Abstract
Hyperaldosteronism is linked to the development and progression of several different cardiovascular diseases. Angiotensin (Ang) II increases aldosterone secretion and adrenal blood flow. Ang II peptide fragments are produced by various peptidases, and these Angs have diverse and vital physiologic roles. Due to the uncharacteristic vasorelaxation of adrenal arteries by Ang II, we tested the hypothesis that Ang II metabolism contributes to its relaxant activity in adrenal arteries. Metabolism of Angs by bovine adrenal cortical arteries and isolated bovine adrenal vascular cells was measured by liquid chromatography-mass spectrometry. The primary Ang metabolites of adrenal arteries are Ang III and Ang (1–7), with Ang IV produced to a lesser extent. Bovine microvascular endothelial cells produced a similar metabolic profile to adrenal arteries, whereas bovine adrenal artery smooth muscle cells exhibited less metabolism. In preconstricted adrenal arteries, Ang II caused relaxation in picomolar concentrations and constrictions at 10nM. Ang-converting enzyme 2 inhibition augmented this relaxation response, whereas aminopeptidase inhibition did not. Ang III was equipotent to Ang II in relaxing adrenal arteries. Ang IV did not cause relaxation. Nitric oxide synthase inhibition enhanced Ang II-induced constriction of adrenal arteries. Aminopeptidase inhibition increased the concentration range for Ang II-induced constriction of adrenal arteries. Ang III and Ang IV did not change the basal tone but caused constriction of adrenal arteries with nitric oxide synthase inhibition. These data indicate that Ang II metabolism modulates the vascular effects of Ang II in the adrenal vasculature.
Elevated circulating levels of aldosterone are linked to the development and progression of several different cardiovascular diseases, including hypertension, congestive heart failure, chronic kidney disease, and stroke (1). Aldosterone, a primary effector molecule of the Ang-aldosterone system, is a mineralocorticoid produced by zona glomerulosa cells of the adrenal gland and is involved in the control of water and electrolyte balance (2). Some forms of hypertension are associated with increased aldosterone secretion and salt and water retention. For example, treatment of patients with resistant hypertension with a mineralocorticoid receptor antagonist significantly lowers blood pressure (3). Evidence is also emerging for nonclassical actions of aldosterone in the heart and blood vessels that result in oxidative stress, inflammation, and fibrosis (4). Thus, understanding aldosterone release has important implications for cardiovascular health.
Aldosterone secretion is stimulated by 3 primary factors: angiotensin II (Ang II), potassium, and adrenocorticotropic hormone. These factors directly stimulate aldosterone biosynthesis in zona glomerulosa cells. In addition, they may partially regulate aldosterone secretion by increasing adrenal blood flow (5–7). Control of adrenal blood flow occurs by regulation of vascular resistance of the adrenal cortical arteries, which are the only resistance arteries in the adrenal gland (8).
Although Ang II is still considered the principal effector peptide of the renin-angiotensin system, there is growing evidence that peptide fragments of Ang II also have diverse and vital physiologic roles. The sequential removal of single amino acids from the N terminus of Ang II by aminopeptidases produces Ang III and Ang IV. There are 2 aminopeptidases, aspartyl aminopeptidase A (APA) and glutamyl APA, that convert Ang II to Ang III by the N-terminal cleavage of aspartic acid (9). Ang III is equipotent to Ang II in stimulating aldosterone release but is a less potent vasoconstrictor (10, 11). Ang III clearance from the plasma occurs at a significantly greater rate than Ang II (12, 13). Thus, the physiological effects of Ang III are likely localized to the site of production.
Ang III is metabolized by aminopeptidase N to Ang IV by the N-terminal cleavage of arginine (14). Although aminopeptidase N is considered the predominant enzyme that produces Ang IV, argininyl aminopeptidase (also termed aminopeptidase B), is another peptidase that converts Ang III to Ang IV (15). Ang IV possesses pressor and renal vasoconstrictor effects, although with less potency than Ang II. However, conflicting results exist regarding whether these effects are mediated by a novel angiotensin type 4 (AT4) receptor, the metallopeptidase insulin-regulated aminopeptidase (16), or by AT1 receptors (17).
Ang (1–7) is produced from Ang II by the C-terminal cleavage of phenylalanine. Ang-converting enzyme 2 (ACE2) is a carboxypeptidase that removes a single amino acid from the N terminus from both Ang I and Ang II, producing Ang (1–9) and Ang (1–7), respectively (18). ACE2 is predominantly expressed in the vascular endothelium of the heart and kidney (19) but is also expressed in a wide variety of tissues (20). Other than ACE2, prolylcarboxypeptidase (also termed angiotensinase C) and prolylendopeptidase also metabolize Ang II to Ang (1–7) (21, 22). Ang (1–7) exerts effects that often counterbalance the actions of Ang II, such as vasodilation and antiproliferation (23). These effects are mediated by the G protein-coupled Mas receptor (24).
Little is known regarding the Ang fragment Ang (2–7). Ang (2–7) exhibits a slight pressor effect in healthy humans (25), although its endogenous production and physiologic role are yet to be determined. Ang (3–7) is an Ang fragment that has been identified in rat brain tissue (26). Ang (3–7) induces a pressor effect in rats when delivered to the rostral ventrolateral medulla and has been proposed to be a potential neuromodulator of the renin-angiotensin system (27). Ang (1–7), Ang II, and Ang IV can be converted to Ang (3–7) by several aminopeptidases and carboxypeptidases (21, 28–33).
Additionally, Ang II is metabolized to Ang A by the decarboxylation of the N-terminal aspartic acid converting it to alanine (34). This novel Ang II metabolite is detectable in human plasma and is increased in patients with renal failure. Ang A has similar AT1 and AT2 receptor agonist activity as Ang II (35). The significance of this association between Ang A and renal failure is still not understood.
