Abstract
Using the retrogradely transported immunotoxin, antidopamine β-hydroxylase-saporin (DSAP), we showed previously that hindbrain catecholamine neurons innervating corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus are required for glucoprivation-induced corticosterone secretion. Here, we examine the metabolic consequences of the DSAP lesion in male rats using indirect calorimetry. Rats injected into the paraventricular nucleus of the hypothalamus with DSAP or saporin (SAP) control did not differ in energy expenditure or locomotor activity under any test condition. However, DSAP rats had a persistently higher respiratory exchange ratio (RER) than SAPs under basal conditions. Systemic 2-deoxy-D-glucose did not alter RER in DSAP rats but rapidly decreased RER in SAP controls, indicating that this DSAP lesion impairs the ability to switch rapidly from carbohydrate to fat metabolism in response to glucoprivic challenge. In SAP controls, 2-deoxy-D-glucose-induced decrease in RER was abolished by adrenalectomy but not adrenal denervation. Furthermore, dexamethasone, a synthetic glucocorticoid, decreased RER in both SAP and DSAP rats. Thus, rapid switching of metabolic substrate use during glucoprivation appears to be due to impairment of the catecholamine-mediated increase in corticosterone secretion. Sustained elevation of basal RER in DSAP rats indicates that catecholamine neurons also influence metabolic functions that conserve glucose under basal conditions.
A continuous glucose supply is essential for the function and survival of neurons in the brain. Maintaining the brain's glucose supply depends on a number of glucoregulatory responses that are elicited during glucoprivation (ie, glucose deficit) (1–6). These include the activation of the hypothalamo-pituitary-adrenocortical system, resulting in secretion of CRH, ACTH, and glucocorticoids (GCs). GCs significantly increase breakdown of triglycerides and release of glycerol into the circulation from different adipose depots both in vivo and in vitro (7–10). GCs also stimulate de novo synthesis of lipids in the liver. Furthermore, palmitate levels and turnover in circulation, indicators of effective adipose tissue lipolysis, are increased by GC treatment with a parallel increase in lipid oxidation and a decreased respiratory quotient (7, 11). GCs appear to increase lipolysis-stimulating hormone-sensitive lipase and lipoprotein lipase in vivo and in vitro, after binding to their cognate intracellular receptors (12–15). Thus, an important role of GC secretion during glucose deficit is to mediate a system-wide switch from carbohydrate to fat metabolism in peripheral tissues, an action that promotes conservation of glucose.
2-Deoxy-D-glucose (2DG), a competitive inhibitor of intracellular glucose metabolism (16), and insulin-induced hypoglycemia produce central glucose deficits that trigger release of GCs. Previous work has shown that the catecholamine neurotransmitters, norepinephrine (NE) and/or epinephrine (E), stimulate release of CRH from parvocellular neurons in the paraventricular nucleus of the hypothalamus (PVH) (17, 18). In addition, previous work has demonstrated that use of the selective retrogradely transported catecholamine immunotoxin, antidopamine-β-hydroxylase (DBH)-saporin (DSAP), destroys hypothalamically-projecting catecholamine neurons required for corticosterone (CORT) secretion in response to systemic 2DG-induced glucoprivation or insulin-induced hypoglycemia in rats (19, 20). Importantly, the secretory deficit produced by this targeted toxin appears to impair the CORT response to glucose deficit selectively, because neither the CORT response to nonglucoprivic challenges nor the circadian rhythm of CORT secretion was impaired by this lesion. Consistent with this result, neurons that secrete CRH were not damaged by this DSAP lesion. Numbers of CRH-expressing neurons were not reduced by DSAP injection, and neither Fos expression nor induction of heterogeneous nuclear RNA in CRH neurons in response to systemic 2DG was impaired (20). Together, these results demonstrate that release of GCs in response to glucose deficit is dependent on hindbrain catecholamine neurons.
In the present experiments, we examine the metabolic consequences of the DSAP lesion. We test the hypothesis that destruction of hypothalamically-projecting hindbrain catecholamine neurons by DSAP significantly alters the metabolic response to systemic glucoprivation and that this alteration results from impairment of glucoprivation-induced CORT secretion. We tested our hypothesis using indirect calorimetry to examine respiratory exchange ratio (RER) (an indicator of the energy substrate being metabolized), energy expenditure (EE) (or metabolic rate), and locomotor activity in DSAP-lesioned and saporin (SAP) control rats. These parameters were recorded 1) under basal conditions, 2) during glucoprivation, 3) after an injection of dexamethasone (DEX), a synthetic GC analog, 4) after adrenal denervation, and 5) after adrenalectomy (ADX).
Materials and Methods
Animals
Male Sprague-Dawley rats were purchased from Simonsen Laboratories and housed individually in an animal care facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were maintained on a 12-hour light, 12-hour dark cycle (lights on at 7 am) with ad libitum access to rodent food (F6 Rodent diet; Harlan Teklad) and tap water (or 0.9% saline after ADX). All experimental procedures were approved by Washington State University Institutional Animal Care and Use Committee, which conforms to National Institutes of Health Guidelines.
DSAP microinjection, adrenal denervation, and ADX surgeries
Rats (12 wk old) were anesthetized using 1.0-mL/kg body weight of ketamine/xylazine/acepromazine cocktail in saline (50-mg/kg ketamine HCl [Fort Dodge Animal Health], 5.0-mg/kg xylazine [Vedco], and 1.0-mg/kg acepromazine [Vedco]) and placed in a stereotaxic device. The immunotoxin, DSAP (82 ng/200 nL; Advanced Targeting Systems) or control unconjugated SAP (17.2 ng/200 nL), dissolved in 0.1M PBS (pH 7.4), was infused bilaterally through a pulled glass capillary pipette (30-μm tip diameter) positioned stereotaxically just dorsal to the targeted site in the PVH. Solutions were delivered using a Picospritzer (Parker). Stereotaxic coordinates for the PVH injection site were 1.8 mm caudal and ± 0.45 mm lateral to bregma and 7.3–7.4 mm ventral to dura mater (21). The amount of unconjugated SAP in the control solution was equal to the amount of SAP present in the DSAP conjugate (21%), as indicated in the manufacturer's product information. A 3-week interval was allowed to elapse between the DSAP injections and further experimentation to permit retrograde transport of the toxin and complete degeneration of lesioned neurons (19). For confirmation of DSAP-induced retrograde lesion in hindbrain catecholamine neurons, DSAP-injected rats were screened for glucoprivic feeding 3 weeks after DSAP injection into PVH. Rats were injected sc with 2DG (200-mg/kg body weight in 0.9% sterile saline; Sigma-Aldrich) or with 0.9% sterile saline as control. Food intake was measured during the 4-hour period after the injection. At the end of experimentation, the DSAP lesion on hindbrain catecholamine neurons was confirmed by quantifying DBH-positive cells in hindbrain catecholamine cell groups. As noted in previously published work comparing SAP and noninjected controls, SAP control injections did not produce behavioral or significant histological signs of toxicity (19).
