rat theca-interstitial cells. Biol Reprod. 2009;81:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63 [DOI] [PubMed] [Google Scholar]
- 20. Farh KK, Grimson A, Jan C, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821 [DOI] [PubMed] [Google Scholar]
- 21. Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E. Deadenylation is a widespread effect of miRNA regulation. RNA. 2009;15:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Behm-Ansmant I, Rehwinkel J, Izaurralde E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb Symp Quant Biol. 2006;71:523–530 [DOI] [PubMed] [Google Scholar]
- 23. Chang SH, Hla T. Gene regulation by RNA binding proteins and microRNAs in angiogenesis. Trends Mol Med. 2011;17:650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat Rev Cancer. 2011;11:644–656 [DOI] [PubMed] [Google Scholar]
- 25. Vo DT, Qiao M, Smith AD, Burns SC, Brenner AJ, Penalva LO. The oncogenic RNA-binding protein Musashi1 is regulated by tumor suppressor miRNAs. RNA Biol. 2011;8:817–828 [DOI] [PubMed] [Google Scholar]
- 26. Mortensen RD, Serra M, Steitz JA, Vasudevan S. Posttranscriptional activation of gene expression in Xenopus laevis oocytes by microRNA-protein complexes (microRNPs). Proc Natl Acad Sci USA. 2011;108:8281–8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishi H, Ono K, Horie T, et al. MicroRNA-27a regulates β cardiac myosin heavy chain gene expression by targeting thyroid hormone receptor β1 in neonatal rat ventricular myocytes. Mol Cell Biol. 2011;31:744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma F, Liu X, Li D, et al. MicroRNA-466l upregulates IL-10 expression in TLR-triggered macrophages by antagonizing RNA-binding protein tristetraprolin-mediated IL-10 mRNA degradation. J Immunol. 2010;184:6053–6059 [DOI] [PubMed] [Google Scholar]
- 29. Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033 [DOI] [PubMed] [Google Scholar]
- 30. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hammell M, Long D, Zhang L, et al. mirWIP: microRNA target prediction based on microRNA-containing ribonucleoprotein-enriched transcripts. Nat Methods. 2008;5:813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khan AA, Betel D, Miller ML, Sander C, Leslie CS, Marks DS. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat Biotechnol. 2009;27:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu X, Chen Z, Yu J, Xia J, Zhou X. MicroRNA profiling and head and neck cancer. Comp Funct Genomics. 2009;837514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tu K, Yu H, Hua YJ, et al. Combinatorial network of primary and secondary microRNA-driven regulatory mechanisms. Nucleic Acids Res. 2009;37:5969–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li X, Mertens-Talcott SU, Zhang S, Kim K, Ball J, Safe S. MicroRNA-27a indirectly regulates estrogen receptor α expression and hormone responsiveness in MCF-7 breast cancer cells. Endocrinology. 2010;151:2462–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pan YZ, Gao W, Yu AM. MicroRNAs regulate CYP3A4 expression via direct and indirect targeting. Drug Metab Dispos. 2009;37:2112–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shahab SW, Matyunina LV, Hill CG, et al. The effects of MicroRNA transfections on global patterns of gene expression in ovarian cancer cells are functionally coordinated. BMC Med Genomics. 2012;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Monk CE, Hutvagner G, Arthur JS. Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS One. 2010;5:e13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romano G, Acunzo M, Garofalo M, et al. MiR-494 is regulated by ERK1/2 and modulates TRAIL-induced apoptosis in non-small-cell lung cancer through BIM down-regulation. Proc Natl Acad Sci USA. 2012;109:16570–16575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Terasawa K, Ichimura A, Sato F, Shimizu K, Tsujimoto G. Sustained activation of ERK1/2 by NGF induces microRNA-221 and 222 in PC12 cells. FEBS J. 2009;276:3269–3276 [DOI] [PubMed] [Google Scholar]
- 41. Lin LF, Chiu SP, Wu MJ, Chen PY, Yen JH. Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLoS One. 2012;7:e43304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98:1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shimano H. Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog Lipid Res. 2001;40:439–452 [DOI] [PubMed] [Google Scholar]
- 44. Esau C, Davis S, Murray SF, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98 [DOI] [PubMed] [Google Scholar]