Ang II, a characteristically potent vasoconstrictor, primarily vasodilates the adrenal vasculature. This primary vasodilation in adrenal arteries is unique to the adrenal circulation and has potentially important implications in the regulation of adrenal blood flow and steroidogenesis after stimulation of Ang II. Our initial studies demonstrated that this Ang II dilation response is mediated by activation of endothelial cell AT2 receptors and subsequent nitric oxide (NO) production (36), with Ang (1–7) having an insignificant role in this dilation response (37). With growing evidence of the importance of Ang metabolites, we examined a potential role of other Ang II metabolites (eg, Ang III and Ang IV) in the dilation response to Ang II in adrenal arteries. The goal of these studies was to 1) simultaneously identify the Ang II metabolites produced by bovine adrenal cortical arteries using an improved liquid chromatography-tandem mass spectrometry (LC-MS/MS) method that detects the novel Ang fragments Ang A, Ang (2–7), and Ang (3–7), 2) fully characterize the constriction and relaxation activity of these Ang metabolites, and 3) examine the role of these various metabolic pathways for Ang II using various peptidase inhibitors.
Our results demonstrate that aminopeptidase metabolism of Ang II to Ang III preserves the relaxation response while removing the constriction response in bovine adrenal arteries. Alternatively, the carboxypeptidase metabolism of Ang II to Ang (1–7) reduces both the relaxation and constriction responses in bovine adrenal arteries. Thus, aminopeptidase metabolism provides a mechanism by which adrenal blood flow increases after Ang II stimulation, whereas ACE2/carboxypeptidase metabolism of Ang II diminishes its vascular actions.
Materials and Methods
Reagents
Losartan, L-nitro-arginine (L-NA), PD123,319, amastatin, all Ang peptides, and buffer reagents were purchased from Sigma-Aldrich. DX-600 was purchased from AnaSpec, cell culture reagents and media from Gibco, and U46619 from Cayman Chemical. The 13C515N1-Ang IV internal standard was synthesized by the Medical College of Wisconsin Protein Nucleic Acid Facility. The 13C515N1-Ang III and 13C515N1-Ang (1–7) internal standards were synthesized by EZBiolab. All of the solvents were high-performance liquid chromatography grade and purchased from Sigma-Aldrich.
Tissue and cell culture
Fresh bovine adrenals were obtained from a local slaughterhouse. Small adrenal cortical arteries closely attached to the adrenal surface (200–300 μm) were dissected and cleaned of connective tissues. Bovine microvascular endothelial cells (BMECs) were isolated and cultured as previously described (38, 39). Bovine adrenal artery smooth muscle cells (BAA-SMCs) were cultured by explant migration. Bovine adrenal arteries were cut into 1-cm segments and cut open longitudinally. The endothelium was removed by gently rubbing the lumen with a sterilized cotton swab. The artery segments were then placed lumen-side down on a tissue culture plate in growth media (medium 199 with 10% fetal bovine serum and 1% L-glutamine). Colonies of SMCs were passaged and cultured. All cells were cultured at 37°C in 5% CO2 in air. Cells used in these studies were between passages 3 and 6.
Ang metabolism
The metabolism of Ang II by BMECs and BAA-SMCs was examined. Each well of a 6-well culture plate was seeded with 1 × 106 cells. The day after, wells were rinsed 3 times with HEPES buffer (130mM NaCl, 5mM KCl, 20mM HEPES, 1mM CaCl2, 2mM MgCl2, and 5.5mM glucose; pH 7.4). Ang II (100nM) was added to each well in 2 mL of HEPES buffer. Cells were incubated at 37°C in 5% CO2 in air for 0, 15, and 30 minutes. Supernatant was removed and extracted the same day. The metabolism of Ang II, Ang III, and Ang (1–7) by adrenal cortical arteries was examined in a similar manner.
Solid-phase extraction
The internal standards (Sar1, Ile8-Ang II, 13C515N1-Ang III, 13C515N1-Ang IV, and 13C515N1-Ang [1–7]; 30 ng) were added to the supernatant. The supernatant was prepared for solid-phase extraction by the addition of ethanol containing 1% trifluoroacetic acid (TFA) to a final volume of 15% followed by adding 1 mL of water containing 1% TFA. The sample was applied to a preconditioned Sep Pak C18 SPE cartridge (Waters Corp) and washed with 20 mL of water containing 1% TFA. Ang peptides were then eluted from the column using 6 mL of methanol containing 1% TFA and dried under a stream of nitrogen gas. The sample was dissolved in 500 μL of 75% acetonitrile/25% water containing 1% TFA, centrifuged, and the supernatant was removed and dried under a stream of nitrogen gas. For liquid chromatography-mass spectrometry (LC-MS), the samples were then dissolved in 30 μL of 50% methanol/50% water containing 3% formic acid and 0.01% TFA, centrifuged, and the supernatant analyzed.
LC-MS analysis
LC-MS and LC-MS/MS were performed using a modification of a previously described method (37, 40). LC-MS/MS was used to identify the peptides, and LC-MS was used to quantify the Ang peptides. Analyses were performed using a Waters-Micromass Quattro micro API electrospray triple quadrupole mass spectrometric system coupled with a Waters 2695 high-performance liquid chromatograph. The mass spectrometer is equipped with a Z-spray dual orthogonal ionization source and is controlled by MassLynx 4.1 software. Samples were separated on a reverse phase C18 column (Jupiter 2.0 × 250 mm; Phenomenex) using water-methanol with 0.3% formic acid as a mobile phase at a flow rate of 0.2 mL per minute. The mobile phase of 20% methanol in water linearly increased to 50% methanol over 30 minutes, followed by a linear increase to 60% methanol over 5 minutes. Positive ion electrospray ionization mass spectrometric conditions were as follows: capillary voltage, 3.0 kV; cone voltage, 20 V; desolvation temperature, 400°C; and source temperature, 100°C. LC-MS analysis was performed in positive electrospray mode in the single-ion recording mode.
Vascular reactivity
Isolated adrenal arterial segments were threaded on 2 stainless steel wires (40 μm in diameter) and mounted on a 4-chamber wire myograph (model 610M; Danish Myo Technology) in physiological saline solution containing 119mM NaCl, 4.7mM KCl, 2.5mM CaCl2, 1.17mM MgSO4, 24mM NaHCO3, 1.18mM KH2PO4, 26μM EDTA, and 5.5mM glucose, bubbled with 95% O2-5% CO2, at 37°C for 30 minutes, as previously described (37). Arteries were gradually stretched to a resting tension of 1mN and stimulated with KCl (60mM) plus U-46619 (100nM) 3 times for 10 minutes at 10-minute intervals to determine the maximal contraction. Arteries were then allowed to equilibrate for another 30 minutes before the initiation of experimental protocols.