Circadian patterns of metabolism and locomotor activity and the responses to 2DG or saline were recorded between 4 and 5 weeks after DSAP or SAP injection. Changes in metabolic parameters and 2DG-induced responses were recorded in adrenal intact, adrenal denervated, and ADX rats, as described for SAP and DSAP injection. Bilateral adrenal denervation was performed under anesthesia by transection of the adrenal nerve (splanchnic) branches at their point of entry into the adrenal glands (22). Changes in metabolic parameters and 2DG-induced responses were recorded beginning 1 week after denervation. Bilateral ADX was subsequently performed under anesthesia, and the changes in metabolic parameters and 2DG-induced responses were recorded beginning 2 weeks later. After ADX, 0.9% saline and water were provided for all rats.
Measurements of hourly feeding, metabolic parameters, and locomotor activity
For automated measurements of daily feeding, metabolic and activity patterns, rats were housed singly in open-circuit Oxymax chambers (Columbus Instruments). The experimental room was maintained at 21 ± 1°C on a 12-hour light, 12-hour dark cycle (light period 7 am to 7 pm). Food (powdered F6 rodent food) and tap water were available ad libitum, except as noted. All rats were acclimatized to the monitoring cages for more than 3 days before the beginning of automated data collection. An equal number of rats from each experimental group (eg, SAP/saline, SAP/2DG, DSAP/saline, and DSAP/2DG) was run simultaneously in each test cycle. Feeding data were collected each minute. For the analysis of daily feeding patterns, 12-hour food intakes during the daytime and nighttime periods were averaged for 2 days. O2 consumed (VO2), CO2 generated (VCO2), x-axis activity (number of beam breaks due to horizontal movements), and z-axis activity (number of beam breaks due to vertical movements) were measured for each rat. Changes as x-axis activity were similar to z-axis changes and are not presented here. Metabolic measures were recorded every 20 minutes. RER was calculated as the ratio of VCO2 over VO2. EE was calculated as EE = (3.815 + 1.232 × RER) × VO2. After normalization with respect to body weight, averages of each parameter over 2 days for the diurnal, and nocturnal periods, respectively, were calculated and compared. For 2DG- or DEX-induced changes in each parameter, comparisons were made between the 3-hour period before and the 6-hour period after each injection. Food was removed at the time of injections (11 am), except in the one experiment where the effect of food on the response to 2DG was specifically tested. Data during the first hour after the injection were discarded due to disruption by handling on rats' activities and metabolic parameter readings.
Drugs
Glucoprivation was produced by sc injection of 2DG (250 mg/kg; Sigma-Aldrich) or saline control. To reveal the effects of exogenous GCs on metabolic parameters, the synthetic GC analog, DEX (Sigma-Aldrich), was dissolved in saline and injected sc at doses of 0.1- and 0.5-mg/kg body weight.
Immunohistochemistry
Rats were killed at the end of experimentation by deep isoflurane anesthesia. After perfusion and fixation in 4% formalin in PBS, brains were sectioned coronally into 4 serial sets (40-μm thickness) and stained using standard avidin-biotin-peroxidase immunohistochemical techniques (23, 24). Cell bodies expressing DBH in hindbrain regions were detected by mouse anti-DBH (1:100 000; Millipore), and numbers of DBH cells/side were counted bilaterally in 3 consecutive coronal sections at each of 4 anatomical levels (see below). Catecholamine cell groups are defined according to the Paxinos and Watson stereotaxic atlas (21). However, we have adopted additional terms to subdivide cell group C1 along its rostral-caudal extent (25, 26). The caudal portion of C1 overlaps cell group A1 and, as in the atlas, is designated as A1/C1 (−13.78 to −13.3 mm). We refer here to the middle portion of C1 as C1m, which extends from −12.98 to −12.5 mm caudal to bregma. The rostral portion (C1r) extends rostrally another 0.5 mm to the caudal border of the facial nucleus (−12.18 to −11.7 mm caudal to bregma). DBH-positive fibers and CRH-positive cells in PVH were detected with mouse anti-DBH antibody (Millipore) and a rabbit anti-CRH antibody (1:1000; Advanced Targeting Systems) to reveal specific and nonspecific effects of the lesion at the injection sites. Numbers of CRH-positive cells/side in PVH were counted bilaterally in 2 consecutive coronal sections at −1.56 to −1.72 mm caudal to bregma.
Statistical analysis
All results are presented as mean ± SEM. For statistical analysis of data, we used unpaired t test, one-way ANOVA, two-way ANOVA, or two-way repeated measures ANOVA with SigmaStat (Systat Software). After significance was determined by ANOVA, multiple comparisons between individual groups were tested using a post hoc Fisher's least significance difference test using P < .05 as the criterion for statistical significant.
Results
Effect of PVH DSAP injections on food intake and body weight
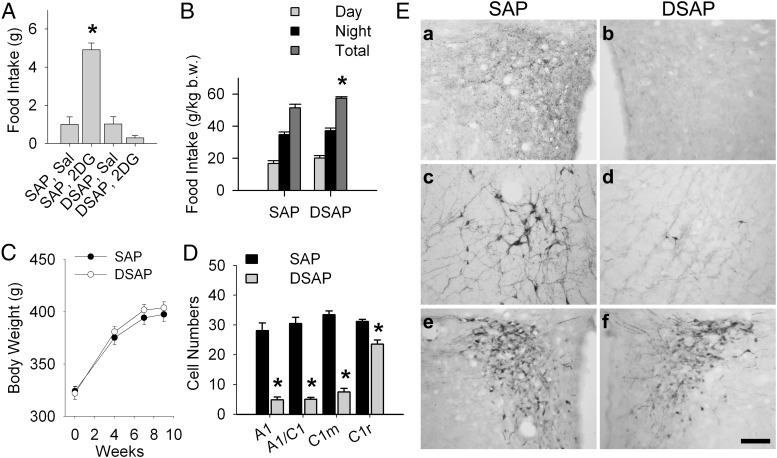
Effects of the DSAP lesion on feeding and body weight are shown in Figure 1. Figure 1A shows that 2DG (200 mg/kg) significantly increased 4-hour food intake in SAP rats (P < .001 vs SAP saline), while failing to enhance food intake in the DSAP rats (n = 8 rats per group), providing preliminary evidence for lesion effectiveness. Figure 1B shows that the DSAP lesion did not disrupt the day-night pattern of spontaneous feeding, although the total 24-hour food intake was slightly but significantly elevated in DSAP rats (P < .01), compared with SAP controls. Body weights throughout the 9-week experimental period did not differ between SAP and DSAP rats (Figure 1C).
