Abstract
Calcium has recently been shown to regulate fibroblast growth factor 23 (FGF-23), a bone-derived phosphate and vitamin D-regulating hormone. To better understand the regulation of FGF-23 by calcium, phosphorus, 1,25 dihydroxyvitamin D3 [1,25(OH)2D], and PTH, we examined FGF-23 expression under basal conditions and in response to PTH, doxercalciferol, or high-calcium diet treatment in Gcm2−/− and Cyp27b1−/− mutant mice. Gcm2−/− mice exhibited low serum PTH and 1,25(OH)2D concentrations, hypocalcemia, and hyperphosphatemia, whereas Cyp27b1−/− mice had high PTH, undetectable 1,25(OH)2D, hypocalcemia, and hypophosphatemia. Serum FGF-23 levels were decreased in both mutant models. Doxercalciferol administration increased serum FGF-23 levels in both mutant models. PTH administration to Gcm2−/− mice also increased serum FGF-23 levels, in association with an increase in both 1,25(OH)2D and calcium concentrations. Multiple regression analysis of pooled data indicated that changes in FGF-23 were positively correlated with serum calcium and 1,25(OH)2D but not related to changes in serum phosphate concentrations. A high-calcium diet also increased serum FGF-23 concentrations in Cyp27b1−/− mice in the absence of 1,25(OH)2D and in Gcm2−/− mice with low PTH. The addition of calcium to the culture media also stimulated FGF-23 message expression in MC3T3-E1 osteoblasts. In addition, FGF-23 promoter activity in cultured osteoblasts was inhibited by the L-calcium-channel inhibitor nifedipine and stimulated by calcium ionophores. The effects of chronic low calcium to prevent 1,25(OH)2D and PTH stimulation of FGF-23 in these mutant mouse models suggest that suppression of FGF-23 plays an important physiological adaptive response to hypocalcemia.
Fibroblast growth factor 23 (FGF-23) is a bone-derived hormone that is essential for maintaining phosphorus and vitamin D homeostasis (1–4). FGF-23 is released into the circulation from osteoblasts and osteocytes and targets FGF receptor/α-Klotho complexes in tissues, including renal tubules in the kidney and chief cells in the parathyroid gland (PTG) (5–8). Excess FGF-23 inhibits renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 [1,25(OH)2D] production leading to hypophosphatemia and suppression of circulating 1,25(OH)2D levels (9–16). In contrast, reductions in FGF-23 cause the syndrome of tumoral calcinosis, characterized by hyperphosphatemia, increased 1,25(OH)2D and soft tissue calcifications (10).
FGF-23 is regulated by an incompletely understood complex interplay between local factors that modulate bone mineralization and systemic factors that control mineral metabolism (17). Several mutations genes that cause impaired bone mineralization result in increased FGF-23 (9, 18–24). The linkage between mineralization and FGF-23 expression appears to be through the activation of paracrine pathways that control FGF-23 gene transcription by osteocytes. Through these mechanisms, FGF-23 released into the circulation and renal handling of phosphate and vitamin D metabolism is coordinated with and functionally linked to the mineralization of bone (9, 18–24).
FGF-23 is also integrally involved in endocrine networks regulating calcium and phosphate homeostasis, including PTH secreted by the parathyroid gland and 1,25(OH)2D produced in the kidney (17). Of these, the 1,25(OH)2D-FGF-23 feedback loop is best characterized. 1,25(OH)2D stimulates FGF-23 gene transcription through putative vitamin D-responsive elements within the FGF-23 promoter (2) (25) as well as through vitamin D-responsive element-independent regulation of FGF-23 production (19, 20, 26). 1,25(OH)2D-mediated increases in circulating FGF-23 suppresses1,25(OH)2D production in the kidney, thereby creating a counterregulatory feedback loop between bone and kidney to protect against vitamin D toxicity (19).
In contrast, there are major gaps in our understanding of regulation of FGF-23 by other systemic factors, including PTH, phosphate, and calcium. For example, the effects of PTH are variable, with some studies showing an effect of PTH, PTH receptor 1 (PTH1R) overexpression in osteocytes, or activating mutations of PTH1R to stimulate FGF-23 expression (21, 27, 28), whereas another study shows that PTH fails to stimulate circulating FGF-23 levels (29). Effects of serum phosphorus on FGF-23 regulation are also unexpectedly complex. Although serum phosphate levels positively correlate with elevations in FGF-23 levels in end-stage renal disease (30), phosphate restriction either fails to lower elevated FGF-23 levels in patients with chronic kidney disease (CKD) (31) or has only modest effects to reduce serum FGF-23 concentrations (32, 33). Acute phosphate loading does also does not increase serum FGF-23 levels (34), whereas chronic phosphate loading may elevate FGF-23 levels after a several weeks (35).
Calcium regulation of FGF-23 expression is also controversial. Calcium had no effect on FGF-23 promoter activity in osteoblast cultures (19); however, ip injections of calcium increased FGF-23 levels in mice with targeted inactivation of PTH and the calcium-sensing receptor (36). FGF-23 levels were also increased in vitamin D receptor-null mice by dietary calcium supplementation (15). In another study in rats with normal renal function, dietary induced hypocalcemia was associated with reductions of circulating FGF-23, despite the high PTH and calcitriol levels (37). Understanding the role of calcium in regulating FGF-23 is not only important because of the physiological implications but may also have pathological relevance because increments in calcium as well as FGF-23 have recently been linked to increased cardiovascular mortality in some observational studies (38–41). The calcium regulation of FGF-23 might provide a novel mechanism linking calcium to cardiovascular outcomes. A full understanding of FGF-23 regulation requires an explanation for the discrepancies in the actions of PTH, 1,25(OH)2D, and phosphate.
It has been difficult to disentangle the complex regulation of FGF-23 by local and systemic factors, primarily due to the limitations of experimental approaches that have failed to control for the simultaneous alterations in multiple factors capable of regulating FGF-23 expression. In the current studies, we used endocrine replacement studies in mutant mouse models to define the independent roles of serum 1,25(OH)2D, PTH, and calcium in the regulation of FGF-23. In particular, we have examined the effects of administration of PTH, active vitamin D analog, or calcium replacement on FGF-23 expression in mutant Cyp27b1−/− and Gcm2−/− mice, which respectively have the inability to produce 1,25(OH)2D and PTH. These studies demonstrated an overriding role for calcium in modulating 1,25(OH)2D and PTH effects on FGF-23 gene expression.
Materials and Methods
Animals and genotyping
All mice were maintained on a standard diet (7912, 0.82% calcium and 0.53% phosphorus; Harlan Teklad) in accordance with the guidelines as detailed in the Guide for Care and Use of Laboratory Animals, prepared by the Institute on Laboratory Animal Resources, National Research Council (Department of Health and Human Services Publication, National Institutes of Health 86–23, National Academy Press, 1996). All studies were approved by the University of Tennessee Institutional Animal Care and Use Committee.
We obtained the Cyp27b1+/− heterozygous mice from Renee St-Arnaud (42) and Gcm2+/− from Gerard Karsenty (43). REDExtract-N-Amp tissue PCR kit (Sigma-Aldrich) was used (2) for DNA extraction and PCR amplification (Supplemental Figure 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) to genotype the mice. Cyp27b1−/− and Gcm2−/− were born with the expected Mendelian frequency. Gcm2−/− mice had decreased survival compared with wild-type littermates with a 90-day survival rate of approximately75%, and there were no differences in survival between wild-type and Cyp27b1−/− mice (Supplemental Figure 1B). Body weights of Cyp27b1−/− mice were significantly lower than wild-type (WT) and Gcm2−/− mice at 12 weeks of age (Supplemental Figure 1, C and D). We also transposed Cyp27b1−/− and Gcm2−/− onto Col4a3−/− mice background that we have previously characterized as a model of progressive CKD (44–46).
For doxercalciferol treatment, mice were subjected to a daily injection of 300 pg/g body weight during a 4-week period. For PTH administration, mice were administered 100 ng/g·d of PTH1–34 through osmotic pump diffusion (Alzet). Finally, animals were also fed a high-calcium (2%) and high-phosphate (1.2%) and lactose (20%)-containing diet that has been previously shown to increase serum calcium levels and normalize bone mineralization in Cyp27b1−/− mice (47).
Serum biochemistry
Calcium was measured using a Calcium CPC Liquicolor kit (Stanbio Laboratories). Phosphorus was measured using the phosphomolybdylate-ascorbic acid method (2). PTH levels were measured using the mouse intact PTH ELISA kit (Immutopics), 1,25(OH)2D and 25OHD levels using the vitamin D enzyme immunoassay kits (Immunodiagnostic Systems), and FGF-23 levels using the FGF-23 ELISA kit (Kainos Laboratories).
RT-PCR
Total RNAs were isolated using TRI-reagent and first-strand cDNA was synthesized according to previously published method (48) from the entire femur. The iCycler iQ real-time PCR detection system and iQ SYBR Green supermix (Bio-Rad Laboratories) were used for real-time quantitative PCR analysis. Primer pairs used in this analysis are shown in Supplemental Table 1. The expression was normalized by glyceraldehyde-3-phosphate dehydrogenase (Gapdh) in the same sample and expressed as fold change of the wild type.