To examine vasorelaxation responses, the arteries were preconstricted to 50%–75% of maximal KCl/U46619 contraction by addition of the thromboxane A2 mimetic U46619 (10nM–30nM). Cumulative concentration responses to Ang II (10fM–10nM), Ang III, and Ang IV (10fM–1μM) were measured. To examine the role of NO in the vascular responses, arteries were pretreated for 10 minutes with L-NA (30μM), an endothelial NO synthase (eNOS) inhibitor. To examine the role of the AT2 receptor, PD123,319 (10μM) was used. Experiments were performed on arteries with intact endothelium. To examine the role Ang II metabolites, the APA/aminopeptidase N inhibitor amastatin (10μM), the selective ACE2 inhibitor DX-600 (1μM), and the Mas receptor antagonist A-779 (10μM) were used.
To examine vasoconstriction, arteries were studied under basal tension. Cumulative concentration responses to Ang II (100pM–100nM), Ang III (100pM–1μM), Ang IV (100pM–1μM), and Ang (1–7) (100pM–1μM) were measured. As with the vasorelaxation studies, L-NA (30μM) and amastatin (10μM) were used to examine the role of NO and Ang II metabolism, respectively, in the vascular responses. Losartan (10μM) was used to examine the role of the AT1 receptor. Experiments were performed on arteries with an intact endothelium.
Data analysis
Data are presented as mean ± SEM. Significant differences between mean values were evaluated by ANOVA followed by the Student-Newman-Keuls multiple comparison test. A value of P < .05 was considered significantly different.
Results
Identification and quantification of Ang metabolites
To characterize the metabolism of Ang II in adrenal cortical arteries and the cellular components of the vasculature, adrenal arteries, BMECs and BAA-SMCs were incubated with Ang II for 15 and 30 minutes. The buffer was then extracted and analyzed for Ang II peptides by LC-MS. To quantify concentration of each Ang peptide, a standard curve was generated by calculating peak area for the following concentrations: 5, 10, 20, 50, 100, 200, and 400 pg/μL. The mass-to-charge ratio (m/z) and elution times for internal standards and Ang peptides are indicated in Table 1.
Table 1.
LC-MS Analysis of Ang Peptides
| Analyte | Peptide | Molecular weight (g) | Mass-to-charge ratio (m/z) | Retention time (min) |
|---|---|---|---|---|
| Internal standards | Sar1, Ile8-Ang II | 967.16 | 484.32 | 21.69 |
| 13C515N1-Ang III | 937.11 | 469.50 | 24.77 | |
| 13C515N1-Ang IV | 780.20 | 391.10 | 28.29 | |
| 13C515N1-Ang (1–7) | 905.03 | 453.50 | 13.63 | |
| Ang peptides | Ang II | 1046.19 | 523.70 | 26.71 |
| Ang A | 1002.20 | 501.54 | 25.76 | |
| Ang III | 931.11 | 465.88 | 24.75 | |
| Ang IV | 774.92 | 387.78 | 28.26 | |
| Ang (1–7) | 899.02 | 450.23 | 13.53 | |
| Ang (2–7) | 783.00 | 392.50 | 5.70 | |
| Ang (3–7) | 627.33 | 314.96 | 13.67 |
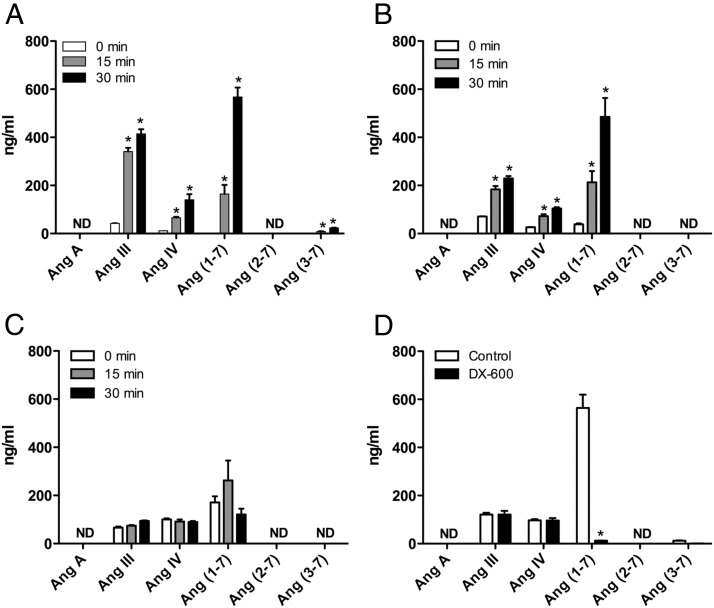
In adrenal cortical arteries and BMECs, Ang II is metabolized to Ang III, Ang IV, and Ang (1–7). LC-MS/MS was performed on the Ang metabolites of adrenal arteries showing multiple reaction monitoring transitions of m/z of 465.9→263.2 (Ang III), 387.8→235.0 (Ang IV), and 450.2→116.1 (Ang [1–7]). Identical transitions were observed with standards of Ang peptides confirming the identity of the Ang II metabolites. Using LC-MS, the primary metabolite of Ang II in adrenal cortical arteries is Ang III at 15 minutes (341.3 ± 15.1 ng/mL) and Ang (1–7) at 30 minutes (567.5 ± 40.0 ng/mL). Ang IV is also produced to a lesser degree, along with a trace of Ang (3–7) at both time points (Figure 1A). When expressed as a percentage of Ang II detected, the amount of Ang metabolites at 30 minutes are as follows: Ang III, 7.72%; Ang IV, 2.61%; Ang (1–7), 10.56%; and Ang (3–7), 0.44%.
Figure 1.
Ang II metabolism of (A) adrenal cortical arteries, (B) BMECs, (C) BAA-SMCs, and (D) BMECs in the presence of the ACE2 inhibitor DX-600 (1μM). Adrenal arteries (30 mg) or 6-well plates seeded with 1 × 106 BMECs or BAA-SMCs were incubated with Ang II (100nM) for 0, 15, or 30 minutes (for D, only the 30-min time point was performed). Supernatant was extracted and analyzed for Ang metabolites by LC-MS. Each value represents the mean ± SEM, n = 4. *, significantly different from respective 0-minute time point, P < .05; ND, not detected.
