Abstract
Children with monocarboxylate transporter 8 (MCT8) deficiency lose weight, even when adequately nourished. Changes in serum markers of thyroid hormone (TH) action compatible with thyrotoxicosis suggested that this might be due to T3 excess in peripheral tissues. Mct8-deficient mice (Mct8KO) replicate the human thyroid phenotype and are thus suitable for metabolic studies so far unavailable in humans. In the current work, compared with wild-type (Wt) mice, Mct8KO mice were leaner due to reduced fat mass. They tended to use more carbohydrates and fewer lipids during the dark phase. Mct8KO mice had increased total energy expenditure (TEE) and food and water intake, with normal total activity, indicating hypermetabolism. To determine whether this is due to the high serum T3, we studied mice deficient in both Mct8 and deiodinase 1 (Mct8D1KO) with serum T3 similar to Wt mice and Wt mice given L-T3 to raise their serum T3 to the level of Mct8KO mice. Contrary to Mct8KO, Mct8D1KO mice had similar fat mass, TEE, and food intake as their D1KO littermates, whereas T3-treated Wt mice showed increased food intake and TEE, similar to Mct8KO mice. In skeletal muscle, Mct8KO mice had increased T3 content and TH action and increased glucose metabolism, which improved in Mct8D1KO mice. These studies indicate that the high serum T3 in MCT8 deficiency increases the TEE and fails to maintain weight despite adequate calorie intake. This is mediated by tissues that are not predominantly MCT8 dependent for TH transport, including skeletal muscle. Normalizing serum T3 level by deleting deiodinase 1 corrects body composition and the metabolic alterations caused by the MCT8 deficiency.
Mutations in the monocarboxylate transporter 8 (MCT8; SLC16A2) gene, coding for a specific thyroid hormone (TH) cell membrane transporter (1), have been recognized to cause a severe form of X-linked mental retardation and psychomotor impairment in more than 200 young males. The syndrome, known also as the Allan-Herndon-Dudley syndrome, comprises severe cognitive deficiency, truncal hypotonia, and poor head control, progressive spastic quadriplegia, diminished muscle mass with weakness, joint contractures, and dystonia (2, 3). However, most patients demonstrate a failure to thrive and an inability to gain weight. Their weight is below the third percentile, and often they require gastric tube feeding (4, 5). In addition to the psychomotor retardation, patients with MCT8 deficiency display a characteristic combination of thyroid function test abnormalities that include high serum concentration of T3, low levels of T4 and rT3, and a normal or slightly elevated concentration of TSH (6, 7).
Although the neurological impairment causing difficulties with feeding could contribute to the inability to gain weight, the hypermetabolism caused by TH excess in some peripheral tissues may play an important role. This was suggested by the responses of several TH-responsive serum markers, such as elevated serum SHBG, ammonium and lactic acid levels, and reduced cholesterol concentration. Of note, three patients showed weight gain closely correlated with the decline of T3 while on treatment with diiodothyropropionic acid (8) or with combined L-T4 and propylthiouracil treatment (5).
Initial studies in Mct8-deficient (Mct8KO) mice demonstrated TH deprivation in the brain resulting from severe impairment of T3 transport reducing its access into neurons, even though the mice do not fully manifest the neuromuscular deficits observed in humans (9, 10). In contrast, the high serum T3, observed in humans and mice, has been shown to increase its supply to mouse tissues, such as liver, which express other TH transporters, thus making them less dependent than brain on TH transport via Mct8. The high liver T3 content in Mct8KO, accumulated through increased uptake or impaired efflux, produces an increase in deiodinase 1 (D1) and alterations in other markers of TH action (ie, increase in serum alkaline phosphatase and decrease in serum cholesterol and liver glutathione S transferase-α2 mRNA) (9, 10). This repetitive cycle of increased liver D1 expression stimulated by T3 is in part responsible for the high serum T3 level and contributes to the low serum T4 concentration. By generating mice with combined Mct8 and D1 deficiencies (Mct8D1KO), we showed that absence of D1 corrected the serum T3 and rT3 abnormalities of Mct8 deficiency and increased the serum T4 concentration, partially correcting the brain depletion of TH (11).
Given that the serum TH abnormalities observed in man have been reproduced in the Mct8KO mouse (9, 10), this mouse model continues to provide insights into the pathophysiology of MCT8 defects. A variable hormonal deficiency among tissues and cell types related to the redundancy of TH membrane transporters raises the possibility that the inability to gain weight observed in patients with MCT8 defects could be due to a TH excess in some peripheral tissues such as muscle. Because direct measurements of metabolism in Mct8-deficient mice and humans are still lacking, we used the Mct8KO mice to determine the whole-body energy homeostasis. To further investigate the metabolic effects of high serum T3 levels in Mct8 deficient mice, we analyzed Mct8D1KO mice, which have normal serum T3 levels, and T3-treated Wt mice, with serum T3 levels similar to Mct8KO mice (Table 1). Thus, we used two complementary experimental approaches to determine the role of excess serum T3 in the hypermetabolism of Mct8 deficiency and the contribution of T3 generated from T4 in peripheral tissues.
Table 1.
Serum Thyroid Function Tests
| Group | TSH, mU/L | T4, μg/dL | T3, ng/dL | rT3, ng/dL |
|---|---|---|---|---|
| Wt | 33.8 ± 6.5 | 3.7 ± 0.2 | 50.7 ± 2.9 | 22.4 ± 1.5 |
| Mct8KO | 74.6 ± 8.1a | 1.1 ± 0.1a | 90.6 ± 2.9a | 3.2 ± 0.4b |
| D1KO | 36.1 ± 7.7 | 5.2 ± 0.1a | 40.1 ± 6.6 | 97.2 ± 6.8a |
| Mct8D1KO | 49.5 ± 7.1c,d | 5.1 ± 0.3a,e | 40.5 ± 3.7e | 17.4 ± 2.1d |
| Wt+T3 | <10a,e | <0.25a,e | 98.6 ± 5.2a | <2.5a |
Data expressed as mean ± SE. Eight to 10 mice were used per group.
P < .001, significant differences in comparison with the Wt.
P < .01, significant differences in comparison with the Wt.
P < .05, significant differences in comparison with the Wt.
P < .05, significant differences when comparing Mct8D1KO or Wt+T3 with Mct8KO mice.
P < .001, significant differences when comparing Mct8D1KO or Wt+T3 with Mct8KO mice.
Materials and Methods
Three separate experiments were performed.
Experiment 1
Body composition was measured by dual-energy x-ray absorptiometry (DEXA) in wild-type (Wt) and Mct8KO mice, five to eight mice for each group. They were subsequently placed in metabolic cages for determination of total energy expenditure (TEE), respiratory exchange ratio (RER), total activity, and food and water intake.