Figure 1.
Food intake, body weight, and DBH-ir and CRH-ir in PVH SAP and DSAP rats. (A) Total 4-hour food intake measured at 3 weeks after saline (Sal) or 2DG (200 mg/kg) injection in SAP and DSAP rats. *, P < .001 vs SAP Sal rats; n = 8 rats for each treatment. (B) Food intake (12-h daytime, 12-h nighttime, and total 24-h feeding) was measured 5 weeks after SAP or DSAP injection and normalized to body weight (b.w.) at the time of the test. *, P < .01 vs SAP rats; n = 8 rats/treatment. (C) Body weight changes in SAP and DSAP rats (n = 8 rats/group) during the 9-week experimental period after SAP or DSAP injection into PVH. (D) Number of DBH-positive cells in ventrolateral medullary catecholamine cell groups in SAP and DSAP rats. *, Ps < .001 vs SAP rats for each region; n = 8 rats/treatment. (E) Representative photomicrographs showing coronal sections through the PVH (a, b, e, and f) and A1/C1 (c and d) from rats injected into the PVH with SAP (left) or DSAP (right). DBH-ir reveals loss of terminal and fiber staining in the PVH (top row), and loss of DBH-positive cells (middle row) in DSAP injected rats, but an intact CRH-ir cell population in PVH of both SAP and DSAP rats (bottom row). Scale bar, 0.5 mm.
Quantification of DBH-immunoreactive (ir) cell bodies in cell groups A1, A1/C1, C1m, and C1r at the conclusion of testing further confirmed the efficacy of the DSAP lesion (P < .001 vs SAP rats) (Figure 1D). Numbers of DBH-positive neurons in the other hindbrain catecholamine regions, ie, A2, C2, and C3, but not A5 or A7, were also significantly decreased in the present study (data not shown), as reported in our previous published work using the same