In BMEC incubations, Ang II is primarily metabolized to Ang III and Ang (1–7) at 15 minutes (184.0 ± 13.4 and 212.8 ± 47.0 ng/mL, respectively). At 30 minutes, Ang (1–7) is the primary metabolite (484.4 ± 78.8 ng/mL). Ang IV is produced to a lesser degree (Figure 1B). Ang (3–7) was not detected in BMEC incubations. In contrast, there is little to no metabolism of Ang II by BAA-SMCs (Figure 1C). Ang A and Ang (2–7) were not detected in any of the samples.
To confirm the inhibition of ACE2 with DX-600, BMEC metabolism of Ang II was examined in the presence of the ACE2 inhibitor DX-600 (1μM). DX-600 nearly ablated the metabolism of Ang II to Ang (1–7) in BMECs (control, 564.0 ± 55.9 ng/mL; DX-600, 12.0 ± 0.9 ng/mL; P < .001) (Figure 1D). DX-600 did not alter the production of other Ang metabolites. Amastatin interfered with ionization of standards and Ang peptides. Thus, confirmation of APA inhibition with amastatin was not possible with our LC-MS technique. However, the concentration used in the following experiments (10μM) is higher than the EC50 and has been shown to effectively inhibit Ang II metabolism in previous studies (41, 42).
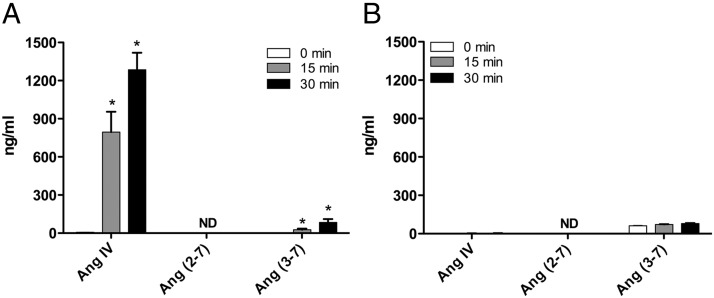
When adrenal cortical arteries were incubated with Ang III, the primary metabolite was Ang IV at 15 minutes (795.5 ± 160.0 ng/mL) and 30 minutes (1285.0 ± 133.7 ng/mL) (Figure 2A). A small amount of Ang (3–7) was produced at both time points. Ang (2–7) was not detected in any of the incubations. When adrenal cortical arteries were incubated with Ang (1–7), no significant metabolism to Ang (2–7) or Ang (3–7) was detected (Figure 2B). A trace amount of Ang (3–7) was detected at both time points but did not increase with incubation time. Ang (2–7) was not detected in any of the incubations.
Figure 2.
Metabolism of (A) Ang III and (B) Ang (1–7) by adrenal cortical arteries. Adrenal arteries (30 mg) were incubated with Ang III or Ang (1–7) (100nM) for 0, 15, or 30 minutes. Supernatant was extracted and analyzed for Ang metabolites by LC-MS. Each value represents the mean ± SEM, n = 4. *, significantly different from respective 0-minute time-point, P < .05; ND, not detected.
Vasorelaxation of adrenal cortical arteries by Ang II and its metabolites
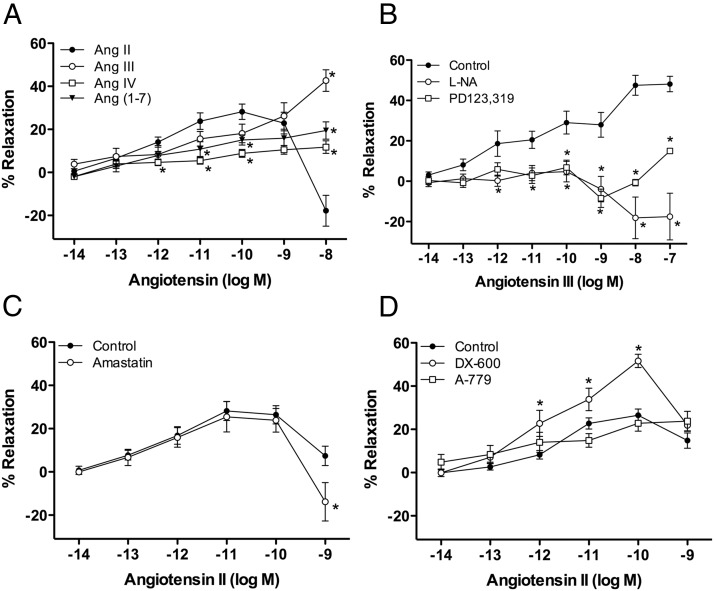
The vasorelaxation responses of Ang peptides were examined in U46619-preconstricted adrenal cortical arteries. Ang II relaxed adrenal arteries in picomolar concentrations, with maximal relaxation at 100pM (28.2 ± 3.6%) (Figure 3A). At higher concentrations (10nM), vasoconstriction by Ang II occurred. Ang III was equipotent to Ang II in stimulating relaxation of adrenal arteries in picomolar concentrations. However, unlike Ang II, Ang III did not produce vasoconstriction at 10nM (Ang II, −17.8 ± 7.1%; Ang III, 42.7 ± 5.0%; P < .0001). Instead, Ang III caused further relaxation in nanomolar concentrations with maximal relaxation at 1μM (48.8 ± 5.3%). The relaxations to Ang III were significantly attenuated by the eNOS inhibitor L-NA (30μM) and the AT2 receptor antagonist PD123,319 (10μM) (Figure 3B). With AT2 receptor blockade or eNOS inhibition, AIII caused constriction at higher concentrations. In contrast, Ang IV failed to cause significant relaxation activity (Figure 3A).
Figure 3.
Relaxation of adrenal cortical arteries to Ang II and its metabolites. Adrenal arteries were preconstricted to 50%–75% of maximal contraction with U46619 (10nM–30nM). Cumulative concentration responses to Ang peptides were measured. Where indicated, vessels were preincubated with pharmacological inhibitors or antagonists for 10 minutes before preconstriction. (A) Ang II, III, and (1–7) relax adrenal arteries, whereas Ang IV does not stimulate relaxation, n = 8–17. (B) Ang III relaxations are significantly attenuated by the eNOS inhibitor L-NA (30μM) and the AT2 receptor antagonist PD123,319 (10μM), n = 7–17. (C) Ang II relaxations are not affected by the aminopeptidase inhibitor amastatin (10μM), but amastatin enhanced the contractile response to Ang II at 10nM, n = 5–6. (D) Ang II relaxations are augmented by the ACE2 inhibitor DX-600 (1μM) but not altered by the Mas receptor antagonist, A-779, n = 4–14. Each value represents the mean ± SEM. *, significantly different from respective control, P < .05.