Experiment 2
To determine the role of the high serum T3 levels on the hypermetabolism associated with Mct8 deficiency, studies were repeated in mice deficient in both Mct8 and D1 (Mct8D1KO), in which serum T3 is not different from that of Wt mice and D1KO littermates, five to eight mice for each group. The results were compared with those obtained in experiment 1.
Experiment 3
Wt mice were treated with T3 to increase the serum T3 levels to those of Mct8KO mice. Five Wt mice received L-T3 in the drinking water at a concentration of 220 ng/mL in 0.01% BSA solution. This treatment was given for 21 days, and for the last 6 days, from the 15th to the 21st day, the mice were placed in metabolic cages. The mice drank on average 11.58 mL/d per 100 g body weight (BW) of water, equating to approximately 2.5 μg per 100g BW of T3 per day.
Experimental animals
Procedures performed in mice were approved by the University of Chicago Institutional Animal Care and Use Committee in which animals were housed and experiments were carried out. Animals were housed in temperature- (22 ± 2°C) and light (12 h light, 12 h dark cycle; lights on at 6:00 am)-controlled conditions and had free access to food and water. Mct8KO, Mct8D1KO, and D1KO mice were generated and genotyped as described (9, 11). Specifically, Wt and Mct8KO male mice were littermates as were D1KO and Mct8D1KO male mice. Because Mct8 is located on the X-chromosome, this was achieved by mating heterozygous Mct8+/− females with Wt males, whereas Mct8+/−D1−/− females were crossed with D1−/− males. Each experiment was performed using different groups of 10- to 12-week-old male C57BL/6J mice. Tissues were obtained at the termination of all experiments.
Body composition
Body composition was measured by DEXA (Lunar PIXImus densitometer system; GE Healthcare) using PIXImus 2 software. The system was calibrated according to the manufacturer's instructions prior to the start of the experiment.
Indirect calorimetry
Animals were placed individually into an eight-cage combined, open circuit indirect calorimetry system (LabMaster system; TSE System), herein referred to as metabolic cages, that measures food and water intake and physical activity continuously as well as O2 uptake and CO2 production at 30-minute intervals (12). Mice were adapted to this environment for 48 hours before starting the recording periods. RER, TEE, and glucose and lipid oxidation were calculated from the O2 consumption (VO2) and CO2 production (VCO2) relative to body weight. In particular, glucose and lipid oxidations were calculated as described (13) with the following modifications: glucose oxidation = 4.55 VCO2 −3.21 VO2, and lipid oxidation = 1.67 VO2 −1.67 VCO2, where VCO2 and VO2 (not corrected for protein oxidation) are in liters per minute per kilogram body weight. Activity monitoring and detection of animal location were performed with infrared sensor pairs arranged in strips for horizontal and vertical activity, detecting every ambulatory movement. The sum of the spontaneous physical activity and the high-frequent activity (equivalent of breathing activity) represents the total activity. Use of infrared sensors for detection of movement allowed continuous recording in both light and dark phases.
Tissue T3 content
Before tissue collection, mice were perfused, under anesthesia, with PBS containing heparin through a needle placed in the left ventricle. Tissues were collected rapidly on dry ice and stored at −80°C. T3 was extracted from muscle and brown adipose tissue using a method described by Morreale de Escobar et al (14), and T3 content was measured by RIA as previously detailed (see supplemental data in Reference 15). Recovery was monitored by the addition of labeled T3 before tissue extraction.
Measurement of tissue mRNAs
Muscle (soleus and gastrocnemius), brown adipose tissue, and cerebrum were collected from 8–10 mice per group, Wt, Mct8KO, D1KO, Mct8D1KO, and T3-treated Wt, to study gene expression. Methods for total RNA extraction from tissue, reverse transcription, and real-time quantitative PCR have been described previously (9). The oligonucleotide primers were designed to cross introns. Primer sequences used for the real-time quantitative PCR are provided in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Results are expressed relative to those in the Wt mice and normalized to RNA polymerase II (RpII) mRNA (16).
Determination of deiodinase 2 (D2) activity
D2 enzymatic activity was performed as described (15) with the following modifications: 100 μg of tissue homogenates (homogenized with Bullet Blender; Next Advance Inc) in 100 μL reaction mixture containing 0.1 M phosphate buffer (pH 7), 1 mM EDTA, 20 mM dithiothreitol, 1 mM propylthiouracil, 100 000 cpm [125I]-T4, and 2 nM unlabeled T4 were incubated at 37°C for 1 hour. Saturating levels of unlabeled T3 (1 μM) were added to the reaction mixture to inhibit the deiodinase 3 enzyme. The enzymatic activity was expressed in femtomoles per hour and milligrams of protein and was corrected for nonenzymatic deiodination observed in the tissue-free controls.
Measurement of serum thyroid function tests
Serum total T4, T3, rT3, and TSH concentrations were measured by RIAs as detailed elsewhere (15).
Statistical analysis
All results are expressed as mean ± SEM. Statistical analysis was performed using a 2-tailed Student's t test for unpaired observations (comparison of values obtained in two groups) or 1-way ANOVA followed by a Newman-Keuls multiple comparison test (comparison among three or more groups). P ≥ .05 was considered not to be significant (NS).