Cell culture and promoter analysis
MC3T3-E1 osteoblast precursor cells were cultured according to American Type Culture Collection guidelines. Briefly, 5 × 104 cells were seeded in 6-cm diameter tissue culture plates in DMEM/F12 media (Life Technologies) with 10% fetal calf serum at 37°C in the presence of 5% CO2 in a humidified incubator. Mouse FGF-23 promoter (mFGF23) DNA was constructed into a pGL3 basic reporter gene (Promega) and introduced into MC3T3-E1 cells using cationic liposomes (LipofectAMINE2000; Life Technologies). Transfection (0.25 μg of mFGF23 promoter plasmid DNA) was carried out for 16–18 hours, and then cells were washed twice with PBS and incubated in fresh medium containing 10% fetal calf serum for 38 hours. To test the effect of extracellular calcium on FGF-23 regulation, cells were switched into calcium-free DMEM medium for 4 hours and then cultured in serum-free DMEM/F12 media for another 12 hours in the presence of indicated concentrations of extracellular calcium. To standardize the transfection efficiency, 0.1 μg of pRL-CMV vector (pRL Renilla reniformis luciferase control reporter vector; Promega) was cotransfected in all experiments. Cells were harvested 72 hours after transfection and lysed in 50 μL of reporter lysis buffer (Promega). A luciferase assay (20 μL of cell lysed) was performed using a dual-luciferase assay kit (Promega), and activity was measured with an Optocomp 1 luminometer (MGM Instruments, Inc. Promoter activity (mean ± SD of duplicate samples in relative light units) is represented by relative light output normalized to pRL-CMV control.
Statistical analysis
Differences among the two groups were tested by Student t test using the Statistica software (Statsoft). The differences were considered statistically significant at P < .05. Spearman correlations and multiple regression analysis were performed to examine the relationship between the measured serum parameters. Collinearity statistics for all of the predictor variables entered in the multiple linear regression analysis indicated that in all cases, tolerance values were greater than 0.7 and the variance inflation factor was less than 2.
Results
Effects of Cyp27b1-mediated reduction in 1,25(OH)2D on FGF-23
Serum 1,25(OH)2D levels were at the lower limit of detection for the assay in Cyp27b1−/− mice (Table 1). Cyp27b1−/− mice exhibited low serum phosphate and calcium levels and markedly elevated serum PTH concentrations compared with WT controls (Table 1). Cyp27b1−/− mice were smaller than Gcm2−/− mice and WT littermates (Supplemental Figure 1). Cyp27b1−/− mice also exhibited marked reductions in cancellous mineralized bone volume and bone mineral density (BMD) as well as diminished cortical thickness and BMD (Supplemental Figure 2), changes consistent with the presence of rickets and osteomalacia (49). Osteoblast (ie, Runx2, PTH1R, Osterix, Osteocalcin, RANKL, and Opg) and osteoclast (TRAP) gene expression markers were increased in Cyp27b1−/− mice (Supplemental Table 2). Thus, loss of Cyp27b1 results in a model of hypocalcemia, hypophosphatemia, elevated PTH, defective bone mineralization, and increased bone turnover.
Table 1.
Serum Biochemistry in 12-Week-Old WT and Mutant Mice
| WT | Cyp27b1−/− | Gcm2−/− | |
|---|---|---|---|
| Serum | |||
| FGF-23, pg/mL | 98.0 ± 31.0 | 1.6 ± 2.5a | 27.3 ± 13.7a,b |
| 1,25(OH)2D, pM | 162.6 ± 48.9 | 5.4 ± 4.1a | 55.3 ± 30.1a,b |
| PTH, pg/mL | 36.1 ± 13.2 | 11309.2 ± 2319.9a | 3.2 ± 3.0a,b |
| 25OHD, pM | 147.3 ± 46.2 | 78.4 ± 12.6a | 145.3 ± 62.4b |
| PO4−, mg/dL | 7.7 ± 1.6 | 6.1 ± 1.2a | 12.5 ± 0.7a,b |
| Ca2+, mg/dL | 9.38 ± 0.92 | 4.9 ± 0.6a | 4.6 ± 0.71a |
| BUN, mg/dL | 26.9 ± 8.1 | 26.1 ± 7.5 | 22.0 ± 6.0 |
| Creatinine, mg/dL | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
Abbreviations: BUN, blood urea nitrogen; Ca2+, calcium; 25OHD, 25-hydroxyvitamin D; PO4, phosphate. Serum biochemistries: n ≥ 7/group. Comparisons were performed using a Student t test. Data are presented as mean ± SD.
P < .05 vs WT.
Compared with Cyp27b1−/− animals.
Serum FGF-23 levels were markedly suppressed in Cyp27b1−/− mice (Table 1). The nearly 50-fold reduction in circulating FGF-23 levels in Cyp27b1−/− mice, however, was disproportionally greater than the reduction in FGF-23 message expression in bone, which was suppressed approximately 2-fold in Cyp27b1−/− compared with controls (Supplemental Table 2). Although PTH is reported to stimulate FGF-23, elevated PTH Cyp27b1−/− mice failed to prevent the decrease in circulating FGF-23, indicating that either the biochemical and/or bone abnormalities in Cyp27b1−/− mice abrogates the ability of PTH to stimulate FGF-23 expression.
Changes in renal gene expression reflected those predicted from the observed changes in serum 1,25(OH)2D, PTH, and FGF-23, except for sodium phosphate cotransporters, in which increments in sodium-phosphate transport protein 2 (Npt2)-a and Npt2c mRNA levels were observed in Cyp27b1−/− mice, despite elevations of PTH, which should suppress their expression (Supplemental Table 2). Thus, reduction in FGF-23 or other effects related to 1,25(OH)2D deficiency offset the effects of PTH to suppress Npt2a and Npt2c expression in the kidney (50). Using primers to the remaining exon in Cyp27b1−/− mice as a means to assess endogenous promoter activity, we found evidence for increased Cyp27b1 promoter activity in Cyp27b1−/− mice. In contrast, we observed decreased Cyp24 mRNA levels in Cyp27b1−/− mice. These changes are consistent with the fact that FGF-23 and 1,25(OH)2D suppress Cyp27b1 and stimulate Cyp24 expression, whereas PTH has the opposite effects on these enzymes. FGF-23 inhibits and 1,25(OH)2D stimulates α-Klotho expression in the kidney (51). We observed reductions of α-Klotho message expression in Cyp27b1−/− mice.
Effects of molecular ablation of PTG on FGF-23
Next, we examined the effects of primary disruption of PTG development and PTH production in Gcm2−/− mice on FGF-23 expression. Consistent with their lack of parathyroid glands, Gcm2−/− had undetectable serum PTH levels (Table 1). In addition, Gcm2−/− mice had reduced serum calcium, increased serum phosphate, and decreased 1,25(OH)2D levels compared with WT mice (Table 1). Gcm2−/− were also smaller than WT littermates (Supplemental Figure 1). At 12 weeks of age, Gcm2−/− mice did not exhibit abnormalities of mineralized bone volume, cortical thickness, or BMD but had a decreased trabecular thickness (Supplemental Figure 2). Low circulating PTH was associated with reductions in the expression of osteoblasts markers in Gcm2−/− mice but did not achieve statistical significance in the current study (Supplemental Table 2). Thus, loss of Gcm2 results in a model of hypocalcemia, decreased 1,25(OH)2D, hyperphosphatemia, decreased PTH, and low bone turnover.
Serum FGF-23 levels were reduced approximately 3-fold in Gcm2−/− mice compared with WT mice (Table 1). Reductions in serum FGF-23 levels were not associated with proportionate reductions in the expression of FGF-23 message in bone in Gcm2−/− mice. Indeed, FGF-23 mRNA expression in bone was not significantly different in Gcm2−/− compared with WT mice. Increased serum phosphate levels in Gcm2−/− mice failed to prevent the reductions in serum FGF-23. Rather, similar to Cyp27b1−/− mice, reductions of serum 1,25(OH)2D levels and hypocalcemia were associated with reduced circulating FGF-23 concentrations in Gcm2−/− mice (Table 1).
PTH-deficient Gcm2−/− mice also had a different pattern of renal gene expression compared with 1,25(OH)2D-deficient Cyp27b1−/− mice. Indeed, Gcm2−/− mice exhibited decreased Cyp27b1 and increased Cyp24 message expression, a profile opposite to that of Cyp27b1−/− mice. Npt2a, but not Npt2c or α-Klotho, were suppressed in Gcm2−/− mice (Supplemental Table 2).
To further determine which factors are regulating serum FGF-23 levels, we performed a multivariable regression analysis using serum phosphate, calcium, PTH, 1,25(OH)2D, and femoral bone volume from all three models (WT, Gcm2−/−, and Cyp27b1−/−) as predictors of FGF-23 concentrations (Supplemental Table 3). 1,25(OH)2D and calcium, but neither increases in serum phosphorus nor PTH, positively correlated with FGF-23 production in this analysis, suggesting that 1,25(OH)2D and calcium have direct and overriding effects on FGF-23 production.