DSAP lesion technique (19, 20, 25, 26). In addition, DBH-ir fibers and terminals in the medial hypothalamus and DBH-ir cell bodies in hindbrain were reduced or eliminated by the retrograde DSAP lesion, as shown for the PVH (Figure 1E, a and b) and A1/C1 (Figure 1E, c and d), respectively. However, CRH-positive cells in the PVH did not appear to differ in DSAP compared with SAP rats (Figure 1E, e and f), 109.3 ± 6.9 in SAP rats vs 104.5 ± 6.1 cells/side in DSAP rats (n = 8/group; P > .6), indicating that they were not damaged nonspecifically by the DSAP injection. These effects of the PVH DSAP injection were similar to those we have reported previously (19, 20).
Metabolic parameters in adrenal intact, adrenal denervated, and ADX rats under basal conditions
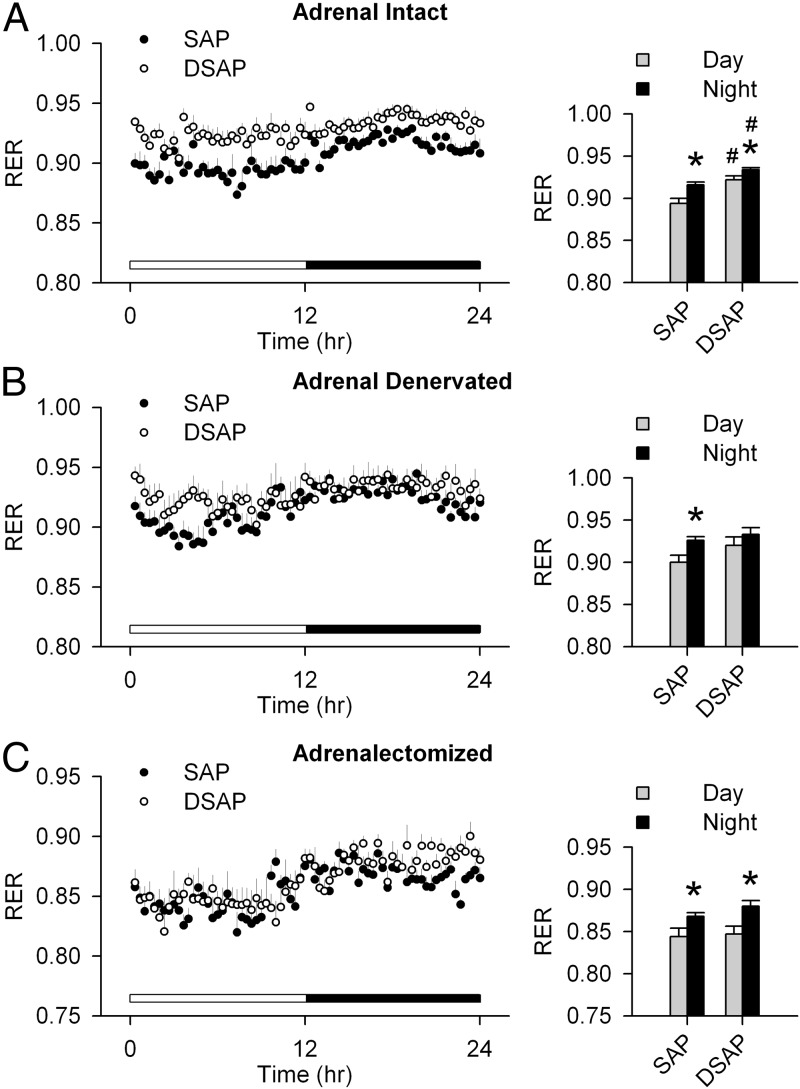
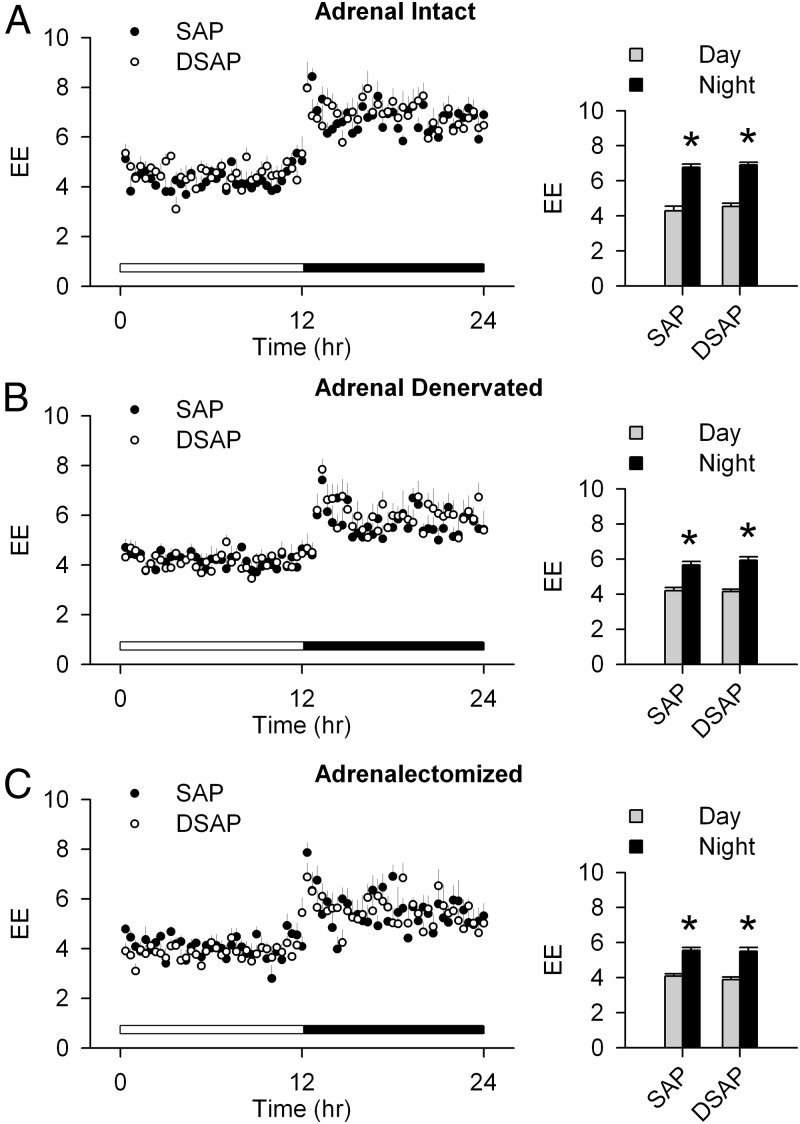
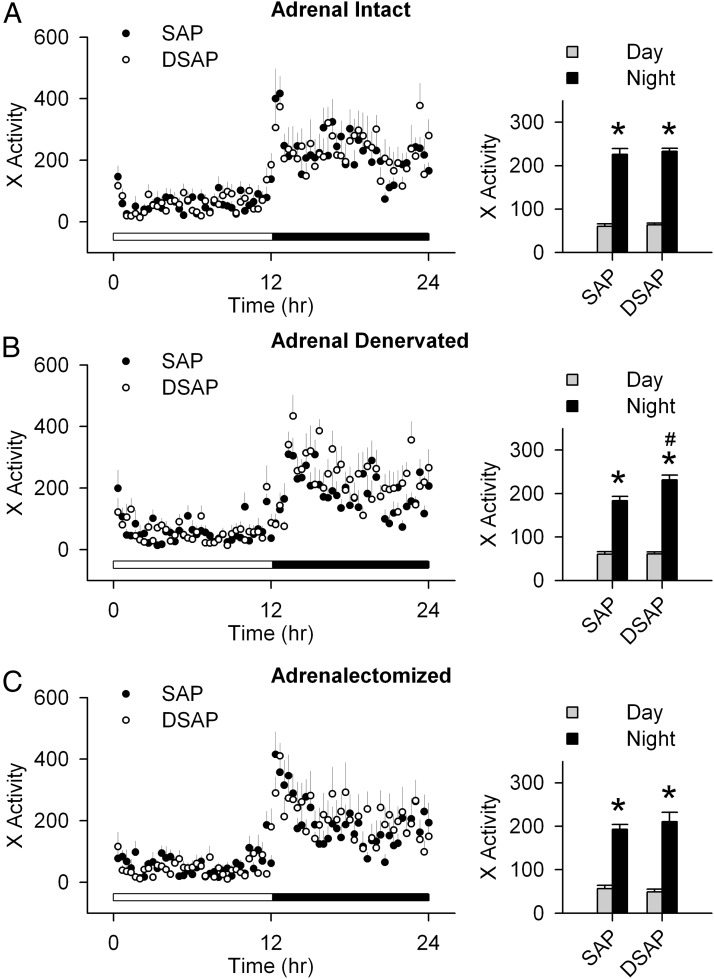
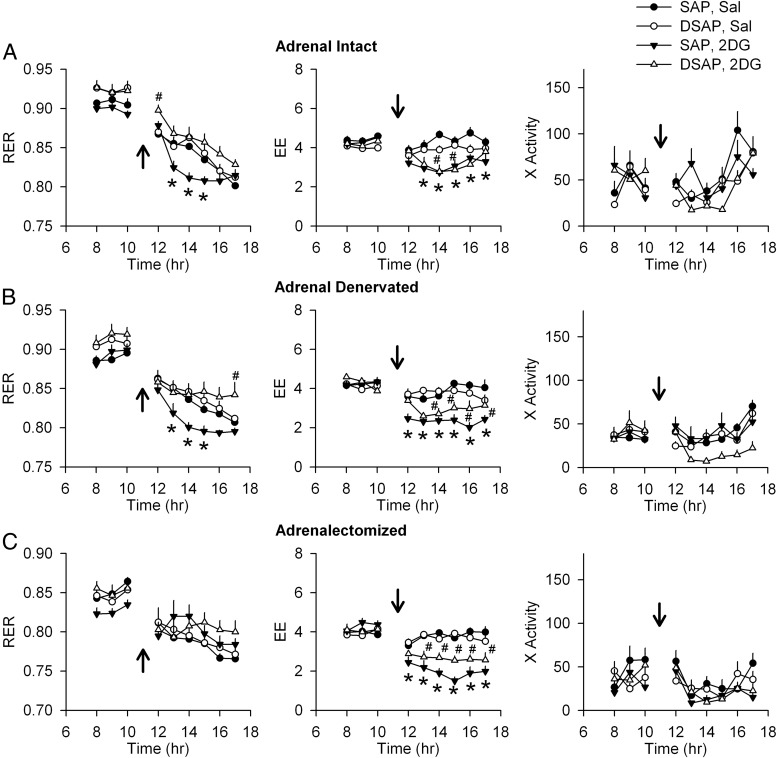
Adrenal intact SAP-injected rats had a clear day-night rhythm, with a higher RER during the night period. The adrenal intact DSAP rats also had a day-night rhythm in RER similar to SAP rats. However, in DSAP rats, both daytime and nighttime RERs were elevated compared with SAP rats (Figure 2A). The day-night difference in RER was lost after adrenal denervation in DSAP rats (Figure 2B). After ADX, RER in SAP and DSAP groups was indistinguishable (Figure 2C). On the other hand, there were no differences between SAP and DSAP rats in EE or locomotor activity under basal conditions (adrenal intact) or after adrenal denervation, or ADX (Figures 3 and 4). Under all conditions, both SAP and DSAP rats showed clear day-night rhythms in EE and activity.