Results
DEXA study
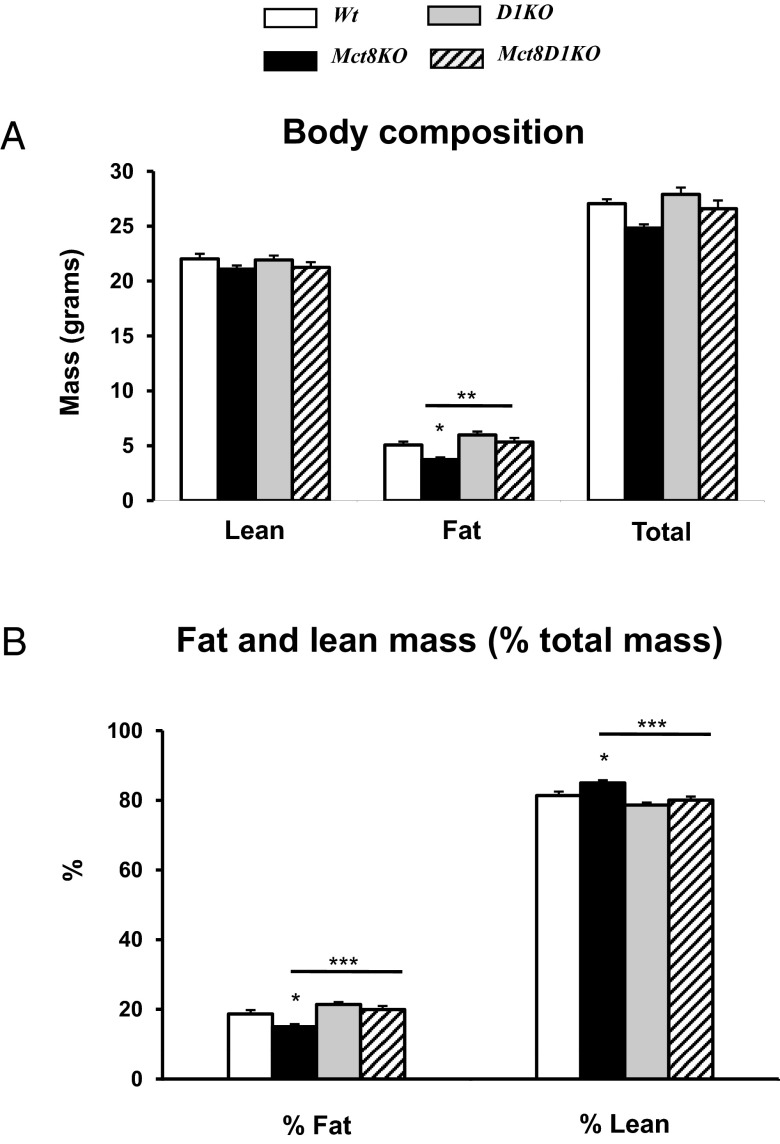
Body composition measurements indicated Mct8KO were leaner than Wt mice. Total body mass was 24.8 ± 0.4 and 27.0 ± 0.4 g in the Mct8KO and Wt mice, respectively (Figure 1A). Although these values were not significantly different, Mct8KO mice had reduced fat mass (15.0% ± 0.8% vs 18.6% ± 1.2%, P < .05) and increased lean mass (85.0% ± 0.8% vs 81.4% ± 1.2%, P < .05) as percentage of total body mass compared with Wt littermates (Figure 1B). In contrast, Mct8D1KO mice with combined Mct8 and D1 deficiency exhibited normal body composition compared with D1KO littermates, having similar total body mass (26.6 ± 0.8 vs 27.9 ± 0.6 g), fat (19.9% ± 1.0% vs 21.4% ± 0.7% total mass) and lean mass (80.1% ± 1.0% vs 78.6% ± 0.7% total mass). All these parameters did not differ from those of Wt mice (Figure 1, A and B).
Figure 1.
DEXA study of Wt, Mct8KO, D1KO, and Mct8D1KO mice. A, Body composition. B, Fat and lean mass expressed as percentage of total body mass. Genotypes are indicated. Asterisks represent P values by ANOVA and Newman-Keuls test. Significant differences compared with Wt animals are above the SEM bars. Comparisons of Mct8KO and Mct8D1KO are indicated by horizontal lines. *, P < .05; **, P < .01; ***, P < .001.
Metabolic cage study
After placement in the metabolic cages, mice were allowed to acclimatize for 2 days with no recording. Subsequently, we gathered data for the following 5 days but used for analyses only data obtained from the last 3 consecutive days. For all considered parameters, results represent mean of the values obtained during the light phase (6:00 am to 6:00 pm), dark phase (6:00 pm to 6:00 am) and during the whole 24-hour period.
All measured parameters had a clear diurnal rhythm in all animal groups, with the highest values registered during the dark phase, corresponding to the period of major activity of the animals. As an example, a TEE flow chart is shown in Figure 2A.
Figure 2.
Metabolic cage study of Wt, Mct8KO, D1KO, and Mct8D1KO mice. A, An example of total TEE flow chart. B, Average 24-hour food intake. C, Average 24-hour water intake. D, Average TEE. Genotypes are indicated. Symbols indicating statistical significances are as indicated in the legend to Figure 1.
Food and water intake
The Mct8KO mice maintained on regular chow were significantly hyperphagic relative to Wt littermates (Figure 2B). Mct8KO mice consumed 21% more regular chow per day than controls. The hyperphagia was associated with increased water intake, 25% more than Wt mice per 24 hours (Figure 2C). Increases in both food and water intake were observed only during the dark phase of the light-dark cycle (data not shown). Food and water intake in mice with Mct8D1KO did not differ from the intakes of the D1KO littermates or Wt mice (Figure 2, B and C).
Motor activity
There were no differences in the mean levels of total activity between Mct8KO and Wt mice in both light and dark phases [average per day: 38318 ± 3756 and 37943 ± 5674 arbitrary unit (AU), respectively]. The same held true for Mct8D1KO mice, which exhibited levels of activity (average per day: 34432 ± 2583 AU), similar to that of Wt mice and D1KO littermates (30531 ± 2701 AU).
Energy expenditure
Mct8KO mice had greater TEE than Wt mice in all periods of the day (Figure 2A), with daily mean values of 19.6 ± 0.8 vs 16.9 ± 0.7 kcal/h·kg BW of Wt (P < .01) (Figure 2D). The association of higher values of TEE with normal levels of motor activity suggests that the increase is not secondary to hyperactivity. TEE normalized in the Mct8D1KO mice during the dark phase and was not different than that in D1KO mice. However, TEE of both Mct8D1KO and D1KO mice was lower than that of Wt mice during the light phase (Figure 2A) with a consequent lower daily mean value (14.6 ± 0.5 and 11.1 ± 0.3 kcal/h·kg BW in the D1KO and Mct8D1KO mice, respectively, Figure 2D), a finding likely related to the absence of D1.