Effects of doxercalciferol treatment on serum FGF-23 levels in Cyp27b1−/− and Gcm2−/− mice
To test the predictions of the above model, we examined the effects of active vitamin D analogs on serum FGF-23 concentrations in Cyp27b1−/− and Gcm2−/− mice. We used doxercalciferol treatment (1α-hydroxyvitamin D2) because this vitamin D analog is a commonly used therapy for secondary hyperparathyroidism in chronic kidney disease (52). We used doses of doxercalciferol reported to normalize serum calcium in Cyp27b1−/− mice (300 pg/g body weight) during a 4-week period (53). Consistent with prior reports that active vitamin D analogs stimulate FGF-23 expression (2), the administration of doxercalciferol to WT mice resulted in an approximately 2-fold increase in serum FGF-23 concentrations after 1 week and a 10-fold increase after 4 weeks of treatment (100.8 ± 39.2 to 1295.6 ± 62.3 pg/mL) (Table 2 and Figure 1A). Bone FGF-23 message levels also increased 2-fold after doxercalciferol administration to WT mice (Figure 1B). Treatment with doxercalciferol resulted in a 4-fold suppression of PTH (38.4 ± 15.6 vs 9.36 ± 5.8 pg/mL). Doxercalciferol treatment was associated with increments in serum calcium and decrements in PTH and phosphate levels in WT mice, suggesting that the increments in FGF-23 caused an inhibition of renal phosphate transport. In bone, doxercalciferol significantly reduced osteoblastic markers, PTH1R, rank ligand [receptor activator of nuclear factor-κB ligand (RANKL)], and osteoclastic marker, tartrate-resistant acid phosphatase (TRAcP) (Supplemental Table 4). Doxercalciferol stimulated Cyp24 and Trpv5 and suppressed Cyp27b1 message levels but had no effect of Napt2a, Napt2c, or α-Klotho message expression in the kidney (Supplemental Table 4).
Table 2.
Serum Biochemistry of 12-Week Old Mice After 4 Weeks of Doxercalciferol Treatment
| Doxercalciferol | WT |
Cyp27b1−/− |
Gcm2−/− |
|||
|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |
| Serum | ||||||
| FGF-23 | 100.8 ± 39.2 | 1295.6 ± 62.3a | 2.9 ± 4.1a | 1054 ± 119b,c | 27.4 ± 16.2a,d | 1399 ± 172b,d |
| PTH | 38.4 ± 15.6 | 9.36 ± 5.8a | 10897.2 ± 2522.5a | 1.12 ± 5.66b,c | 1.0 ± 1.4a,d | 0 ± 0 |
| PO4− | 7.2 ± 1.8 | 5.5 ± 0.7a | 5.8 ± 1.3a | 7.2 ± 0.6b | 12.8 ± 0.9a,d | 7.7 ± 0.3b |
| Ca2+ | 8.9 ± 0.6 | 10.6 ± 0.5a | 4.5 ± 0.7a | 9.8 ± 0.7b | 4.3 ± 0.8a | 9.5 ± 0.6b |
| BUN | 27.8 ± 7.9 | 23.14 ± 9.2 | 21.9 ± 3.8 | 26.2 ± 8.4 | 22.5 ± 6.2 | 28.7 ± 936 |
Abbreviations: BUN, blood urea nitrogen; Ca2+, calcium; PO4, phosphate. Serum biochemistry was n ≥ 5/group. Comparisons were performed using a Student t test. Units were as follows: FGF-23, picograms per milliliter; PTH, picograms per milliliter; PO4−); BUN, mg/dl. Data are presented as mean ± SD.
P < .05 compared with untreated WT.
Compared with untreated animals of the similar genotype.
Compared with treated WT.
Compared with Cyp27b1−/− animals administered a similar treatment.
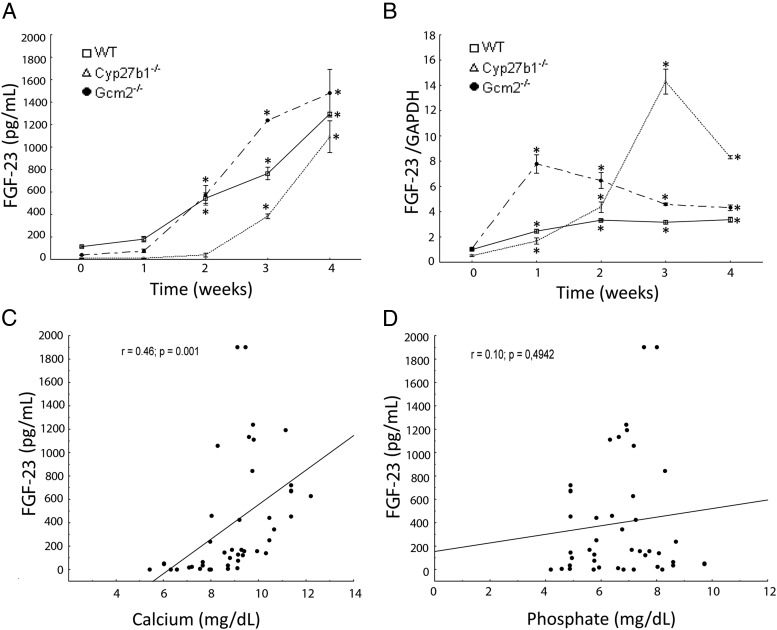
Figure 1.
Effects of doxercalciferol administration on the phenotype of 12-week-old WT and mutant mice. Time-dependent changes in serum FGF-23 concentrations (A) and FGF-23 mRNA expression (B) in bone after doxercalciferol administration. Comparisons were performed using a Student's t test (n ≥ 5/group). *, P < .05 vs untreated control. Data are presented as mean ± SD. Spearman correlations between serum FGF-23 and calcium (C) and phosphate (D) concentrations in doxercalciferol-treated mice are shown.
Doxercalciferol rescued the biochemical and bone abnormalities in Cyp27b1−/− mice, as previously reported (52). The administration of doxercalciferol increased serum calcium levels (4.5 ± 0.7 vs 9.8 ± 0.7) and reduced PTH levels (10 897.2 ± 2522.5 to 1.12 ± 5.66) in Cyp27b1−/− after 4 weeks of treatment (Table 2 and Figure 1A). Treatment with doxercalciferol stimulated FGF-23 serum levels to 3 times and FGF-23 message expression in bone to 14 times normal values by week 3 (Figure 1, A and B). However, the time course was delayed and the magnitude of the response differed from that observed in wild-type mice. Indeed, elevations of FGF-23 message levels in bone in Cyp27b1−/− mice required 3 weeks of treatment with doxercalciferol to achieve the magnitude of expression observed after 1 week of treatment in wild-type mice. The increase in FGF-23 after doxercalciferol treatment correlated with elevations in serum calcium concentrations but not phosphate (Figure 1, C and D). These data, along with the association between calcium and FGF-23 observed in multivariate analysis, implicate a role of calcium in regulating serum FGF-23 levels.
Doxercalciferol administration normalized bone abnormalities identified by microcomputed tomography in Cyp27b1−/− mice (Supplemental Figure 2, A and B). In addition, doxercalciferol treatment of Cyp27b1−/− mice reduced the elevations in Runx2, osteocalcin, and RANKL observed in untreated Cyp27b1−/− mice (Supplemental Table 4). The bone resorptive marker TRAcP was also normalized in Cyp27b1−/− treated with doxercalciferol. With regard to the kidney, doxercalciferol treatment decreased Cyp27b1 expression; stimulated Cyp24a1, Npt2a, and transient receptor potential cation channel subfamily V member 5 (TRPV5) expression; and had no effect on α-Klotho expression in Cyp27b1−/− mice (Supplemental Table 4).
Treatment with doxercalciferol resulted in a time-dependent increase in serum FGF-23 and expression of FGF-23 mRNA in bone (Figure 1, A and B), consistent with prior reports (2). However, there were temporal differences in the responses between Gcm2−/− mice with low PTH and that observed in Cyp27b1−/− with high PTH. The magnitude of the response was greater in Gcm2−/− than Cyp27b1−/− mice. Serum FGF-23 levels were 4-fold higher in Gcm2−/− mice compared with Cyp27b1−/− mice after 3 weeks of doxercalciferol treatment (1240 vs 342 pg/mL). In contrast, FGF-23 mRNA expression in bone increased less in Gcm2−/− mice (∼5-fold), compared with the 24-fold increase in Cyp27b1−/− mice. This nonconcordance between alterations in serum FGF-23 and FGF-23 mRNA levels indicates complex transcription and posttranscriptional regulation of FGF-23.