Figure 2.
RER during day and night in intact (A), adrenal denervated (B), and ADX (C) SAP and DSAP rats (left panels). Day and night periods are indicated by white and black bars, respectively, above the x-axis. Averages over 2 days for the diurnal and nocturnal periods are presented as bar graphs (right panels). Adrenal intact DSAP rats had a higher RER during both day and night than SAP rats. ADX abolished that effect but did not disrupt the day-night rhythm of the RER; n = 7–8 rats/group. *, P < .05 vs daytime within the same treatment group; #, P < .05 vs SAP rats.
Figure 3.
Day-night changes of EE (or metabolic rate, in kcal/kg·h) in SAP and DSAP rats under adrenal intact (A), adrenal denervated (B), and ADX (C) conditions. Bar graphs show EE, normalized for body weight, and averaged over 2 days for the diurnal and nocturnal periods; n = 7–8 rats/group. *, P < .05 vs daytime within the same treatment group.
Figure 4.
Day-night measures of x-axis locomotor activity (in counts/20 min) in SAP and DSAP rats under adrenal intact (A), adrenal denervated (B), and ADX (C) conditions. Averages over 2 days for the diurnal and nocturnal periods, respectively, were calculated and are presented as bar graphs (right panels); n = 7–8 rats/group. *, P < .05 vs daytime within the same treatment group; #, P < .05 vs SAP rats.
Glucoprivation-induced changes in metabolic parameters when food was absent
In this experiment, metabolic parameters were investigated before and during the 6-hour period after 2DG or saline injection in SAP and DSAP rats. Food was removed immediately after 2DG or saline injection. As shown in Figure 5A, RER gradually decreased in both saline-injected SAP and DSAP groups during the 6-hour period of food deprivation. In contrast, RER fell abruptly after 2DG injection in SAP, but not in DSAP rats. In DSAP rats, RER fell slowly after 2DG injection, and its rate of decline was similar to saline-injected SAP rats. This occurred despite the fact that systemic 2DG decreased EE to a similar degree during hours 2–6 after 2DG injection in both SAP and DSAP rats (Figure 5A). Locomotor activity was not significantly altered from the saline baseline condition by the 2DG injection in either SAP or DSAP rats. The 2DG-induced decrease in RER was still observed in SAP rats after adrenal denervation (Figure 5B), but was completely abolished by ADX (Figure 5C). On the other hand, EE was significantly reduced after 2DG, compared with saline treatment, in both in SAP and DSAP rats in adrenal intact, adrenal denervated, and ADX conditions (Figure 5, B and C). However, 2DG reduced EE somewhat more in SAP than in DSAP rats at several, but not all, time points. This 2DG-induced suppression of EE was significantly (P < .05) greater in SAP than in DSAP groups at 12 and 16 hours (ie, 1 and 5 h after 2DG injection) in adrenal denervated rats and at 14 and 15 hours (ie, 3 and 4 h after injection in the ADX rats). Locomotor activity was not altered by either adrenal denervation or ADX compared with baseline and did not differ between SAP and DSAP rats.
Figure 5.
Effects of 2DG on metabolic parameters and locomotor activity. SAP and DSAP rats were injected with saline (Sal) or 2DG (250 mg/kg) at the time indicated by arrows, and food was removed. The lights were on between 7 am and 7 pm. Changes of RER (VCO2/VO2), EE (kcal/kg·h), and x-axis activity (counts/20 min) under adrenal intact (A), adrenal denervated (B), and ADX (C) conditions are shown. RER values decreased gradually in SAP/Sal, DSAP/Sal, and DSAP/2DG rats due to food removal. However, RER values (left column) fell abruptly in adrenal intact SAP/2DG rats (A). These effects were not significantly altered by adrenal denervation (B), but effects of 2DG on RER in SAP rats were eliminated by ADX (C). EE fell in all 2DG-treated rats (middle column), compared with Sal. Activity did not differ between groups. *, P < .05, SAP 2DG vs SAP Sal; #, P < .05, DSAP 2DG vs DSAP Sal (two-way repeated measures ANOVA; n = 7–8 rats/group).
Glucoprivation-induced changes in metabolic parameters when food was available
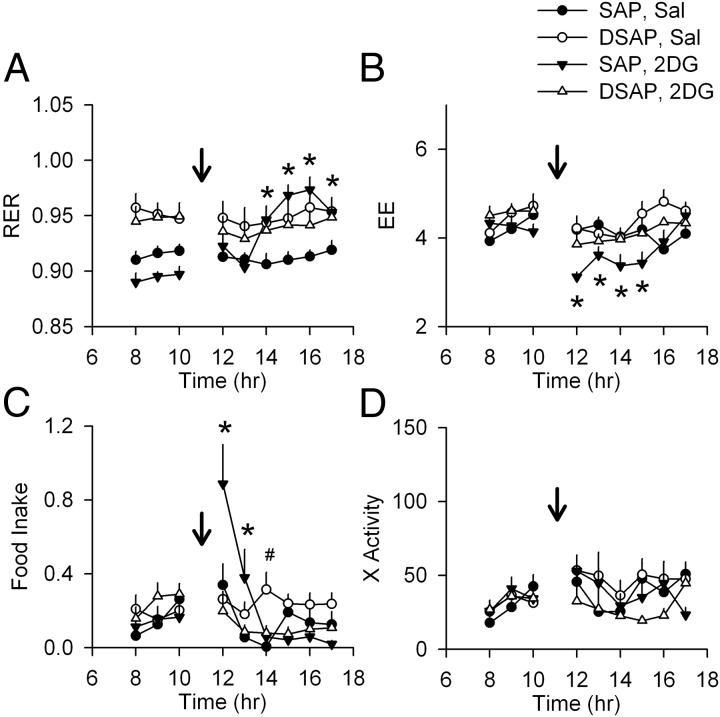
As shown in Figure 6, when food was available ad libitum during the entire experiment, food intake was increased in SAP rats within the first 2 hours after 2DG injection, followed by an increase in RER during hours 3–6, indicating a postabsorptive increase in carbohydrate use. During hours 1–4 after 2DG injection, EE was decreased in SAP rats. In contrast, 2DG did not produce any changes in RER or EE in DSAP rats. Although food was available, 2DG did not stimulate food intake in the DSAP animals, and hence, RER was not increased by postingestive factors. No significant changes in locomotion were observed between or within groups.