RER and substrate oxidation
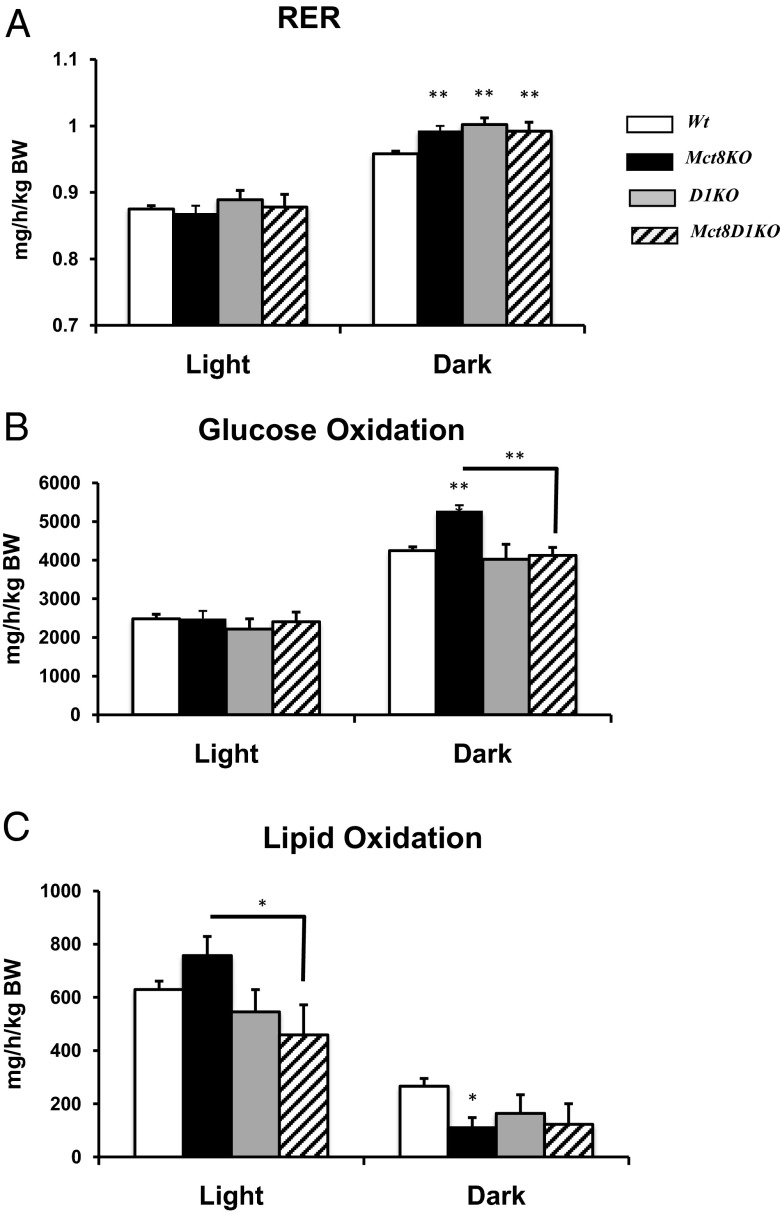
To evaluate whether there were differences in substrate oxidation in mice lacking Mct8, we measured the RER, an indicator of metabolic fuel preference. The 24-hour average RER was similar in all four genotypes; thus, we further analyzed RER in the light and dark phases separately and found significant higher RER in Mct8KO, D1KO, and Mct8D1KO during the dark phase (Figure 3A).
Figure 3.
RER and substrate oxidation. A, RER, B, Glucose oxidation and C, Lipid oxidation in Wt, Mct8KO, D1KO, and Mct8D1KO mice. Genotypes are indicated. Symbols indicating statistical significances are as indicated in the legend to Figure 1.
Further analysis of glucose and lipid oxidation shows that during the dark phase, there is a significant increase (P < .001) in glucose oxidation and decreased (P < .05) lipid oxidation in Mct8KO vs Wt littermates (Figure 3, B and C). In the combined Mct8D1KO, the glucose oxidation normalized (Figure 3B), whereas lipid oxidation remains lower, although it did not reach significance (Figure 3C). The RER in Mct8KO, D1KO, and Mct8D1KO during the dark phase was close to 1, indicating that these mice are preferentially using carbohydrates as fuel during this interval.
TH status and glucose metabolism in skeletal muscle (SM), brown adipose tissue (BAT), and cerebrum
The finding that the normalization of serum T3 levels in the Mct8D1KO mice ameliorated the phenotype of Mct8 deficiency indicates that the perturbation of TH status could be in part responsible for the observed hypermetabolism. It is known that TH plays an important role in energy expenditure and basal metabolic rate (17) both centrally and in periphery. Therefore, to investigate further, we assessed TH status and glucose metabolism in SM, BAT, and cerebrum.
Effect on SM
Data on muscle T3 content are shown in Figure 4A. The SM of Mct8KO mice contained 30% more T3 than Wt animals: 22.2 ± 2.1 vs 16.6 ± 1.6 pg/mg protein, respectively. Deletion of D1 reduced muscle T3 content, making the values in Mct8D1KO mice intermediate between those in Wt and Mct8KO mice. The muscle T3 content was 13.8 ± 0.9 and 18.1 ± 1.4 pg/mg of protein in the D1KO and Mct8D1KO mice, respectively. In general and for all genotypes, skeletal muscle T3 content followed the concentrations observed in serum, indicating that TH uptake by skeletal muscle can occur independently of Mct8. In fact, we found that Wt mice have higher expression of Mct10 than Mct8 in muscle, both soleus (4.6 times) and gastrocnemius (40 times), and likely compensated for the absence of Mct8 in Mct8KO mice (Supplemental Figure 1).
Figure 4.
Measurements in skeletal muscle. A, T3 content. B, Gene expression in soleus. C, Gene expression in gastrocnemius. Genotypes are indicated. Symbols indicating statistical significances are as in the legend to Figure 1. In addition, # and ##, P < .05 and P < .01, respectively, calculated by a 2-tailed Student's t test compared with Wt.
We next measured the SM mRNA levels of well-documented markers of TH action and genes involved in glucose metabolism. These included the uncoupling protein-3 (Ucp3); the uncoupling protein-2 (Ucp2); the major isoforms of myosin heavy chain (Mhc); hairless (Hr); monocarboxylate transporter 1 (Mct1) and 4 (Mct4), which facilitate lactic acid and pyruvate transport; and pyruvate kinase muscle isoform (Pkm). Gene expression was studied separately in two different types of mouse SM: soleus, with predominantly slow-oxidative type I fibers, which control slow movement and posture, and gastrocnemius, with predominantly fast-glycolytic type IIB fibers, which control rapid movement (18). As expected on the basis of the tissue T3 content, compared with Wt mice, Mct8KO mice had evidence of increased TH action (Figure 4, B and C) with increased Hr expression in both muscle types. Mct8KO mice exhibited in the soleus a reduction in MhcI with a significant increase in MhcIId mRNA levels, and in the gastrocnemius a reduction in MhcIIa with similar MhcIId as compared with the Wt mice (Figure 4C). These differences were more pronounced when the results were expressed as ratio between IId/I and IId/IIa isoforms of Mhc (Figure 4, B and C).