Doxercalciferol treatment increased trabecular bone volume by increasing the trabecular number (Supplemental Figure 2, A and B) in Gcm2−/− mice while slightly increasing the total mineralized volume, as a result of decreased osteoclastogenesis due to a decrease in TRAcP and an increase in osteoprotegerin expression (Supplemental Table 4). With regard to the kidney, similarly to WT and Cyp27b1−/−, doxercalciferol treatment in Gcm2−/− mice decreased Cyp27b1 and stimulated Cyp24a1 expression (Supplemental Table 4). TRPV5 expression increased by approximately 50%, whereas Npt2c decreased by 5-fold in Gcm2−/− mice treated with doxercalciferol.
Effect of PTH treatment of WT and Gcm2−/− mice
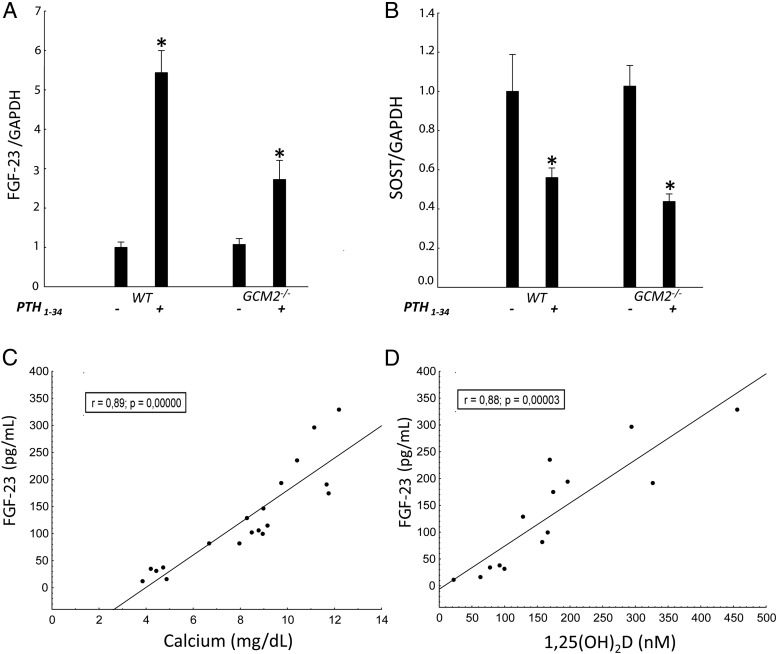
Next, we administered PTH1–34 at a dose of 100 ng/g·d by sc minipump infusion to WT and Gcm2−/− mice for 4 weeks. In WT mice, PTH administration resulted in a 2-fold increase in serum FGF-23 levels (Table 3) and a 5-fold increase in FGF-23 mRNA levels in bone (Figure 2A), in association with concomitant elevations of serum calcium, reductions in serum phosphate, and a 2-fold elevation in serum 1,25(OH)2D concentrations (Table 2). In Gcm2−/− mice, PTH administration increased serum FGF-23 levels 6-fold (Table 2), but this increment was associated with only a 3-fold increment in FGF-23 mRNA expression in bone (Figure 2A). In contrast, PTH administration had equal effects to suppress Sost mRNA expression in bone, reducing expression of this PTH responsive gene by 40% in both wild-type and Gcm2−/− mice (Figure 2B). PTH administration to Gcm2−/− mice also decreased serum phosphate concentrations from 12.8 ± 0.9 to 5.3 ± 1.2 mg/dL, corrected the hypocalcemia and increased serum 1,25(OH)2D levels. The elevations in FGF-23 correlated with increments in serum calcium (Figure 2C) and 1,25(OH)2D levels (Figure 2D) in PTH-treated mice.
Table 3.
Serum Biochemistry of 12-Week Old Mice After 4 Weeks of rPTH1–34 Treatment
| rPTH1–34 | WT |
Gcm2−/− |
||
|---|---|---|---|---|
| − | + | − | + | |
| Serum | ||||
| FGF-23 | 91.9 ± 32.2 | 180.8 ± 41.3a | 29.5 ± 15.3a | 203.6 ± 54.9b |
| 1,25(OH)2D | 164.7 ± 45.4 | 292.3 ± 69.4a | 48.5 ± 28.4a | 196.7 ± 64.9b,c |
| PO4− | 7.5 ± 1.8 | 5.0 ± 1.1a | 12.8 ± 0.9a | 5.3 ± 1.24b |
| Ca2+ | 9.5 ± 1.1 | 11.2 ± 0.8a | 4.5 ± 0.6a | 9.3 ± 2.0b,c |
| BUN | 29.3 ± 7.2 | 23.0 ± 3.5 | 28.5 ± 8.0 | 26.0 ± 4.1 |
Abbreviations: BUN, blood urea nitrogen; Ca2+, calcium; PO4, phosphate. Serum biochemistry was n ≥ 5/group. Comparisons were performed using a Student t test. Units were as follows: FGF-23, picograms per milliliter; 1,25(OH)2D; picomoles; PO4−); BUN, mg/dl. Data are presented as mean ± SD.
P < .05 compared with untreated WT.
Compared with untreated animals of the similar genotype.
Compared with treated WT.
Figure 2.
Effects of rPTH1–34 administration on the phenotype of 12-week-old WT and mutant mice. FGF-23 (A) and Sost (B) mRNA expression as assessed in the femurs and expressed as fold change of WT mice (n ≥ 3/group). Comparisons were performed using a Student t test. *, P < .05 vs untreated WT. Data are presented as mean ± SD. Spearman correlations between serum FGF-23 and calcium (C) and 1,25(OH)2D (D) concentrations are shown.
With regard to bone, PTH resulted in an increase in trabecular bone volume and a reduction in BMD (Supplemental Figure 2, C and D). PTH treatment increased the expression of bone remodeling markers in WT and Gcm2−/− mice including Runx2, Osx, Ocn, RANKL, and TRAcp as well as the expression of PTH1R (Supplemental Table 5).
In the kidney, PTH1–34 administration increased both Cyp27b1 and Cyp24 in WT mice. PTH administration also stimulated Cyp27b1 in Gcm2−/− mice but did not further elevate Cyp24 mRNA levels in these mice. PTH had no effect on the either α-Klotho or TRPV5 expression in either WT or Gcm2−/− mice. Interestingly, PTH reduced the expression of Npt2c but not Npt2a in WT and Gcm2−/− mice, consistent with the importance of nontranscriptional, posttranslational control of sodium-phosphate cotransporters in the brush border membranes in regulating phosphate transport (Supplemental Table 5).
Effects of calcium supplements on FGF-23 production in vivo
Next, we examined the possibility that alterations in serum calcium may regulate FGF-23 expression in the absence of 1,25(OH)2D and PTH. To accomplish this, we administered a rescue diet to WT, Cyp27b1−/−, and Gcm2−/− mice. This high-calcium (2%) and high-phosphate (1.2%) and lactose (20%)-containing diet has been previously shown to increase serum calcium levels and normalize bone mineralization in Cyp27b1−/− mice (47). This diet was started in 3-week-old mice, just after weaning and continued for 9 weeks. This regimen increased serum calcium levels in Cyp27b1−/− mice (from 4.7 ± 0.5 to 9.1 ± 0.4 mg/dL) and Gcm2−/− mice (from 4.5 ± 0.4 to 8.4 ± 0.8 mg/dL) but had no effect on serum calcium in WT animals (Table 4). In addition, the rescue diet reduced serum phosphate levels in WT (from 6.9 ± 1.5 to 6.1 ± 1.2 mg/dL) and Gcm2−/− (from 12.7 ± 0.9 to 8.4 ± 1.7 mg/dL) mice and increased serum phosphate (from 6.0 ± 0.8 to 7.5 ± 0.7 mg/dL) in Cyp27b1−/− mice. The rescue diet suppressed PTH levels in Cyp27b1−/− mice (from 12834.1 ± 3147.3 to 37.0.±22.6 mg/dL) but had no effect on the normal PTH levels in WT mice or the suppressed PTH levels in Gcm2−/− mice.
Table 4.
Serum Biochemistry and Expression Fold Changes in Bone and Renal Transcripts of 12-Week-Old Mice After 9 Weeks of a High-Calcium Diet Administration
| Rescue Diet | WT |
Cyp27b1−/− |
Gcm2−/− |
|||
|---|---|---|---|---|---|---|
| − | + | − | + | − | + | |
| Serum | ||||||
| FGF-23 | 83.5 ± 25.3 | 282.7 ± 40.2a | 5.5 ± 3.8b | 121.8 ± 23.7ac | 29.4 ± 15.3b | 115.9 ± 22.4ac |
| PTH | 32.2 ± 14.3 | 30.6 ± 15.4 | 12834.1 ± 3147.3b | 37.0.±22.6a | 1.2 ± 1.7b | 2.1 ± 3.3acd |
| PO4− | 6.9 ± 1.5 | 6.1 ± 1.2 | 6.0 ± 0.8b | 7.5 ± 0.7ac | 12.7 ± 0.9b | 8.4 ± 1.7a |
| Ca2+ | 9.5 ± 1.2 | 8.9 ± 0.5 | 4.7 ± 0.5b | 9.1 ± 0.4a | 4.5 ± 0.4b | 8.4 ± 0.8a |
| BUN | 24.5 ± 9.4 | 20.9 ± 6.8 | 28.6 ± 10.5 | 22.1 ± 3.7 | 25.3 ± 7.1 | 22.4 ± 8.2 |
Abbreviations: BUN, blood urea nitrogen; Ca2+, calcium; PO4, phosphate. Serum biochemistry was n ≥ 5/group. Comparisons were performed using a Student t test. Data are presented as mean ± SD. Units were as follows: FGF-23, picograms per milliliter; PTH, picograms per milliliter; 1,25(OH)2D, picomoles; PO4−, Ca2+, and BUN, milligrams per deciliter.