Figure 6.
Effects of 2DG on metabolic parameters and locomotor activity in SAP and DSAP rats during ad libitum access to food. Food was available ad libitum during the entire experiment. Changes of RER (VCO2/VO2), EE (kcal/kg·h), food intake (g/20min), and x-axis activity (counts/20 min) after saline (Sal) or 2DG injection (250 mg/kg, indicated by arrows) are shown in A–D, respectively (lights on from 7 am to 7 pm). In SAP rats given 2DG, RER fell briefly and then increased above the level of the other groups (A), presumably due to food intake and absorption (C). EE also initially fell in this group but was restored to control level upon eating. DSAP rats did not increase their food intake above their own predrug level in response to 2DG. *, P < .05, 2DG vs Sal in SAP rats; #, P < .05, 2DG vs Sal in DSAP rats, after two-way repeated measures ANOVA; n = 8 rats/group.
Effects of DEX injection on metabolic parameters
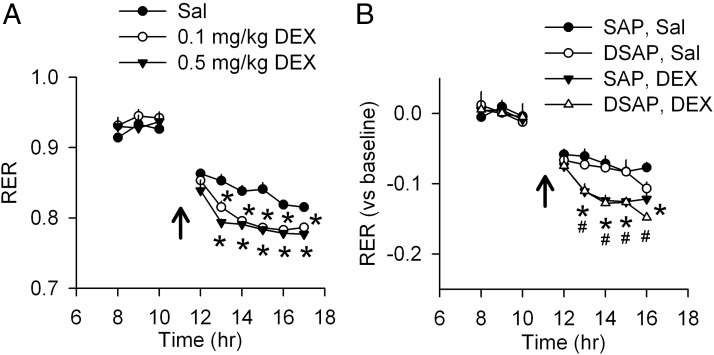
Effects of DEX on metabolic parameters in normal and PVH DSAP rats are shown in Figure 7. DEX injection (0.1 and 0.5 mg/kg) decreased RER in intact controls (Figure 7A), producing an effect similar to 2DG (Figure 5A), but had no effects on EE or locomotor activity (Supplemental Figure 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Similarly, DEX (0.5 mg/kg) decreased RER in both PVH SAP and DSAP rats (Figure 7B) but had no effects on EE or locomotor activity (Supplemental Figure 1B).
Figure 7.
Effects of DEX on metabolic parameters. (A) Saline (Sal) or DEX (at 0.1 or 0.5 mg/kg) was injected in naïve rats at the time indicated by arrows (lights on from 7 am to 7 pm). Food was removed after the injection. Changes in RER (VCO2/VO2) are shown. *, P < .05 vs Sal-injected rats, after two-way repeated measures ANOVA. No difference in EE or locomotor activity was found between treatments (data not shown); n = 5–6 rats/group. (B) Effects of DEX on RER in SAP and DSAP rats. Sal or DEX (0.5 mg/kg) was injected in PVH SAP and DSAP rats at the time as indicated by arrows. Food was removed after the injection. Changes of RER (VCO2/VO2), minus baseline (averages of 3 h before the injection), are shown. *, P < .05, DEX vs Sal in SAP rats; # P < .05, DEX vs Sal in DSAP rats, after two-way repeated measures ANOVA. No difference on EE or locomotor activity was found between treatments (data not shown); n = 6–8 rats/group.
Discussion
A novel finding in the present study is that the hindbrain catecholamine neurons that innervate the hypothalamus exert important control of metabolic substrate selection under basal conditions, as well as during glucoprivation. Under basal conditions, rats in which the NE and E innervation of the PVH and medial hypothalamus was eliminated by DSAP exhibited higher RER than SAP controls during both daytime and nighttime, indicating persistently enhanced carbohydrate use. In contrast to RER, locomotor activity and EE did not differ between groups. The increased RER in DSAP rats was not due to a difference in macronutrient intake, because all rats were fed the same mixed nutrient diet during the experimental period. Rather, the elevated RER appears to reflect the loss of a centrally mediated control of metabolic substrate selection that requires hindbrain catecholamine neurons.
During a 6-hour period of food deprivation after saline injection, RER gradually decreased in both SAP and DSAP groups. In contrast, 2DG caused an abrupt decrease in RER in SAP controls, indicating that SAP rats actively switched from carbohydrate to fat metabolism during glucoprivic challenge. In DSAP-lesioned rats, the rate of decline in RER was not altered by 2DG and declined at the same rate as after saline treatment. The absence of an acute drop in RER during glucoprivation in DSAP rats occurred despite the fact that systemic 2DG decreased total EE to a similar degree in both SAP and DSAP rats during the 2- to 6-hour period after 2DG injection. Together, these data indicate that the rapid decrease in RER in SAP rats in response to 2DG was not a direct result of inhibited glucose metabolism by 2DG, or the rapid decline would have occurred in 2DG-injected DSAP rats, as well as in SAP controls. Furthermore, the rapid decline in RER in SAP rats was not caused by a change in locomotor activity, because this parameter was not altered by glucoprivation in either SAP or DSAP rats. Rather, the data suggest that the decline in RER after 2DG in SAP controls was the result of an active switch of metabolic substrate selection from carbohydrate to fat, triggered by glucoprivation and mediated by hindbrain catecholamine neurons.
The contributions of CORT to fat metabolism are widely recognized but still poorly understood. However, in both humans and rodents, CORT increases lipolysis by stimulating transcription of lipolytic enzymes, leading to increased lipolysis and elevated circulating free fatty acids levels and glycerol (7, 9, 10), increased lipid oxidation, and a decrease in RER (7, 11). In addition, GCs are lipogenic, especially in specific adipose depots. It has been shown recently that GCs exert lipolytic and lipogenic effects simultaneously and that the lipogenic effects result from stimulation of preadipocyte differentiation in visceral adipose depots and the associated increase in visceral adipose mass (10).