The results are in agreement with the higher muscle T3 content producing a hyperthyroid state in muscle of Mct8-deficient mice because increasing levels of TH induce a consecutive shift from MhcI to MhcIIa, to MhcIId, and to MhcIIb, stimulating the next gene in the sequence and reducing or shutting down the expression of the former (18–20). We did not find any difference on Ucp2, MhcIIb mRNA levels between mice of two genotypes (data not shown). In soleus Ucp3 mRNA was increased by 68% in Mct8KO mice as compared with the Wt littermates, whereas it was unchanged in gastrocnemius (data not shown). For lactate transporters Mct1 expression was increased in soleus and Mct4 expression was increased in gastrocnemius of Mct8KO mice compared with Wt, indicating increased shuffling of lactate between muscle fibers. Although Mct1 facilitates the uptake of lactate to be used as a respiratory fuel in soleus, which is primarily oxidative, Mct4 is the main transporter for efflux of lactic acid from a predominantly white fiber type muscle such as gastrocnemius, which is primarily glycolytic. Mct4 overexpression in Mct8KO mice indicates relative increased lactic acid generation through glycolysis (21), as supported by the concomitant increased expression of Pkm in gastrocnemius in these mice (Figure 4C).
D1 deletion corrected the abnormalities related to Mct8 defects. Mct8D1KO mice showed in soleus normal Hr, Ucp3, and Mct1 mRNA levels and normal MhcIId to MhcI mRNA ratio (Figure 4B). Hr and Mct4 expression in gastrocnemius was no different from Wt in Mct8D1KO (Figure 4C). It is of interest to note that in the absence of D1, in gastrocnemius the mRNA levels of both MhcIIa and MhcIId were higher than in Wt and Mct8KO mice. The resulting ratio of MhcIId to MhcIIa is similar in these two genotypes and higher than the Wt. This is not well understood and needs further investigation.
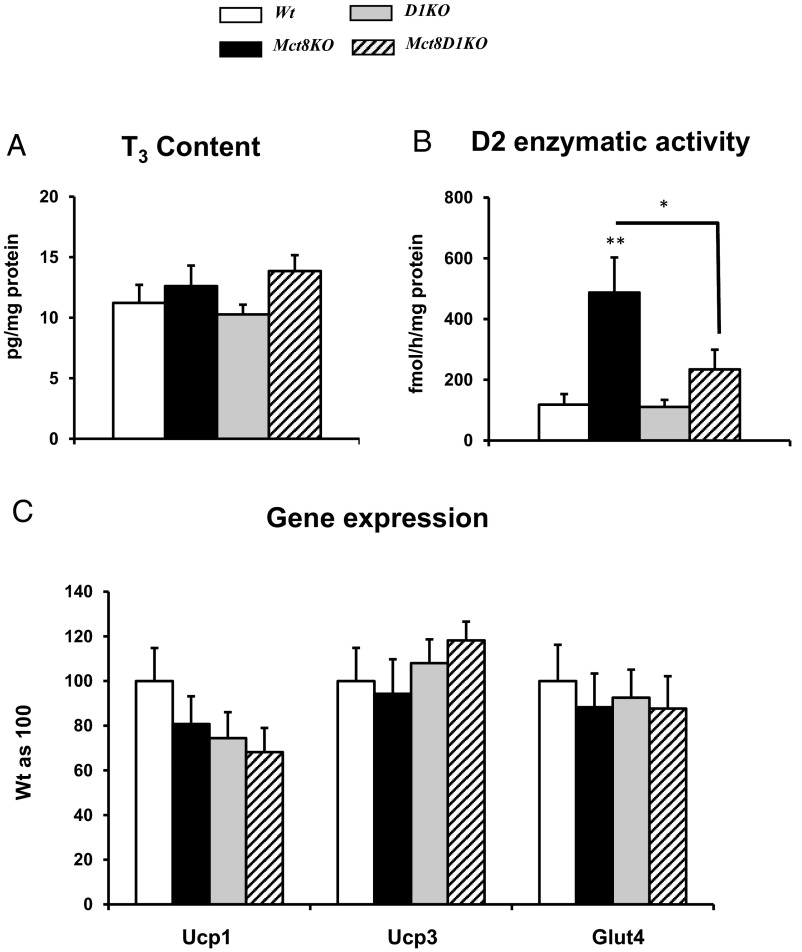
Effects on BAT (Figure 5)
Figure 5.
Measurements in BAT. A, T3 content. B, D2 enzymatic activity. C, Gene expression. Genotypes are indicated. Symbols indicating statistical significances are as in the legend to Figure 1.
To evaluate the TH status and action in BAT, we measured T3 content and expression of TH-regulated genes, namely the uncoupling protein-1 (Ucp1), Ucp3, and Glut4. D2 enzymatic activity was also measured as an indicator of TH status. No differences were found in T3 content and in the expression of all studied genes in all genotypes (Figure 5, A and C). Whereas D2 activity was increased in the Mct8KO mice as compared with the Wt controls (Figure 5B), the differences in D2 activity were abrogated in Mct8D1KO mice, probably as consequence of increased T4 availability.
Effect on cerebrum (Supplemental Figure 2)
The TH status of the brain of Mct8KO mice has been previously reported (9–11), showing decreased T3 content and expression of TH target genes. Considering the finding of overall increased glucose oxidation in Mct8KO mice (Figure 3B) and the fact that the brain is a major site for glucose consumption, we measured the expression of genes involved in glucose transport and metabolism: glucose transporter Glut1, Mct1, and Mct2, which shuffle lactic acid between astrocytes and neurons (21), and Ucp2, and Pkm, which is the predominant Pk isoform in brain (Supplemental Figure 2). Glut1, Ucp2, and Mct1 show similar expression in the two genotypes, with Mct2 and Pkm showing decreased expression in the Mct8KO mice compared with Wt littermates. This indicates similar or decreased glucose metabolism in the cerebrum consistent with the observed overall brain hypothyroidism.