Compared with untreated animals of the similar genotype.
P < .05 compared with untreated WT.
Compared with treated WT.
Compared with Cyp27b1−/− animals administered a similar treatment.
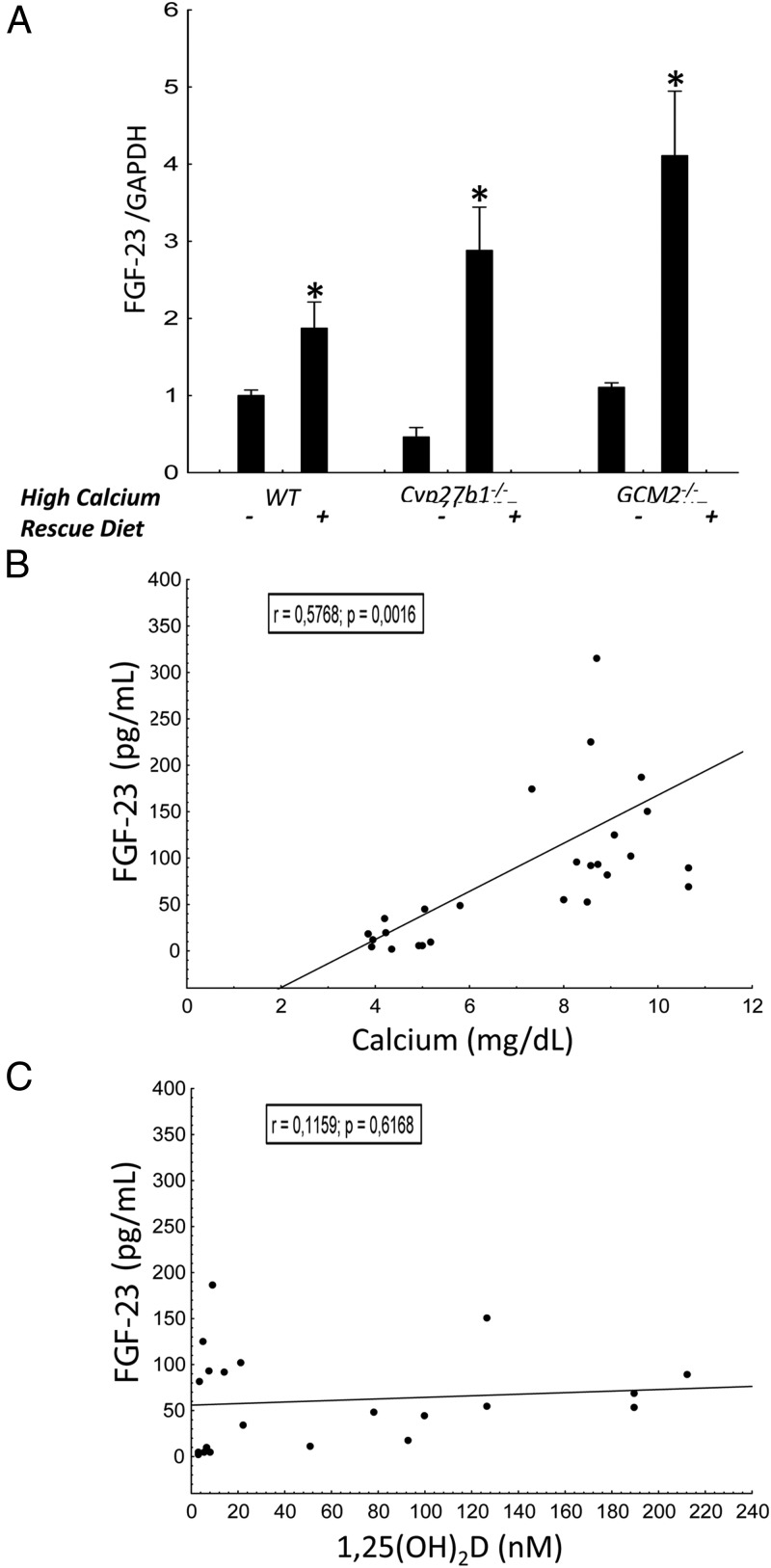
The rescue diet increased serum FGF-23 levels (Table 3) and FGF-23 mRNA expression (Figure 3A) in all three groups. In WT mice, FGF-23 concentrations increased approximately 3-fold above the levels observed in mice treated with a normal diet and normalized serum FGF-23 levels in Cyp27b1−/− (from 5.5 ± 3.8 to 121.8 ± 23.7 mg/dL) and Gcm2−/− (from 29.4 ± 15.3 to 115.9 ± 22.4 mg/dL) mice. The increase in FGF-23 levels correlated with increments in serum calcium (Figure 3B) but not with 1,25(OH)2D (Figure 3C) concentrations.
Figure 3.
Effects of 9-week high-calcium diet administration on the phenotype of 12-week-old WT and mutant mice. A, FGF-23 mRNA expression as assessed in the femurs and expressed as fold change of WT mice (n ≥ 3/group). Comparisons were performed using a Student t test. *, P < .05 vs untreated WT. Data are presented as mean ± SD. Spearman correlations between serum FGF-23 and calcium (B) and 1,25(OH)2D (C) are shown.
The administration of the rescue diet decreased bone remodeling expression markers in WT and Gcm2−/− femurs (Supplemental Table 6), with the exception of osteocalcin and normalized the expression of these bone markers in Cyp27b1−/− mice. The administration of the rescue diet had limited impact on bone mineralization and microarchitecture in WT and Gcm2−/− mice but increased BMD and trabecular bone volume in Cyp27b1−/− mice (Supplemental Figure 2, E and F). With regard to the kidney, Cyp27b1 expression was reduced in both WT and Cyp27b1−/− mice (Supplemental Table 6), whereas Cyp24a1 expression was increased in both groups. α-Klotho, the FGF-23 coreceptor, was increased in all three treated groups compared with the untreated controls of similar genotype, whereas the expression of TRPV5 decreased by approximately 50% in all three groups.
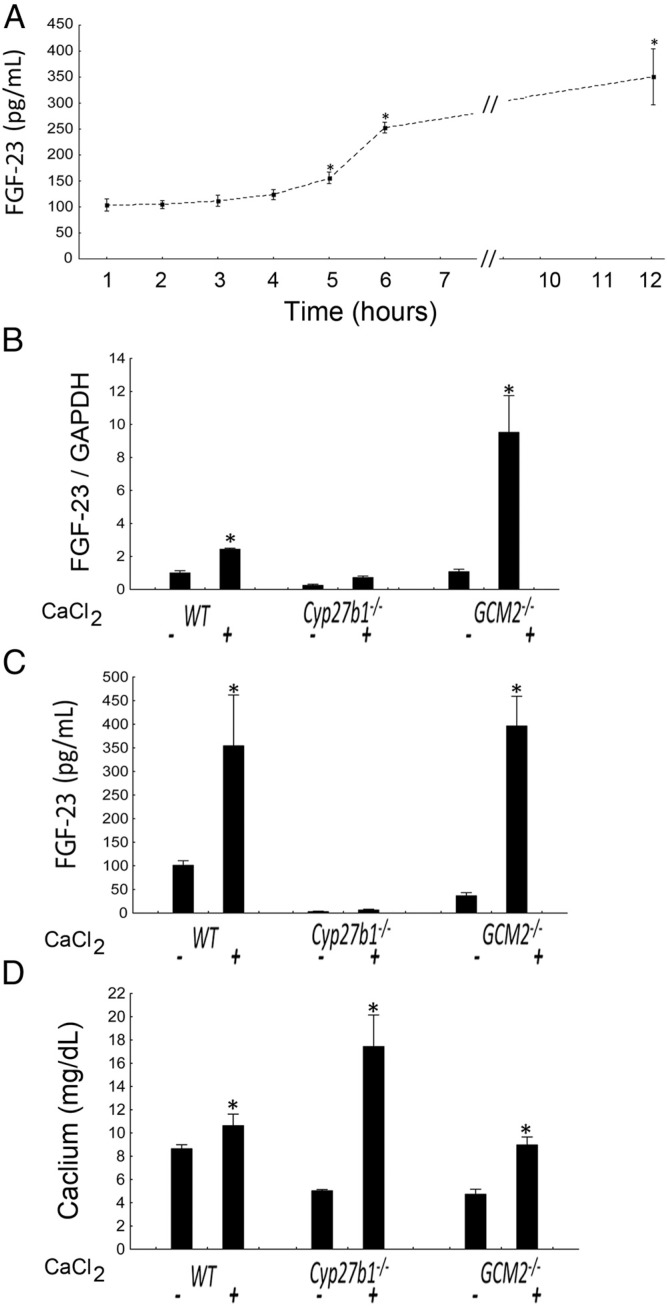
To detect if acute administration of calcium was equally able to stimulate FGF23 production, we administered the animals with a Ca-chloride bolus and observed that after 12 hours the circulating levels of FGF-23 significantly increased in all the groups, with the exception of Cyp27b1−/− mice (Figure 4), and in parallel with increase in serum calcium levels. The absence of FGF-23 stimulation in Cyp27b1−/− mice may relate to their bone defect and the incapacity to produce FGF23 until bone mineralization has reached a certain threshold. Regardless, the fact that calcium is able to stimulate FGF-23 in such a short period of time suggests that these effects are direct.