Ang (1–7) also stimulated the relaxation of adrenal arteries, although to lessor extent. These relaxations were significantly less than Ang II-stimulated relaxations at 10pM and 100pM. Additionally, as with Ang III, constriction to Ang (1–7) was not observed at 10nM (Ang II, −17.8 ± 7.1%; Ang (1–7), 19.5 ± 4.0%; P = .0006) (Figure 3A). The Mas receptor antagonist A-779 (10μM) ablated the relaxation response to Ang (1–7) (data not shown).
The aminopeptidase inhibitor amastatin (10μM) had no effect on the relaxation response to Ang II as compared with vehicle control, with a maximal relaxation response at 10pM (25.5 ± 7.0%) (Figure 3C). Amastatin increased the constrictor response at the highest concentration of Ang II. In contrast, the ACE2 inhibitor DX-600 (1μM) augmented the relaxation response to Ang II with a maximal relaxation response at 100pM (51.6 ± 3.1%) (Figure 3D). Additionally, the Mas receptor antagonist A-779 (10μM) had no effect on the relaxation response to Ang II (Figure 3D).
Vasoconstriction of adrenal cortical arteries by Ang II and its metabolites
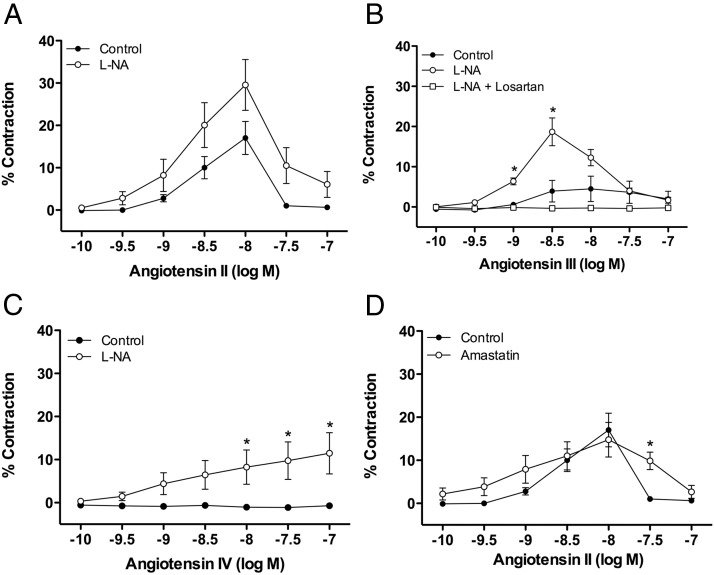
The vasoconstriction response of Ang peptides was examined in adrenal cortical arteries under basal tone. Ang II significantly constricted adrenal arteries, with maximal constriction at 10nM (17.0 ± 3.9%) (Figure 4A). This constriction response did not persist with higher concentrations of Ang II. This lack of persistent constriction is due to tachyphylaxis, because naïve arteries exposed to Ang II (100nM) caused a significant constriction (12.76 ± 4.0%) (43). The eNOS inhibitor L-NA (30μM) increased the constriction response to Ang II, although this increase was not statistically significant (maximal constriction at 10nM, 29.5 ± 6.0%). The duration of the constriction to Ang II was also increased by L-NA treatment (data not shown). Ang III caused a slight, but not significant, constriction at concentrations greater than 100nM, with maximal constriction at 10nM (4.5 ± 3.2%) (Figure 4B). L-NA significantly increased the constrictions to Ang III (maximal constriction at 1nM, 18.7 ± 3.5%). Losartan ablated this constriction response, indicating that Ang III constriction is mediated by AT1 receptors. Similarly, Ang IV caused no vasoconstriction under basal conditions. However, a significant constriction response occurred in the presence of L-NA (maximal at 100nM, 11.5 ± 4.8%) (Figure 4C). Ang (1–7) was without constrictor activity in the absence or presence of L-NA (data not shown).
Figure 4.
Constriction of adrenal cortical arteries to Ang II and its metabolites. Adrenal arteries were studied under basal tension. Cumulative concentration responses to Ang peptides were measured. Where indicated, vessels were preincubated with pharmacological inhibitors for 10 minutes before the concentration response. (A) Ang II contracts adrenal arteries and causes a greater constriction response in the presence of the eNOS inhibitor L-NA (30μM), n = 7–16. (B) Ang III did not cause significant constrictions alone but caused constrictions in the presence of L-NA. Losartan (10μM) blocks constrictions to Ang III, n = 4–16. (C) Similarly, Ang IV did not cause significant constriction alone but causes constriction in the presence of L-NA, n = 8–16. (D) The aminopeptidase inhibitor amastatin (10μM) increased the concentration range that Ang II caused constrictions, n = 7–8. Each value represents the mean ± SEM. *, significantly different from respective control, P < .05.
The aminopeptidase inhibitor amastatin (10μM) did not alter the magnitude of the constrictions to Ang II. However, amastatin did increase the concentration range that Ang II caused constrictions, with significant constrictions persisting at 30nM (Figure 4D).
Discussion
The primary Ang II metabolites of bovine adrenal cortical arteries are Ang (1–7) and Ang III, with Ang IV produced to a lesser extent. The metabolism of Ang II in BMECs reflected a similar metabolic profile to adrenal arteries, whereas BAA-SMCs exhibited less metabolism of Ang II. These data indicate that the endothelium is the primary source of Ang II metabolism in the adrenal vasculature. This observation parallels that of the conversion of Ang I to Ang II by ACE, which is also predominantly endothelium dependent (44). This observation is also in agreement with published studies that demonstrate the expression of APA in kidney glomerular endothelial cells (45, 46).
Ang (2–7) production was not detected in adrenal arteries, BMECs, and BAA-SMCs, whereas Ang (3–7) was only detected in minor amounts in adrenal arteries. These data suggest that Ang III and Ang IV are likely poor substrates for the carboxypeptidases that produce Ang (1–7) in the adrenal vasculature. Similarly, Ang (1–7) is likely a poor substrate for the aminopeptidases that produce Ang III and Ang IV in the adrenal vasculature. Additionally, Ang A formation was not detected in adrenal arteries, BMECs, or BAA-SMCs. Thus, the vasculature is not a source of Ang A.