T3 treatment
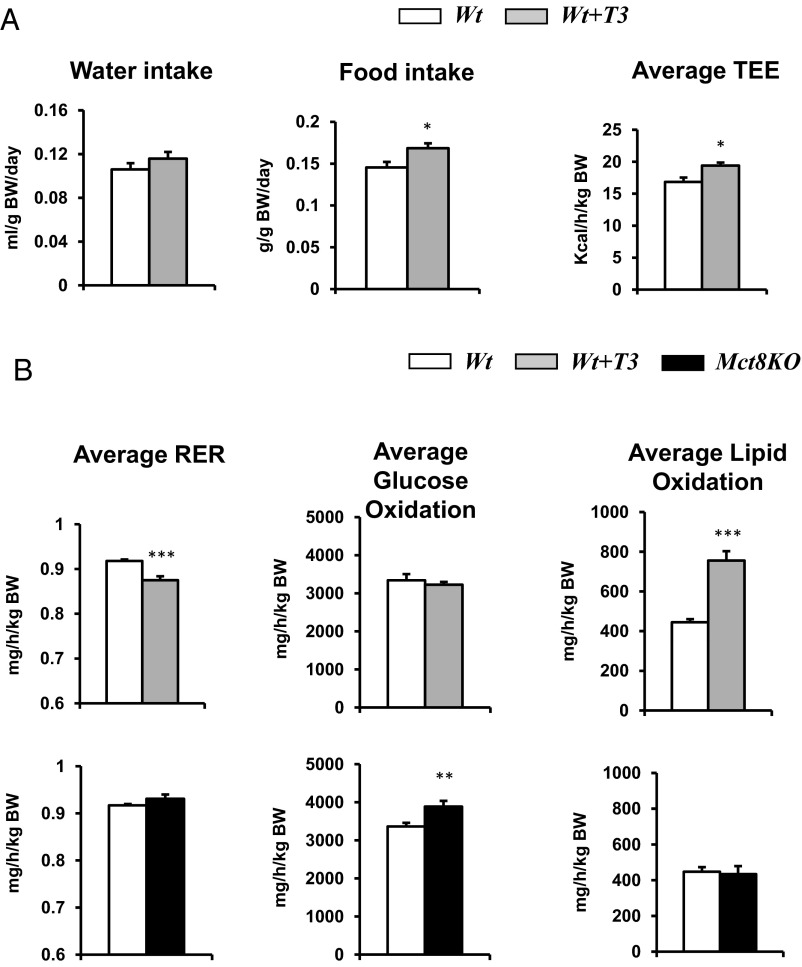
To assess whether the observed differences in the metabolic profile of the Mct8KO mice correlate with peripheral hyperthyroidism, their baseline serum T3 levels (90.6 ± 2.9 ng/dL) were replicated in Wt mice by treating them with T3 (98.6 ± 5.2 ng/dL) (see Table 1). T3-treated Wt mice displayed increased food and water intake, increased energy expenditure (Figure 6A), together with normal total activity (data not shown), similar to Mct8KO mice. However, in Wt T3-treated mice, 24-hour average RER was decreased, glucose oxidation was normal, and lipid oxidation was increased compared with Wt untreated mice (Figure 6B). For Mct8KO mice, 24-hour average RER and lipid oxidation were normal, whereas glucose oxidation was increased. This indicates that some aspects of the hypermetabolism observed in the Mct8KO mice are replicated in the Wt T3-treated mice, whereas there are some differences in substrate preference.
Figure 6.
Metabolic cage study of Wt T3-treated mice. A, Average 24-hour food intake, water intake, and TEE in Wt T3-treated mice compared with Wt. B, Average 24-hour RER and glucose and lipid oxidation in Wt T3-treated and Mct8KO mice compared with Wt. Genotypes are indicated. Statistical analyses were performed by a 2-tailed Student's t test for unpaired observations. *, P < .05; **, P < .01; ***, P < .001.
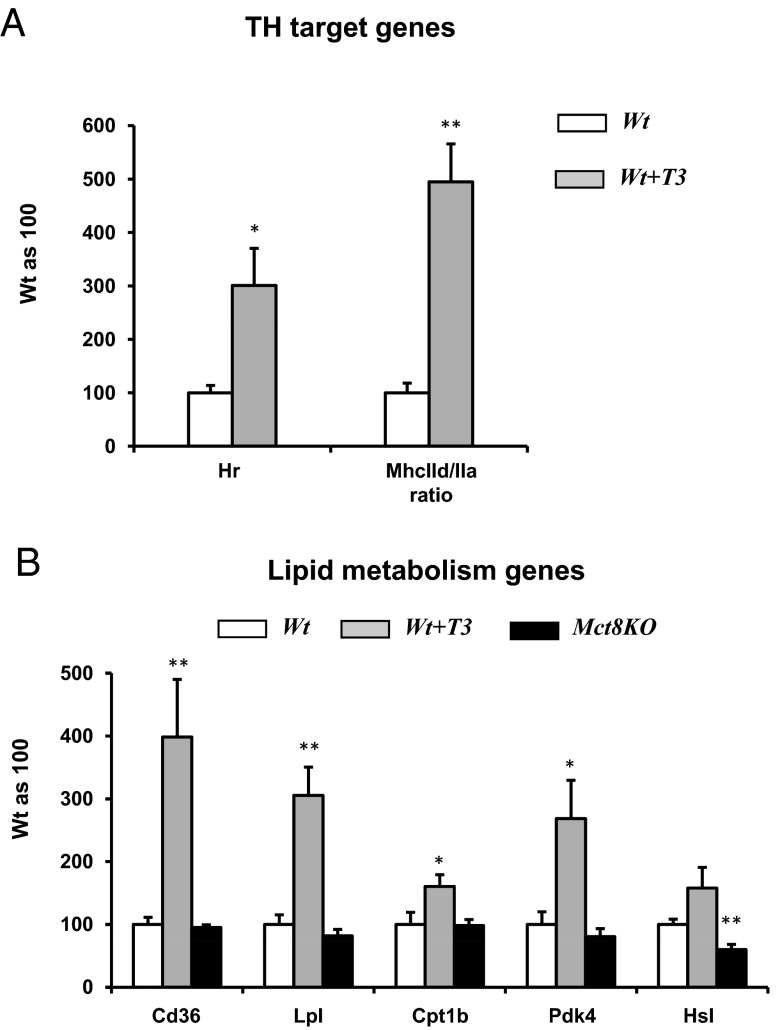
The study of gene expression in the Wt T3-treated mice showed increase in Hr expression and MhcIId to MhcIIa ratio in gastrocnemius, confirming the hyperthyroid status obtained through T3 treatment (Figure 7A). Considering the finding of increased lipid oxidation in Wt T3-treated mice, the expression of genes involved in lipid metabolism (22) was studied in gastrocnemius (Figure 7B). Some of the genes studied include fatty acid translocase/Cd36 (Cd36), which facilitates fatty acid uptake into cells; lipoprotein lipase (Lpl); carnitin palmitoyltransferase 1b (Cpt1b), which is required to transport long-chain fatty acyl-CoAs from the cytoplasm into the mitochondria for β-oxidation; pyruvate dehydrogenase kinase 4 (Pdk4), which when increased determines the preference for lipid over glucose oxidation; and hormone-sensitive lipase (Hsl) (23, 24). When compared with Wt littermates, T3-treated mice had increased expression of all genes studied, although not significant for Hsl, in agreement with the finding of increased lipid oxidation in T3-treated mice. Mct8KO at baseline had normal expression of Cd36, Lpl, Cpt1b, and Pdk4 but significantly low expression of Hsl, the latter one possibly reflecting, at least in part, the decreased lipid oxidation observed during the dark phase (Figure 3C).