Figure 4.
Effects of a single CaCl2 3% solution-200 μL administration on FGF-23 production in WT, Cyp27b1−/−, and Gcm2−/− mice. A, Time course of FGF-23 after injection in WT animals (n ≥ 5/group). B, FGF-23 mRNA expression as assessed in the femurs and expressed as fold change of WT mice after 12 hours (n ≥ 3/group). C, Circulating FGF-23 levels after 12 hours (n ≥ 5/group). D, Circulating calcium levels after 12 hours (n ≥ 5/group). Comparisons were performed using a Student t test. *, P < .05 vs untreated WT.
Finally, we examined the effects genetic ablation of Cyp27b1 and Gcm2, as well as both dietary calcium supplementation and acute ip injections of calcium chloride, in the Col4a3−/− mouse model of CKD (Supplemental Table 7 and Supplemental Figure 3). We have previously shown that Col4a3−/− mice have markedly elevations of FGF-23 (46). Similar to observations in mice with normal renal function, we found that hypocalcemia was associated with decreased FGF-23 in both Cyp27b1−/−Col4a3−/− and Gcm2−/−Col4a3−/− mice. Moreover, dramatically elevated PTH levels in Cyp27b1−/−Col4a3−/− did not result in increased FGF-23 production (Supplemental Table 7). Administration of doxercalciferol or the high-calcium rescue diet further increased FGF-23 levels in Col4a3−/− mice and restored FGF23 levels in compound Cyp27b1−/−Col4a3−/− and Gcm2−/−Col4a3−/− mice. Because calcium is still able to stimulate FGF-23 in our CKD model indicates that this mechanism is still active in the settings of the disease.
Effects of calcium supplements on FGF-23 production in vitro
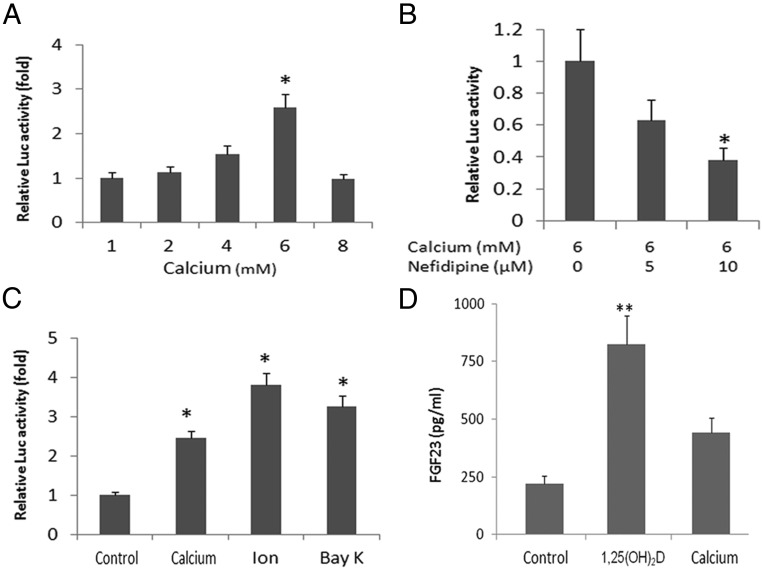
To study the mechanisms of regulation of FGF-23 by extracellular calcium, we cultured MC3T3-E1 cells and examined FGF-23 promoter activities in response to changes of extracellular calcium concentrations. Addition of calcium to the culture medium resulted in a dose-dependent increase in FGF-23 promoter activity in MC3T3-E1 osteoblasts, achieving a maximal 2.5-fold increase in promoter activity at a calcium concentration of 6 mM (Figure 5A). The effect of extracellular calcium (6 mM) to stimulate FGF-23 promoter activity was blocked by nifedipine, an L-type calcium channel blocker, also in a dose-dependent manner (Figure 5B). Calcium ionophores, ionomycin, and Bay K8466 at concentrations of 1 and 10 μM, respectively, significantly increased FGF-23 promoter activity in MC3T3-E1 osteoblasts transfected with the FGF-23 promoter (Figure 5C).
Figure 5.
Up-regulation of FGF-23 promoter activity by a high level of extracellular calcium. MC3T3–E1 (3–5 × 104) cells were seeded in 6-cm diameter tissue culture plates in MEM-α (Life Technologies) with 10% fetal calf serum at 37°C in the presence of 5% CO2 in a humidified incubator. Cells were plated 18 hours before transfection and fed with fresh medium 4 hours before transfection. Transfection (0.25 μg of mFGF-23 promoter plasmid DNA) was carried out for 16–18 hours, and then cells were washed twice with PBS and incubated in fresh medium containing 10% fetal calf serum for 38 hours. To test the effect of extracellular calcium on FGF-23 regulation, the cells cited above were switched into calcium-free DMEM medium for 4 hours and then cultured in serum-free DMEM/F12 media for another 12–18 hours in the presence of indicated concentrations of extracellular calcium. A, Up-regulation of FGF-23 promoter activity by a high level of extracellular calcium (1–6 mM). B, Nifedipine (10 μM) blocked the effect of extracellular calcium on FGF-23 promoter activity in a dose-dependent manner. C, Ionomycin (Ion; 1 μM) and Bay K8466 (BAY; 10 μM) stimulated FGF-23 promoter activity. D, 1,25(OH)2D3 and calcium stimulated secretion of FGF-23 by MC3T3–E1 cells cultured in a differentiation medium. *, P < .05 compared with controls.
We also demonstrated that MC3T3-E1 cells secreted FGF-23 protein into the media (Figure 5D). Both 1,25 dihydroxyvitamin D3 (1,25-(OH)2D3; 100 nM) and calcium (6 mM) added to cells cultured under conditions that promote osteoblasts differentiation [ie, medium containing α-MEM, ascorbic acid (50 μg/ml) and β-glycerolphosphate (10 mM)] resulted in increased FGF-23 levels. In contrast, 1,25(OH)2D3 (100 nM), PTH (100 nM), or phosphate (3 mM) had no effect on the FGF23 promoter activity in osteoblastic MC3T3-E1 cells cultured in the medium containing low calcium (1 mM) (data not shown). In addition, cotransfection of a calcium-sensing receptor (CaSR), a class C G protein-coupled receptor, which senses extracellular levels of calcium, did not affect FGF-23 promoter activities (Supplemental Figure 4).
Discussion
We found an essential role of calcium in the regulation of FGF-23. By comparing FGF-23 regulation in mutant Cyp27b1−/− mice, which lack the ability to synthesize 1,25(OH)2D, and Gcm2−/− mice, which fail to develop parathyroid glands, we discovered that 1,25(OH)2D and PTH could stimulate FGF-23 only if hypocalcemia was not present. An increase in both serum FGF-23 levels and bone expression of FGF-23 mRNA after doxercalciferol administration were delayed in Cyp27b1−/− compared with wild-type mice, indicating that 1,25(OH)2D regulation of FGF-23 is modified by concurrent hypocalcemia, impaired bone mineralization or other factors. Moreover, high circulating PTH concentrations do not stimulate serum FGF-23 concentrations in the absence of 1,25(OH)2D, and the presence of hypocalcemia. A role of calcium in determining the circulating FGF-23 levels is further supported by the strong correlation between serum calcium and serum FGF-23 concentrations in the mutant mouse models. An in vivo role of calcium in regulating FGF-23 was also demonstrated by the specific effect of a high-calcium rescue diet to increase serum FGF-23 concentrations in Cyp27b1−/− mice and Gcm2−/− mice. Calcium-mediated increase in serum FGF-23 levels appears to be due, at least in part, to an increase in FGF-23 gene transcription, as evidenced by the effect of a high-calcium diet to increase bone FGF-23 mRNA levels. The in vivo studies were not designed to determine whether the effects of calcium are direct or indirect.