In preconstricted adrenal cortical arteries, Ang II has biphasic vascular effects (36, 37). In picomolar concentrations, Ang II causes vasorelaxation through activation of endothelial AT2 receptors and release of NO (36). In nanomolar concentrations, the overriding vascular effect of Ang II is vasoconstriction. Our results indicate that Ang III has similar vasorelaxation activity in adrenal arteries as Ang II, which is ablated by AT2 receptor antagonism by PD123,319 and eNOS inhibition with L-NA. However, unlike Ang II, Ang III caused a continued relaxation response in nanomolar concentrations. Overall, these data indicate that metabolism of Ang II to Ang III will be without effect on relaxation at low concentrations of Ang II and will favor vasorelaxation at higher concentrations of Ang II.
Our observation that Ang III relaxes adrenal arteries through AT2 receptors agrees with AT2 receptors mediating Ang III natriuresis in rats (47, 48). However, there are differences in the importance of Ang II metabolism between the adrenal gland and the kidney. The metabolism of Ang II to Ang III is not necessary for AT2 receptor-mediated vasorelaxation of bovine adrenal arteries, whereas Ang III formation is critical for the AT2 receptor-mediated natriuresis in rats (49).
Under basal tension, Ang II constricted adrenal arteries in nanomolar concentrations. These constrictions were transient, with tachyphylaxis occurring at 30nM. Inhibition of eNOS by L-NA enhanced the constriction of adrenal arteries by Ang II. Ang III did not induce constriction in adrenal arteries under basal tension in preconstricted arteries. However, in the presence of the eNOS inhibitor L-NA, Ang III constricted adrenal arteries, and these constrictions were inhibited by the AT1 receptor antagonist losartan. Thus, like Ang II, Ang III constrictions are mediated by AT1 receptor activation, and relaxations are mediated by AT2 receptors. As with Ang III, Ang IV did not cause constriction under basal conditions but constricted adrenal arteries in the presence of L-NA. In contrast, Ang (1–7) did not cause constriction under basal conditions or in the presence of L-NA. These data reinforce the importance of NO in limiting vasoconstriction in the adrenal vasculature. In pathological conditions, such as hypertension, endothelial dysfunction is commonly associated with a decreased bioavailability of NO (50). Thus, endothelial dysfunction will likely exacerbate the vasoconstriction to Ang II and reverse the vascular effects of Ang III and Ang IV from relaxation to constriction.
Because both Ang II and Ang III cause AT1 receptor-mediated constriction and AT2 receptor-mediated relaxation, treatment with AT1 receptor antagonists will inhibit constriction, allowing unopposed dilation and an increase in adrenal blood flow. Increases in adrenal blood flow may increase aldosterone secretion (5–7) and lead to “aldosterone escape” with AT1 receptor blockade.
We characterized the vasorelaxation by Ang (1–7) in adrenal cortical arteries. Ang (1–7) was a 1000-fold less potent than Ang II in relaxing adrenal arteries. Ang (1–7)-induced relaxations are mediated by stimulation of endothelial Mas receptors and the release of NO (37). In the current study, the ACE2 inhibitor DX-600 enhanced the relaxation response to Ang II in adrenal arteries. This finding is consistent with ACE2 inactivating Ang II by its metabolism to the less potent Ang (1–7). In contrast, the aminopeptidase inhibitor amastatin did not alter Ang II relaxations in adrenal arteries. Because Ang II and Ang III are equipotent in causing relaxation, it is not surprising that inhibition of APA does not alter Ang II relaxation. However, amastatin did enhance the constriction induced by Ang II at 1nM in preconstricted arteries and increased the concentration range in which Ang II caused constrictions in adrenal arteries under basal tension. These data agree with Ang II, but not Ang III, causing constriction of adrenal arteries.
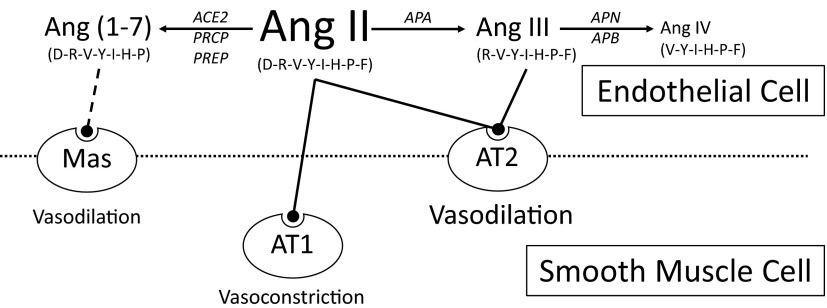
These data provide novel insights into the role of these 2 Ang II metabolic pathways in the adrenal gland (Figure 5). N-terminal metabolism of Ang II by aminopeptidases results in the primary production of Ang III. Ang III is equipotent to Ang II in stimulating vasorelaxation of adrenal arteries and aldosterone production in zona glomerulosa cells (51). However, unlike Ang II, Ang III did not stimulate vasoconstriction in adrenal arteries. A vasoconstriction response to Ang III was only uncovered when the vasorelaxation response was blocked by NOS inhibition or AT2 receptor blockade. Thus, the metabolism of Ang II by aminopeptidases augments the vasorelaxation of adrenal arteries. Additionally, as indicated by previous studies, Ang III is equipotent to Ang II in stimulating aldosterone release (10, 11). Thus, aminopeptidase metabolism then provides a mechanism by which an increase in adrenal blood flow occurs in response to Ang II, a classic vasoconstrictor, while still preserving the stimulation of aldosterone secretion.
Figure 5.
The role of Ang II and its metabolites in the adrenal gland vasculature. Ang II and Ang III dilate adrenal arteries by AT2 receptor-mediated NO release and constrict arteries by AT1 receptor activation. The metabolism of Ang II by aminopeptidases to Ang III augments the vasorelaxation of adrenal arteries. Alternatively, the metabolism of Ang II by carboxypeptidases to Ang (1–7) acts as an inactivation pathway in the adrenal gland by attenuating vasorelaxation. Amino acid sequence of each Ang peptide is indicated in parentheses. PRCP, prolylcarboxypeptidase; PREP, prolylendopeptidase; APN, aminopeptidase N; APB, aminopeptidase B.