Figure 7.
Gene expression in gastrocnemius of Wt T3-treated mice. A, TH target genes in Wt T3-treated mice compared with Wt. B, Lipid metabolism genes in Wt T3-treated and Mct8KO mice compared with Wt. Statistical analyses were performed by a 2-tailed Student's t test for unpaired observations. *, P < .05; **, P < .01.
Discussion
Several observations suggest that, in patients with inactivating mutations of MCT8 gene, failure to gain weight may be the consequence of a hypermetabolic state resulting from elevated serum T3 levels. A recent publication showed a close correlation between T3 levels and body mass index in two twins with MCT8 deficiency, who showed an improvement in an SD score of weight with the decline of T3 during treatment with the TH analog diiodothyropropionic acid (8). A similar observation was reported by Wemeau et al (5) in a child with MCT8 deficiency in whom T3 generation was decreased during replacement therapy with levothyroxine by treatment with propylthiouracil, which reduces the conversion of T4 to T3 by D1 peripherally. The undernourished 16-year-old boy had previously difficulty to maintain his weight, even after placement of a gastric feeding tube. When T3 levels normalized, there was a significant weight gain. The reduction of serum T3 also resulted in a normalization of the low cholesterol and the high SHBG levels. These serum alterations, which are markers of TH action in peripheral tissues, have also been reported in other patients with MCT8 gene mutations (4, 25). The increase of muscle metabolism in MCT8-deficient patients, as assessed by serum markers, is in agreement with the data found in mice.
Because detailed metabolic evaluation is lacking in individuals with MCT8 defects, we used the mouse model of Mct8 deficiency to study the whole-body energy homeostasis in this condition. Previous studies have showed that the Mct8KO mice have high serum T3 levels (Table 1) and the other thyroid function test abnormalities described in humans with MCT8 gene mutations (9, 10). In addition, they present a combination of tissue-selective hypothyroidism and hyperthyroidism (9, 10).
The present work shows that the Mct8KO mice are lean, have increased metabolism, and are hyperphagic, with the tendency to use as fuel more carbohydrates and less lipids during the dark phase and have higher RER during this time. The leanness in the presence of a normal activity is most likely a consequence of the increased energy expenditure, whereas the food intake is increased for substrate replenishment.
Of note, D1KO mice had normal food intake but decreased TEE compared with Wt; however, they have normal body composition paradoxically without an increase in fat mass, as one would predict. The reason for this finding is unclear. D1 was shown to have a functional role in white adipose tissue, being involved in the metabolism and/or accumulation of adipose tissue, with a stimulatory effect of leptin on D1 activity (26). Therefore, the relative lack of increased fat mass in D1KO mice could be due to this role of D1 in white adipose tissue.
The study of the Wt mice treated with L-T3 to have serum T3 levels similar to those of untreated Mct8KO mice showed that some of the metabolic parameters, such as increased food and water intake, increased energy expenditure in a setting of normal level of total activity, were similar in these groups of mice. However, other parameters were different between the two groups, such as the 24-hour average RER, and glucose and lipid oxidation. In agreement with the finding of increased lipid oxidation in Wt T3-treated mice, the expression of Cd36, Lpl, Cpt1b, and Pdk4, genes involved in lipid metabolism, was increased. The decreased Hsl expression observed in Mct8KO mice possibly reflects the decreased lipid oxidation observed during the dark phase. Some of the observed differences between the Wt T3-treated and Mct8KO mice could be in part due to different thyroid function tests because T3-treated Wt mice have undetectable TSH and T4 compared with the Mct8KO mice (Table 1). Also, we cannot exclude the possibility that the unique tissue-specific TH availability characteristic of the MCT8 defect has intricate consequences on the metabolic homeostasis, compared with global hyperthyroidism. This requires further studies.
The involvement of TH in the regulation of energy expenditure and the metabolic rate is well known (17), being in part dependent on central control from the brain as well as on peripheral tissues. As previously shown (27), the TH status of the brain can modulate the metabolic status in peripheral tissues through sympathetic and parasympathetic pathways. Considering the central hypothyroidism in Mct8KO mice (9, 10), compared with a relative state of hyperthyroidism in Wt T3-treated mice based on undetectable TSH, the autonomic outflow of the brain to metabolic organs is expected to be different. In fact, our data on gene expression involving glucose transport and metabolism in cerebrum (Glut1, Mct1 and Mct2, Ucp2, and Pkm) indicate similar or decreased glucose metabolism in the cerebrum of Mct8KO mice, consistent with the previously reported brain hypothyroidism. Although it is likely that brain metabolism contributes to the global metabolism measured in these mice, it seems that peripheral tissues such as muscle have a bigger contribution because the overall TEE is actually increased in Mct8KO mice.
Muscle and BAT are peripheral tissues playing important roles on the metabolism and energy expenditure. The higher T3 content in muscle of Mct8KO mice compared with Wt mice was associated with markers of increased TH action including increased Hr gene expression and the switch in the expression of the myosin isoforms. Thus, it seems that as for liver and kidney, Mct8 is not critical for TH transport into muscle, and other transporters can compensate for its lack. This is supported by a recent study showing that in addition to MCT8, other transporters are expressed in human skeletal muscle, including MCT10 (28). In fact, we find that mice have higher expression of Mct10 than Mct8 in muscle and likely compensates for the absence of Mct8 in Mct8KO mice.
The study of genes involved in glucose metabolism showed increased expression of Pkm and Mct4 in the gastrocnemius of Mct8KO mice, indicating increased glycolysis, and efflux of lactic acid, whereas the increased expression of Ucp3 and Mct1 in soleus indicates increased lactate uptake and overall increased mitochondrial oxidative capacity (21). Overall, muscle manifests a hyperthyroid status with increased glucose metabolism and energy expenditure.
Contrary to muscle, BAT seems to respond to the low serum T4 levels of Mct8 deficiency because Mct8KO mice show a significant increase in BAT D2 enzymatic activity. These results are in agreement with the estimated important contribution of D2 to T3 levels in BAT, even at room temperature (29). The finding of similar T3 levels and mRNA level of genes regulated by TH in Mct8KO and Wt mice is likely due to this compensatory increase in D2.