It is also widely recognized that the output of the hypothalamo-pituitary-adrenocortical axis is increased by systemic 2DG-induced glucoprivation and insulin-induced hypoglycemia in rodents and humans (1, 20, 27–29), leading to an increase in fatty acid oxidation in peripheral tissues. Moreover, evidence that catecholamine neurons stimulate CRH is substantial. Both NE and E neurons innervate the parvocellular area of the PVH (30, 31), which contains CRH neurons. Systemic glucoprivation increases NE turnover in the hypothalamus (32–34). Paraventricular hypothalamic injection of catecholamines or neuropeptide Y, which are coexpressed in many ascending catecholamine neurons (35–37), increases CRH and CORT levels (17, 18) and CRH gene expression in vitro (38, 39). Furthermore, it is now known that the postsynaptic effect of the NE signal is transduced by activation of the MAPK signaling cascade in CRH neurons (40, 41). Cannula mapping studies examining brainstem sites from caudal medulla to the optic chiasm have shown that CORT levels are elevated by localized injection of the glucoprivic agent, 5-thioglucose, into hindbrain sites coextensive with NE and E cell groups A1, A2, and C1–C3 (42). Finally, it has been demonstrated that injection of DSAP, into the PVH area, severely and selectively impairs the glucoprivation-induced increase in circulating CORT levels, CRH hnRNA, or c-fos mRNA expression in CRH cell bodies without damaging CRH neurons themselves and without impairing the CORT response to swim stress or the circadian rhythm of CORT secretion (20). These results demonstrate that glucoprivation-induced CORT secretion requires the catecholamine innervation of the PVH.
We found that the GC receptor agonist, DEX, mimicked the effect of 2DG in producing a rapid fall in RER in both SAP and DSAP rats, confirming the hypothesis that the fall in RER is mediated by CORT. This response to DEX in DSAP rats suggests that the absence of the RER response to glucoprivation in these rats was not due to insensitivity to CORT. In light of these effects, it is puzzling that ADX increased basal fat metabolism. We cannot explain this result but suggest that it may reflect the restructuring of peripheral metabolism in response to loss of both the adrenal medulla and the adrenal cortex.
As noted, EE did not differ between SAP and DSAP rats under basal conditions and was decreased to a similar degree by 2DG in both groups when food was withheld. However, in contrast to 2DG, DEX had no effect on EE, as shown previously (7). Published work suggests that decreased thermogenesis may be the major mechanism contributing to reduction of EE during both central and peripheral glucoprivation (43–45), potentially providing an important means of conserving energy during glucoprivation. Decreased thermogenesis during glucoprivation arises from inhibition of sympathetic nerve activity in brown adipose tissue (46, 47), an effect requiring hindbrain serotonin neurons (48) and, as we show here, not dependent on CORT secretion.
Although 2DG-induced decrease in EE was similar in adrenal intact SAP and DSAP rats, we noted a somewhat attenuated 2DG-induced reduction in EE in DSAP, compared with SAP rats, after adrenal denervation and ADX (Figure 5, B and C). If reduced EE is a homeostatic response that conserves energy in the face of reduced glucose availability, as it appears to be, then a possible explanation for the difference in EE between ADX/denervated SAP and DSAP rats is that intact catecholamine neuron projections in SAP rats sustain an ability to reduce EE in response to glucoprivic challenge, whereas DSAP rats, due to destruction of ascending catecholamine systems, exhibit impairment of their ability to suppress EE. Active suppression of EE by catecholamine neurons could be mediated through their projections to dorsomedial hypothalamic or caudal raphe neurons involved in thermogenesis, as discussed above, or other aspects of metabolic control that promote energy conservation.
The results reported here make clear the fact that in response to glucoprivation, CORT effects on peripheral metabolism are rapid and profound, as would be expected of an actively mediated response. Indeed, the CORT response proactively conserves glucose. The importance of the rapid CORT-mediated increase in fat metabolism is readily understandable in the context of experimentally induced glucoprivation, in which the development of glucose deficit is also rapid and profound. However, this chain of responses is also relevant to more physiological scenarios associated with glucose deficit, such as starvation, in which glucose deficit develops gradually and is prolonged. In such a situation, the mobilization and use of fat not only preserves body carbohydrate stores but helps to maintain conditions that foster the synthesis of ketone bodies (49, 50) that can serve as a supplementary fuel for the brain over a prolonged period of glucose shortage. In addition to stimulating lipolysis in mature adipocytes, CORT (as noted above) simultaneously increases adipogenesis and increased fat cell mass (primarily in visceral adipose tissue depots) by stimulating preadipocyte differentiation (10), an effect that would provide additional storage capacity for fat when it is available in excess.
In summary, previous results have shown that hindbrain catecholamine neurons are required for key responses to glucoprivation, including those conferring immediate and those conferring longer range protection against severe central glucose deficit (5). Results from the present study reveal yet another role of these catecholamine neurons. They indicate that control of CORT secretion by hindbrain catecholamine neurons is required for rapid switching of metabolic substrate use from carbohydrate to fat during glucoprivation. The results also suggest a previously unrecognized role for hindbrain catecholamine neurons in promoting fat oxidation even under basal, nonglucoprivic conditions. This response, which would favor conservation and storage of glucose, also appears to require catecholamine-mediated stimulation of CORT secretion.
Acknowledgments
This work was supported by National Institutes of Health Grants DK040498 and DK081546 (to S.R.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADX
- adrenalectomy
- CORT
- corticosterone
- DBH
- dopamine-β-hydroxylase
- DSAP
- anti-DBH-saporin
- DEX
- dexamethasone
- 2DG
- 2-deoxy-D-glucose
- E
- epinephrine
- EE
- energy expenditure
- GC
- glucocorticoid
- ir
- immunoreactivity
- NE
- norepinephrine
- PVH
- paraventricular nucleus of the hypothalamus
- RER
- respiratory exchange ratio
- SAP
- saporin
- VCO2
- CO2 generation
- VO2
- O2 consumption.