A possible direct effect of calcium to regulate FGF-23 expression was identified by the effects of extracellular calcium to stimulate both FGF-23 promoter activity and FGF-23 protein secretion in MC3T3-E1 osteoblasts. Such a direct stimulation of FGF-23 production by osteoblasts is at variance with our prior investigations that failed to show an effect of extracellular calcium to regulate FGF-23 expression in ROS17/2.8 osteoblasts (19). The variable response is likely a limitation of the clonal cell lines used to study FGF-23, which can show variable responses to factors known to stimulate FGF-23 expression in vivo (54). Although it is tempting to speculate that extracellular calcium may regulate FGF-23 through the CasR or the related G-protein receptor, class C6A in bone, overexpression of these receptors failed to activate FGF23 promoter activity, consistent with other studies that have excluded are role of CasR in regulating FGF-23 (36, 55). Rather, our studies suggest involvement of L-type voltage-sensitive calcium channels in regulating FGF-23 expression, as evidenced by the fact that calcium stimulation of FGF-23 promoter activity in osteoblasts was inhibited by nifedipine. Bone mineralization and turnover has also been shown to regulate FGF-23 expression (22). Despite the evidence linking mineralization with FGF-23 expression, we found no specific alterations in bone microarchitecture in calcium-treated mice to account for the increase in FGF-23. Further studies investigating effects of calcium on local factors regulating FGF-23 expression in bone are warranted. Finally, the magnitude of the change in FGF-23 mRNA levels was not proportional to the increase in serum FGF-23 levels, suggesting an additional role for posttranscriptional regulation of FGF-23.
Alterations in FGF-23 also correlate with changes in serum calcium in various clinical settings (27, 56, 57). For example, a significant positive correlation between FGF-23 levels with serum-corrected calcium and intact PTH levels in patients with primary hyperparathyroidism (58). In addition, dietary deprivation and surgical parathyroidectomy models to induce hypocalcemia levels resulted in reduced circulating FGF-23 concentrations in rats and that the administration of calcium gluconate rapidly increased serum FGF-23 in parathyroidectomized rats (37). In vitamin D receptor-null mice, calcium supplementation also has been reported to increase FGF-23 mRNA levels (15), similar to our findings in Cyp27b1−/− mice in the current study. Although a controversial association, use of dietary calcium supplements has recently been linked to increased cardiovascular mortality (59), and similarly, FGF-23 is linked to cardiovascular mortality in CKD (40) as well as to a weaker extent in the general population (60). Further studies are warranted to determine whether FGF-23 is a mediator of the adverse effects of calcium on cardiovascular outcomes.
The observation that calcium regulates FGF-23 has important physiological implications by defining a role for FGF-23 in calcium homeostasis. Because FGF-23 suppresses 1,25(OH)2D production, the presence of circulating FGF-23 in settings of hypocalcaemia would be maladaptive because FGF-23 would counter the calcemic effects of PTH. Our findings indicate that this does not occur because hypocalcemia leads to reductions in FGF-23 production by bone and thereby adds another layer of protection against hypocalcemia by removing FGF-23 suppression of 1,25(OH)2D production by the kidney. In contrast, in the setting of hypercalcemia, the high circuiting calcium levels would suppress PTH but stimulate FGF-23. FGF-23 would further mitigate the hypercalcemia by exerting potential additive effects to directly suppress PTH expression from the PTG (27) as well as act to suppress 1,25(OH)2D production by the kidney. In addition, reductions in FGF-23 would be predicted to increase α-Klotho expression in the kidney and consequent enhancement of TRPC5-dependent distal tubule calcium absorption. In this regard, FGF-23 and 1,25(OH)2D had opposite effects on α-Klotho expression, with FGF-23 inhibiting and 1,25(OH)2D stimulating α-Klotho message levels in the kidney (61). α-Klotho expression was decreased in Cyp27b1−/− mice with low 1,25(OH)2D and FGF-23 levels, and α-Klotho expression was increased in response to doxercalciferol, suggesting that 1,25(OH)2 is a more important regulator of α-Klotho than FGF-23.
Our results also provide additional insights into the complexities of PTH regulation of FGF-23 production (27, 28, 62). PTH can directly stimulate FGF-23 production in bone through activation of PTH receptor osteoblasts (27, 63, 64), as evidenced by the effects of activating mutations of the PTH1R and GNAS genes (21) and overexpression of a constitutively active PTH receptor in osteocytes (6, 27, 57, 65–67) to stimulate serum FGF-23 concentrations (27, 28, 62). PTH may also indirectly increase FGF-23 through the stimulation of 1,25(OH)2D production (68) or indirectly due to PTH regulation of paracrine bone factors, such as suppression of sclerostin (SOST) (69).
In the current study, we show that the effects of 1,25(OH)2D predominate over actions of PTH in regulation of FGF-23, such that high levels of PTH fail to stimulate FGF-23 in Cyp27b1−/− mice, similar to vitamin D receptor−/− mice in which serum FGF-23 is undetectable despite elevations of PTH (70). Indeed, we found a negative correlation between PTH and FGF-23 by multiple regression analysis, consistent with studies showing that administration of PTH may not stimulate FGF-23 levels in some experimental settings (29). Previous studies in healthy volunteers have also indicated that infusion of PTH1–34 may increase FGF-23 through the actions of 1,25(OH)2D (71), although calcium effects have not been observed. Collectively these findings indicate that PTH stimulation of FGF-23 is dependent on the concurrent calcium and 1,25(OH)2D functions. Other studies show that PTH regulation of FGF-23 is also modified by whether PTH induces a net anabolic or catabolic effect on bone remodeling (22, 27), possibly through actions of PTH to suppress SOST (27). Finally, it is important to note that absence of PTH in Gcm2−/− mice does not impact upon FGF23 mRNA expression, suggesting that PTH may regulate posttranslational modifications of FG23 and secretion. This discrepancy between circulating intact FGF-23 levels and FGF-23 mRNA levels is also observed when animals are administered with doxercalciferol, further emphasizing that FGF-23 is transcriptionally and posttranscriptionally regulated.
An important regulatory role of serum phosphate is not supported by our findings. In this regard, we found that hyperphosphatemia is associated with low circulating FGF-23 levels in Gcm2−/− mice, and serum phosphate, in contrast to calcium and 1,25(OH)2D, did not predict changes in FGF-23 levels in the multivariable regression analysis. Thus, the increased phosphate content of the high-calcium rescue diet is unlikely to be responsible for the observed changes in serum FGF-23. Other data suggest that high serum phosphate per se does not stimulate FGF-23 to the degree observed in the current study. Indeed, the administration of high dietary phosphate to healthy human volunteers resulted in only a 12% increase in serum FGF-23 levels (72). Thus, although FGF-23 regulates serum phosphate, phosphate is not a major regulator of FGF-23 compared with calcium and 1,25(OH)2D. In contrast, PTH, 1,25(OH)2D and FGF-23 discordantly regulate renal Npt2c and Npt2a message expression in mutant mice. Indeed, in Cyp27b1−/− mice, Npt2c, but not Npt2a, expression was significantly elevated. Either doxercalciferol or PTH treatment suppressed Npt2c in both Cyp27b1−/− and Gcm2−/− mice, whereas doxercalciferol and PTH, respectively, increased Npt2a message in Cyp27b1−/− and Gcm2−/− mice. The significance of the discordant changes in Npt2a and Npt2c expression is uncertain because the posttranscriptional mechanism leading to the insertion of Npt2a and Npt2c are more important that transcriptional control of renal phosphate transport by PTH and FGF-23 (73).
In conclusion, these findings provide new insights into the physiological role of calcium in regulation of FGF-23 and a role of FGF-23 in calcium homeostasis. In addition, to the function of FGF-23 to act as a counterregulatory hormone to mitigate vitamin D toxicity, our studies show that suppression of FGF-23 is a physiological adaptive response to protect the organism against hypocalcemia by removing the FGF-23-mediated suppression of Cyp27b1 and stimulating α-Klotho expression, thereby enhancing circulating 1,25(OH)2D levels and distal tubule calcium transport through stabilization of membrane TRPV5, respectively.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1-AR45955 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- CaSR
- calcium-sensing receptor
- CKD
- chronic kidney disease
- FGF-23
- fibroblast growth factor 23
- mFGF23
- mouse FGF-23 promoter
- Npt2
- sodium-phosphate transport protein 2
- 1,25(OH)2D
- 1,25 dihydroxyvitamin D3
- 1,25-(OH)2D3
- 1,25 dihydroxyvitamin D3
- PTG
- parathyroid gland
- PTH1R
- PTH receptor 1
- RANKL
- receptor activator of nuclear factor-κB ligand
- SOST
- suppression of sclerostin
- TRAcP
- tartrate-resistant acid phosphatase
- TRPV5
- transient receptor potential cation channel subfamily V member 5
- WT
- wild type.