When we performed the same studies in mice deficient in both Mct8 and D1, (Mct8D1KO), which have normal serum T3 levels (Table 1) and normal cerebrum T3 content (11), the body composition and food intake normalized. In the combined Mct8D1KO, the glucose oxidation normalized, whereas lipid oxidation remained lower. The muscle of Mct8D1KO mice showed a euthyroid state in terms of Hr expression and the metabolic genes studied, in agreement with the metabolic cage data and DEXA study. These data support the hypothesis that normalization of serum T3 concentration and that of brain T3 levels can ameliorate the metabolic phenotype of Mct8 deficiency.
In conclusion, this study demonstrates that in MCT8 defects, failure to maintain normal weight despite increased caloric intake is in part due to increased energy expenditure associated with high serum T3 levels. This is true for tissues that are not predominantly MCT8 dependent for TH transport. Skeletal muscle is among these tissues, manifesting thyrotoxic increase in energy expenditure. Also, the tissue-specific thyroid hormone availability characteristic of MCT8 defect plays a role in the observed metabolic homeostasis.
Acknowledgments
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
This work was supported by in part by Grants DK15070, DK091016, and DK020595 from the National Institute of Diabetes and Digestive and Kidney Diseases and the Smile Foundation with support from the Sherman family.
Current address for C.D.C.: Dipartimento di Medicina Clinica e Sperimentale, Sezione di Endocrinologia, Universita' di Pisa, via Paradisa 2, Pisa 56124, Italy.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AU
- arbitrary unit
- BAT
- brown adipose tissue
- BW
- body weight
- D1
- deiodinase 1
- D2
- deiodinase 2
- DEXA
- dual-energy x-ray absorptiometry
- KO
- knockout
- MCT8
- monocarboxylate transporter 8
- RER
- respiratory exchange ratio
- SM
- skeletal muscle
- TEE
- total energy expenditure
- TH
- thyroid hormone
- VCO2
- CO2 production
- VO2
- O2 consumption
- Wt
- wild type.
References
- 1. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40128–40135 [DOI] [PubMed] [Google Scholar]
- 2. Schwartz CE, Stevenson RE. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab. 2007;21:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25(1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herzovich V, Vaiani E, Marino R, et al. Unexpected peripheral markers of thyroid function in a patient with a novel mutation of the MCT8 thyroid hormone transporter gene. Horm Res. 2006;67:1–6 [DOI] [PubMed] [Google Scholar]
- 5. Wemeau JL, Pigeyre M, Proust-Lemoine E, et al. Beneficial effects of propylthiouracil plus L-thyroxine treatment in a patient with a mutation in MCT8. J Clin Endocrinol Metab. 2008;93:2084–2088 [DOI] [PubMed] [Google Scholar]
- 6. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet. 2004;74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friesema EC, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364:1435–1437 [DOI] [PubMed] [Google Scholar]
- 8. Verge CF, Konrad D, Cohen M, et al. Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab. 2012;97(12):4515–4523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043 [DOI] [PubMed] [Google Scholar]
- 10. Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liao XH, Di Cosmo C, Dumitrescu AM, et al. Distinct roles of deiodinases on the phenotype of Mct8 defect: a comparison of eight different mouse genotypes. Endocrinology. 2011;152:1180–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong KE, Szeto FL, Zhang W, et al. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab. 2009;296:E820–E828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishihara K, Oyaizu S, Fukuchi Y, et al. A soybean peptide isolate diet promotes postprandial carbohydrate oxidation and energy expenditure in type II diabetic mice. J Nutr. 2003;133:752–757 [DOI] [PubMed] [Google Scholar]
- 14. Morreale de Escobar G, Pastor R, Obregon MJ, Escobar del Rey F. Effects of maternal hypothyroidism on the weight and thyroid hormone content of rat embryonic tissues, before and after onset of fetal thyroid function. Endocrinology. 1985;117:1890–1900 [DOI] [PubMed] [Google Scholar]
- 15. Ferrara AM, Liao XH, Gil-Ibanez P, et al. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154:2533–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862 [DOI] [PubMed] [Google Scholar]
- 17. Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144 [DOI] [PubMed] [Google Scholar]
- 18. Simonides WS, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid. 2008;18:205–216 [DOI] [PubMed] [Google Scholar]
- 19. Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986;231:597–600 [DOI] [PubMed] [Google Scholar]
- 20. Mahdavi V, Izumo S, Nadal-Ginard B. Developmental and hormonal regulation of sarcomeric myosin heavy chain gene family. Circ Res. 1987;60:804–814 [DOI] [PubMed] [Google Scholar]
- 21. Halestrap AP, Wilson MC. The monocarboxylate transporter family—role and regulation. IUBMB Life. 2012;64:109–119 [DOI] [PubMed] [Google Scholar]
- 22. Jeukendrup AE. Regulation of fat metabolism in skeletal muscle. Ann NY Acad Sci. 2002;967:217–235 [DOI] [PubMed] [Google Scholar]
- 23. Harris RA, Bowker-Kinley MM, Huang B, Wu P. Regulation of the activity of the pyruvate dehydrogenase complex. Adv Enzyme Regul. 2002;42:249–259 [DOI] [PubMed] [Google Scholar]
- 24. Attia RR, Connnaughton S, Boone LR, et al. Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by thyroid hormone: role of the peroxisome proliferator-activated receptor γ coactivator (PGC-1α). J Biol Chem. 2010;285:2375–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boccone L, Mariotti S, Dessi V, Pruna D, Meloni A, Loudianos G. Allan-Herndon-Dudley syndrome (AHDS) caused by a novel SLC16A2 gene mutation showing severe neurologic features and unexpectedly low TRH-stimulated serum TSH. Eur J Med Genet. 2010;53:392–395 [DOI] [PubMed] [Google Scholar]
- 26. Macek Jilkova Z, Pavelka S, Flachs P, Hensler M, Kus V, Kopecky J. Modulation of type I iodothyronine 5′-deiodinase activity in white adipose tissue by nutrition: possible involvement of leptin. Physiol Res. 2010;59:561–569 [DOI] [PubMed] [Google Scholar]
- 27. Fliers E, Klieverik LP, Kalsbeek A. Novel neural pathways for metabolic effects of thyroid hormone. Trends Endocrinol Metab. 2010;21:230–236 [DOI] [PubMed] [Google Scholar]
- 28. Visser WE, Heemstra KA, Swagemakers SM, et al. Physiological thyroid hormone levels regulate numerous skeletal muscle transcripts. J Clin Endocrinol Metab. 2009;94:3487–3496 [DOI] [PubMed] [Google Scholar]
- 29. Bianco AC, Silva JE. Nuclear 3,5,3′-triiodothyronine (T3) in brown adipose tissue: receptor occupancy and sources of T3 as determined by in vivo techniques. Endocrinology. 1987;120:55–62 [DOI] [PubMed] [Google Scholar]