Alternatively, C-terminal metabolism of Ang II by carboxypeptidases results in the primary production of Ang (1–7). Although Ang (1–7) stimulates the relaxation of adrenal arteries, it is 1000-fold less potent than Ang II and Ang III (37). Additionally, Ang (1–7) does not stimulate aldosterone production from zona glomerulosa cells (52). Thus, unlike aminopeptidase metabolism, the metabolism of Ang II by carboxypeptidases acts as an inactivation pathway in the adrenal gland by attenuating both vasorelaxation and aldosterone production.
In conclusion, adrenal blood flow is an important component in the regulation of aldosterone secretion. Ang II, an established vasoconstrictor, predominately causes vasorelaxation in bovine adrenal cortical arteries. The metabolism of Ang II in adrenal arteries is one potential mechanism by which Ang II preferentially induces vascular relaxation. Our studies indicate that the endothelium is primarily responsible for Ang II metabolism in adrenal arteries. The 2 endothelial metabolic pathways of Ang II, aminopeptidase and carboxypeptidase, have divergent effects in the adrenal gland. Aminopeptidase metabolism of Ang II provides a mechanism by which adrenal blood flow may be maintained or increased. Alternatively, the metabolism of Ang II by carboxypeptidases provides a mechanism by which the vascular effects of Ang II are inactivated.
Acknowledgments
We thank Dr Kasem Nithipatikom and Ms Marilyn Isbel for their mass spectrometry technical assistance, Dr Kathryn Gauthier for her scientific review and preparation, and Ms Gretchen Barg for secretarial assistance.
This work was supported by the National Institute of Health Grant HL-83297 and by a postdoctoral fellowship from the Midwest Affiliate of the American Heart Association.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACE2
- Ang-converting enzyme 2
- Ang
- angiotensin
- APA
- aminopeptidase A
- AT
- angiotensin type
- BAA-SMC
- bovine adrenal artery smooth muscle cell
- BMEC
- bovine microvascular endothelial cell
- eNOS
- endothelial NO synthase
- LC-MS
- liquid chromatography-mass spectrometry
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- L-NA
- L-nitro-arginine
- NO
- nitric oxide
- TFA
- trifluoroacetic acid.
References
- 1. Calhoun DA. Aldosterone and cardiovascular disease: smoke and fire. Circulation. 2006;114:2572–2574 [DOI] [PubMed] [Google Scholar]
- 2. Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev. 2005;10:7–13 [DOI] [PubMed] [Google Scholar]
- 3. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896 [DOI] [PubMed] [Google Scholar]
- 4. Funder J. Mineralocorticoids and cardiac fibrosis: the decade in review. Clin Exp Pharmacol Physiol. 2001;28:1002–1006 [DOI] [PubMed] [Google Scholar]
- 5. Hinson JP, Vinson GP, Kapas S, Teja R. The relationship between adrenal vascular events and steroid secretion: the role of mast cells and endothelin. J Steroid Biochem Mol Biol. 1991;40:381–389 [DOI] [PubMed] [Google Scholar]
- 6. Hinson JP, Vinson GP, Whitehouse BJ, Price GM. Effects of stimulation on steroid output and perfusion medium flow rate in the isolated perfused rat adrenal gland in situ. J Endocrinol. 1986;109:279–285 [DOI] [PubMed] [Google Scholar]
- 7. Vinson GP, Pudney JA, Whitehouse BJ. The mammalian adrenal circulation and the relationship between adrenal blood flow and steroidogenesis. J Endocrinol. 1985;105:285–294 [DOI] [PubMed] [Google Scholar]
- 8. Jasper MS, McDermott P, Gann DS, Engeland WC. Measurement of blood flow to the adrenal capsule, cortex and medulla in dogs after hemorrhage by fluorescent microspheres. J Auton Nerv Syst. 1990;30:159–167 [DOI] [PubMed] [Google Scholar]
- 9. Reaux A, Fournie-Zaluski MC, Llorens-Cortes C. Angiotensin III: a central regulator of vasopressin release and blood pressure. Trends Endocrinol Metab. 2001;12:157–162 [DOI] [PubMed] [Google Scholar]
- 10. Campbell WB, Pettinger WA. Organ specificity of angiotensin II and des-aspartyl angiotensin II in the concious rat. J Phamacol Exp Ther. 1976;198:450–455 [PubMed] [Google Scholar]
- 11. Peach MJ, Chiu AT. Stimulation and inhibition of aldosterone biosynthesis in vitro by angiotensin II and analogs. Circ Res. 1974;34/35:I-7-I-13 [Google Scholar]
- 12. Plovsing RR, Wamberg C, Sandgaard NC, et al. Effects of truncated angiotensins in humans after double blockade of the renin system. Am J Physiol Regul Integr Comp Physiol. 2003;285:R981–R991 [DOI] [PubMed] [Google Scholar]
- 13. Wamberg C, Plovsing RR, Sandgaard NC, Bie P. Effects of different angiotensins during acute, double blockade of the renin system in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2003;285:R971–R980 [DOI] [PubMed] [Google Scholar]
- 14. Ardaillou R, Chansel D. Synthesis and effects of active fragments of angiotensin II. Kidney Int. 1997;52:1458–1468 [DOI] [PubMed] [Google Scholar]
- 15. Ramírez-Expósito MJ, García MJ, Mayas MD, Ramírez M, Martínez-Martos JM. Differential effects of dietary cholesterol on aminopeptidase A, B and M in the frontal cortex of male and female mice. Nutr Neurosci. 2001;4:461–468 [DOI] [PubMed] [Google Scholar]
- 16. Albiston AL, McDowall SG, Matsacos D, et al. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem. 2001;276:48623–48626 [DOI] [PubMed] [Google Scholar]
- 17. Yang R, Walther T, Gembardt F, et al. Renal vasoconstrictor and pressor responses to angiotensin IV in mice are AT1a-receptor mediated. J Hypertens. 2010;28:487–494 [DOI] [PubMed] [Google Scholar]
- 18. Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9 [DOI] [PubMed] [Google Scholar]
- 19. Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296:F398–F405 [DOI] [PubMed] [Google Scholar]