References
- 1. Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426 [DOI] [PubMed] [Google Scholar]
- 2. Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49 [DOI] [PubMed] [Google Scholar]
- 3. Stubbs J, Liu S, Quarles LD. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin Dialysis. 2007;20:302–308 [DOI] [PubMed] [Google Scholar]
- 4. Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498 [DOI] [PubMed] [Google Scholar]
- 5. Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774 [DOI] [PubMed] [Google Scholar]
- 7. Yu X, Ibrahimi OA, Goetz R, et al. Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology. 2005;146:4647–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phillips CM, Goumidi L, Bertrais S, et al. Leptin receptor polymorphisms interact with polyunsaturated fatty acids to augment risk of insulin resistance and metabolic syndrome in adults. J Nutr. 2010;140:238–244 [DOI] [PubMed] [Google Scholar]
- 9. Quarles LD. Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest. 2003;112:642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–1647 [DOI] [PubMed] [Google Scholar]
- 12. Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086 [DOI] [PubMed] [Google Scholar]
- 15. Shimada T, Yamazaki Y, Takahashi M, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Renal Physiol. 2005;289:F1088–F1095 [DOI] [PubMed] [Google Scholar]
- 16. Tomiyama K, Maeda R, Urakawa I, et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci USA. 2010;107:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quarles LD. The bone and beyond: ’Dem bones’ are made for more than walking. Nat Med. 2011;17:428–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goebel S, Lienau J, Rammoser U, et al. FGF23 is a putative marker for bone healing and regeneration. J Orthop Res. 2009;27(9):1141–1146 [DOI] [PubMed] [Google Scholar]
- 19. Liu S, Tang W, Zhou J, Stubbs JR, et al. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315 [DOI] [PubMed] [Google Scholar]
- 20. Kolek OI, Hines ER, Jones MD, et al. 1α,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–G1042 [DOI] [PubMed] [Google Scholar]
- 21. Brown WW, Juppner H, Langman CB, et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2009;94:17–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology. 2009;150:4835–4845 [DOI] [PubMed] [Google Scholar]
- 23. Oliver GJ, Maruthappu M, Shalhoub J. How do we continue to attract the best candidates to the surgical profession? Int J Surg. 2012;10:102–103 [DOI] [PubMed] [Google Scholar]
- 24. Shalhoub J, Davies KJ, Hasan N, Thapar A, Sharma P, Davies AH. The utility of collaborative biobanks for cardiovascular research. Angiology. 2012;63:367–377 [DOI] [PubMed] [Google Scholar]
- 25. Wohrle S, Bonny O, Beluch N, et al. FGF receptors control vitamin D and phosphate homeostasis by mediating renal FGF-23 signaling and regulating FGF-23 expression in bone. J Bone Miner Res. 2011;26:2486–2497 [DOI] [PubMed] [Google Scholar]
- 26. Barthel TK, Mathern DR, Whitfield GK, et al. 1,25-Dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol. 2007;103:381–388 [DOI] [PubMed] [Google Scholar]
- 27. Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299:F882–F889 [DOI] [PubMed] [Google Scholar]
- 28. Rhee Y, Bivi N, Farrow E, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49(4):636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gutierrez OM, Smith KT, Barchi-Chung A, Patel NM, Isakova T, Wolf M. (1–34) Parathyroid hormone infusion acutely lowers fibroblast growth factor 23 concentrations in adult volunteers. Clin J Am Soc Nephrol. 2012;7:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234 [DOI] [PubMed] [Google Scholar]
- 31. Isakova T, Gutierrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2010;26(2):584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oliveira RB, Cancela AL, Graciolli FG, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol. 2010;6(2):257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishida Y, Taketani Y, Yamanaka-Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147 [DOI] [PubMed] [Google Scholar]
- 35. Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–5364 [DOI] [PubMed] [Google Scholar]
- 36. Quinn SJ, Thomsen AR, Pang JL, et al. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am J Physiol Endocrinol Metab. 2013;304:E310–E320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodriguez-Ortiz ME, Lopez I, Munoz-Castaneda JR, et al. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012;23:1190–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xiao Q, Murphy RA, Houston DK, Harris TB, Chow WH, Park Y. Dietary and supplemental calcium intake and cardiovascular disease mortality: the National Institutes of Health-AARP diet and health study. JAMA Intern Med. 2013;173:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leifsson BG, Ahren B. Serum calcium and survival in a large health screening program. J Clin Endocrinol Metab. 1996;81:2149–2153 [DOI] [PubMed] [Google Scholar]
- 40. Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michaelsson K, Melhus H, Warensjo Lemming E, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ. 2013;346:f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dardenne O, Prud'homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1α-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141 [DOI] [PubMed] [Google Scholar]
- 43. Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111:1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dai B, David V, Alshayeb HM, et al. Assessment of 24,25(OH)2D levels does not support FGF23-mediated catabolism of vitamin D metabolites. Kidney Int. 2012;82:1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dai B, David V, Martin A, et al. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PloS One. 2012;7:e44161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stubbs JR, He N, Idiculla A, et al. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. 2012;27:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dardenne O, Prud'homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). Bone. 2003;32:332–340 [DOI] [PubMed] [Google Scholar]
- 48. Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295:E254–E261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dardenne O, Prudhomme J, Hacking SA, Glorieux FH, St-Arnaud R. Rescue of the pseudo-vitamin D deficiency rickets phenotype of CYP27B1-deficient mice by treatment with 1,25-dihydroxyvitamin D3: biochemical, histomorphometric, and biomechanical analyses. J Bone Miner Res. 2003;18:637–643 [DOI] [PubMed] [Google Scholar]
- 50. Li H, Martin A, David V, Quarles LD. Compound deletion of Fgfr3 and Fgfr4 partially rescues the Hyp mouse phenotype. Am J Physiol Endocrinol Metab. 2011;300:E508–E517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Forster RE, Jurutka PW, Hsieh JC, et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coburn JW, Maung HM, Elangovan L, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43:877–890 [DOI] [PubMed] [Google Scholar]
- 53. St-Arnaud R, Arabian A, Akhouayri O, Knutson JC, Strugnell SA. Differential effects of oral doxercalciferol [Hectorol(R)] or paricalcitol [Zemplar(R)] in the Cyp27b1-null mouse model of uremia. Nephron Exp Nephrol. 2011;119:e67–e74 [DOI] [PubMed] [Google Scholar]
- 54. Saini RK, Kaneko I, Jurutka PW, et al. 1,25-Dihydroxyvitamin D(3) regulation of fibroblast growth factor-23 expression in bone cells: evidence for primary and secondary mechanisms modulated by leptin and interleukin-6. Calcif Tissue Int. 2013;92:339–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Finch JL, Tokumoto M, Nakamura H, et al. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1315–F1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Evenepoel P, Viaene L, Meijers B. PTH, FGF23, and calcium: it takes three to tango? Kidney Int. 2011;80:1377. [DOI] [PubMed] [Google Scholar]
- 57. Sato T, Tominaga Y, Ueki T, et al. Total parathyroidectomy reduces elevated circulating fibroblast growth factor 23 in advanced secondary hyperparathyroidism. Am J Kidney Dis. 2004;44:481–487 [PubMed] [Google Scholar]
- 58. Kobayashi K, Imanishi Y, Miyauchi A, et al. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154:93–99 [DOI] [PubMed] [Google Scholar]
- 59. Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg). Heart. 2012;98:920–925 [DOI] [PubMed] [Google Scholar]
- 60. Westerberg PA, Tivesten A, Karlsson MK, et al. Fibroblast growth factor 23, mineral metabolism and mortality among elderly men (Swedish MrOs). BMC Nephrol. 2013;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haussler MR, Haussler CA, Whitfield GK, et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J Steroid Biochem Mol Biol. 2010;121:88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rhee Y, Allen MR, Condon K, et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res. 2011;26(5):1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu S, Tang W, Fang J, Ren J, Li H, Xiao Z, Quarles LD. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jean G, Bresson E, Terrat JC, et al. Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant. 2009;24:948–955 [DOI] [PubMed] [Google Scholar]
- 65. Kawata T, Imanishi Y, Kobayashi K, et al. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688 [DOI] [PubMed] [Google Scholar]
- 66. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51 [DOI] [PubMed] [Google Scholar]
- 67. Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99 [DOI] [PubMed] [Google Scholar]
- 68. Lopez I, Rodriguez-Ortiz ME, Almaden Y, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475–482 [DOI] [PubMed] [Google Scholar]
- 69. Onuchic L, Ferraz-de-Souza B, Mendonca BB, Correa PH, Martin RM. Potential effects of alendronate on fibroblast growth factor 23 levels and effective control of hypercalciuria in an adult with Jansen's metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 2012;97:1098–1103 [DOI] [PubMed] [Google Scholar]
- 70. Yu X, Sabbagh Y, Davis SI, Demay MB, White KE. Genetic dissection of phosphate- and vitamin D-mediated regulation of circulating Fgf23 concentrations. Bone. 2005;36:971–977 [DOI] [PubMed] [Google Scholar]
- 71. Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ. Effects of hPTH(1–34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res. 2009;24:1681–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vervloet MG, van Ittersum FJ, Buttler RM, Heijboer AC, Blankenstein MA, ter Wee PM. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011;6:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weinman EJ, Boddeti A, Cunningham R, et al. NHERF-1 is required for renal adaptation to a low-phosphate diet. Am J Physiol Renal Physiol. 2003;285:F1225–F1232 [DOI] [PubMed] [Google Scholar]