- 20. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110 [DOI] [PubMed] [Google Scholar]
- 21. Welches WR, Santos RA, Chappell MC, Brosnihan KB, Greene LJ, Ferrario CM. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J Hypertens. 1991;9:631–638 [DOI] [PubMed] [Google Scholar]
- 22. Yang HY, Erdös EG, Chiang TS. New enzymatic route for the inactivation of angiotensin. Nature. 1968;218:1224–1226 [DOI] [PubMed] [Google Scholar]
- 23. Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1–7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521 [DOI] [PubMed] [Google Scholar]
- 24. Santos RA, Simoes e Silva AC, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kono T, Taniguchi A, Imura H, Oseko F, Khosla MC. Pressor activity of angiotensin II-(2–7)-hexapeptide in man. Endocrinol Jpn. 1985;32:767–769 [DOI] [PubMed] [Google Scholar]
- 26. Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1–7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–16523 [PubMed] [Google Scholar]
- 27. Ferreira PM, Souza Dos Santos RA, Campagnole-Santos MJ. Angiotensin-(3–7) pressor effect at the rostral ventrolateral medulla. Regul Pept. 2007;141:168–174 [DOI] [PubMed] [Google Scholar]
- 28. Chansel D, Czekalski S, Vandermeersch S, Ruffet E, Fournié-Zaluski MC, Ardaillou R. Characterization of angiotensin IV-degrading enzymes and receptors on rat mesangial cells. Am J Physiol. 1998;275:F535–F542 [DOI] [PubMed] [Google Scholar]
- 29. Chappell MC, Tallant EA, Brosnihan KB, Ferrario CM. Processing of angiotensin peptides by NG108–15 neuroblastoma x glioma hybrid cell line. Peptides. 1990;11:375–380 [DOI] [PubMed] [Google Scholar]
- 30. Greene LJ, Spadaro AC, Martins AR, Perussi De Jesus WD, Camargo AC. Brain endo-oligopeptidase B: a post-proline cleaving enzyme that inactivates angiotensin I and II. Hypertension. 1982;4:178–184 [DOI] [PubMed] [Google Scholar]
- 31. Handa RK. Metabolism alters the selectivity of angiotensin-(1–7) receptor ligands for angiotensin receptors. J Am Soc Nephrol. 2000;11:1377–1386 [DOI] [PubMed] [Google Scholar]
- 32. Neves LA, Almeida AP, Khosla MC, Santos RA. Metabolism of angiotensin I in isolated rat hearts. Effect of angiotensin converting enzyme inhibitors. Biochem Pharmacol. 1995;50:1451–1459 [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696 [DOI] [PubMed] [Google Scholar]
- 34. Jankowski V, Vanholder R, van der Giet M, et al. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol. 2007;27:297–302 [DOI] [PubMed] [Google Scholar]
- 35. Yang R, Gembardt F, Walther T, et al. Pressor and renal effects of angiotensin A. J Hypertens. 2009;27:S453–S453 [Google Scholar]
- 36. Gauthier KM, Zhang DX, Edwards EM, Holmes B, Campbell WB. Angiotensin II dilates bovine adrenal cortical arterioles: role of endothelial nitric oxide. Endocrinology. 2005;146:3319–3324 [DOI] [PubMed] [Google Scholar]
- 37. Gauthier KM, Zhang DX, Cui L, Nithipatikom K, Campbell WB. Angiotensin II relaxations of bovine adrenal cortical arteries: role of angiotensin II metabolites and endothelial nitric oxide. Hypertension. 2008;52:150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Folkman J, Haudenschild CC, Zetter BR. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci USA. 1979;76:5217–5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosolowsky LJ, Hanke CJ, Campbell WB. Adrenal capillary endothelial cells stimulate aldosterone release through a protein that is distinct from endothelin. Endocrinology. 1999;140:4411–4418 [DOI] [PubMed] [Google Scholar]
- 40. Cui L, Nithipatikom K, Campbell WB. Simultaneous analysis of angiotensin peptides by LC-MS and LC-MS/MS: metabolism by bovine adrenal endothelial cells. Anal Biochem. 2007;369:27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abhold RH, Sullivan MJ, Wright JW, Harding JW. Binding, degradation and pressor activity of angiotensins II and III after aminopeptidase inhibition with amastatin and bestatin. J Pharmacol Exp Ther. 1987;242:957–962 [PubMed] [Google Scholar]
- 42. Ahmad S, Ward PE. Role of aminopeptidase activity in the regulation of the pressor activity of circulating angiotensins. J Pharmacol Exp Ther. 1990;252:643–650 [PubMed] [Google Scholar]
- 43. Fasciolo JC, Binia A. Angiotensin I, II, and II tachyphylaxis in the mesenteric vascular circuit of the rat. Hypertension. 1981;3:II-166–170 [DOI] [PubMed] [Google Scholar]
- 44. Gohlke P, Bünning P, Unger T. Distribution and metabolism of angiotensin I and II in the blood vessel wall. Hypertension. 1992;20:151–157 [DOI] [PubMed] [Google Scholar]
- 45. Song L, Healy DP. Kidney aminopeptidase A and hypertension, part II: effects of angiotensin II. Hypertension. 1999;33:746–752 [DOI] [PubMed] [Google Scholar]
- 46. Song L, Ye M, Troyanovskaya M, Wilk E, Wilk S, Healy DP. Rat kidney glutamyl aminopeptidase (aminopeptidase A): molecular identity and cellular localization. Am J Physiol. 1994;267:F546–F557 [DOI] [PubMed] [Google Scholar]
- 47. Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544 [DOI] [PubMed] [Google Scholar]
- 48. Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension. 2009;53:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51:460–465 [DOI] [PubMed] [Google Scholar]
- 50. Montezano AC, Touyz RM. Molecular mechanisms of hypertension-reactive oxygen species and antioxidants: a basic science update for the clinician. Can J Cardiol. 2012;28:288–295 [DOI] [PubMed] [Google Scholar]
- 51. Campbell WB, Brooks SN, Pettinger WA. Angiotensin II- and angiotensin 3-induced aldosterone release vivo in the rat. Science. 1974;184:994–996 [DOI] [PubMed] [Google Scholar]
- 52. Yatabe J, Yoneda M, Yatabe MS, et al. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011;152:1582–1588 [DOI] [PubMed] [Google Scholar]