References
- 1. Smith GP, Root AW. Effect of feeding on hormonal responses to 2-deoxy-D-glucose in conscious monkeys. Endocrinology. 1969;85:963–966 [DOI] [PubMed] [Google Scholar]
- 2. Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Adv Pharmacol. 1998;42:620–622 [DOI] [PubMed] [Google Scholar]
- 3. Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2010;31:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda). 2007;22:241–251 [DOI] [PubMed] [Google Scholar]
- 5. Ritter S, Li AJ, Wang Q, Dinh TT. Minireview: the value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit. Endocrinology. 2011;152:4019–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beall C, Ashford ML, McCrimmon RJ. The physiology and pathophysiology of the neural control of the counterregulatory response. Am J Physiol Regul Integr Comp Physiol. 2012;302:R215–R223 [DOI] [PubMed] [Google Scholar]
- 7. Djurhuus CB, Gravholt CH, Nielsen S, et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab. 2002;283:E172–E177 [DOI] [PubMed] [Google Scholar]
- 8. Novelli M, Pocai A, Chiellini C, Maffei M, Masiello P. Free fatty acids as mediators of adaptive compensatory responses to insulin resistance in dexamethasone-treated rats. Diabetes Metab Res Rev. 2008;24:155–164 [DOI] [PubMed] [Google Scholar]
- 9. Xu C, He J, Jiang H, et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol Endocrinol. 2009;23:1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell JE, Peckett AJ, D'souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am J Physiol Cell Physiol. 2011;300:C198–C209 [DOI] [PubMed] [Google Scholar]
- 11. Divertie GD, Jensen MD, Miles JM. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991;40:1228–1232 [DOI] [PubMed] [Google Scholar]
- 12. Slavin BG, Ong JM, Kern PA. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res. 1994;35:1535–1541 [PubMed] [Google Scholar]
- 13. Ottosson M, Vikman-Adolfsson K, Enerbäck S, Olivecrona G, Björntorp P. The effects of cortisol on the regulation of lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1994;79:820–825 [DOI] [PubMed] [Google Scholar]
- 14. Samra JS, Clark ML, Humphreys SM, MacDonald IA, Bannister PA, Frayn KN. Effects of physiological hypercortisolemia on the regulation of lipolysis in subcutaneous adipose tissue. J Clin Endocrinol Metab. 1998;83:626–631 [DOI] [PubMed] [Google Scholar]
- 15. Wang JC, Gray NE, Kuo T, Harris CA. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci. 2012;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism. 1962;11:1098–1112 [PubMed] [Google Scholar]
- 17. Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res Bull. 1988;21:905–912 [DOI] [PubMed] [Google Scholar]
- 18. Kakui N, Kitamura K. Direct evidence that stimulation of neuropeptide Y Y5 receptor activates hypothalamo-pituitary-adrenal axis in conscious rats via both corticotropin-releasing factor- and arginine vasopressin-dependent pathway. Endocrinology. 2007;148:2854–2862 [DOI] [PubMed] [Google Scholar]
- 19. Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216 [DOI] [PubMed] [Google Scholar]
- 20. Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–1367 [DOI] [PubMed] [Google Scholar]
- 21. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed Academic Press, San Diego; 2007 [Google Scholar]
- 22. Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res. 1998;805:41–54 [DOI] [PubMed] [Google Scholar]
- 23. Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–1191 [DOI] [PubMed] [Google Scholar]
- 24. Li AJ, Wang Q, Dinh TT, Ritter S. Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J Neurosci. 2009;29:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci. 2004;19:2147–2154 [DOI] [PubMed] [Google Scholar]
- 26. Li AJ, Wang Q, Ritter S. Differential responsiveness of dopamine-β-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology. 2006;147:3428–3434 [DOI] [PubMed] [Google Scholar]
- 27. Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest. 1996;98:680–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis SN, Shavers C, Davis B, Costa F. Prevention of an increase in plasma cortisol during hypoglycemia preserves subsequent counterregulatory responses. J Clin Invest. 1997;100:429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGregor VP, Banarer S, Cryer PE. Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. Am J Physiol Endocrinol Metab. 2002;282:E770–E777 [DOI] [PubMed] [Google Scholar]
- 30. Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325 [DOI] [PubMed] [Google Scholar]
- 31. Füzesi T, Wittmann G, Liposits Z, Lechan RM, Fekete C. Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucleus of the rat. Endocrinology. 2007;148:5442–5450 [DOI] [PubMed] [Google Scholar]
- 32. Bellin SI, Ritter S. Insulin-induced elevation of hypothalamic norepinephrine turnover persists after glucorestoration unless feeding occurs. Brain Res. 1981;217:327–337 [DOI] [PubMed] [Google Scholar]
- 33. Rowland NE. Effects of glucose and fat antimetabolites on norepinephrine turnover in rat hypothalamus and brainstem. Brain Res. 1992;595:291–294 [DOI] [PubMed] [Google Scholar]
- 34. Beverly JL, De Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am J Physiol Regul Integr Comp Physiol. 2001;280:R563–R569 [DOI] [PubMed] [Google Scholar]
- 35. Everitt BJ, Hökfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984;11:443–462 [DOI] [PubMed] [Google Scholar]
- 36. Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O'Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181 [DOI] [PubMed] [Google Scholar]
- 37. Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153 [DOI] [PubMed] [Google Scholar]
- 38. Plotsky PM, Cunningham ET, Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10:437–458 [DOI] [PubMed] [Google Scholar]
- 39. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629 [DOI] [PubMed] [Google Scholar]
- 40. Khan AM, Ponzio TA, Sanchez-Watts G, Stanley BG, Hatton GI, Watts AG. Catecholaminergic control of mitogen-activated protein kinase signaling in paraventricular neuroendocrine neurons in vivo and in vitro: a proposed role during glycemic challenges. J Neurosci. 2007;27:7344–7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan AM, Kaminski KL, Sanchez-Watts G, et al. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. J Neurosci. 2011;31:18479–18491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1792–R1798 [DOI] [PubMed] [Google Scholar]
- 43. Freinkel N, Metzger BE, Harris E, Robinson S, Mager M. The hypothermia of hypoglycemia. Studies with 2-deoxy-D-glucose in normal human subjects and mice. N Engl J Med. 1972;287:841–845 [DOI] [PubMed] [Google Scholar]
- 44. Thompson DA, Lilavivathana U, Campbell RG, Welle SL, Craig AB. Thermoregulatory and related responses to 2-deoxy-D-glucose administration in humans. Am J Physiol. 1980;239:R291–R295 [DOI] [PubMed] [Google Scholar]
- 45. Pénicaud L, Thompson DA, Le Magnen J. Effects of 2-deoxy-D-glucose on food and water intake and body temperature in rats. Physiol Behav. 1986;36:431–435 [DOI] [PubMed] [Google Scholar]
- 46. Egawa M, Yoshimatsu H, Bray GA. Effects of 2-deoxy-D-glucose on sympathetic nerve activity to interscapular brown adipose tissue. Am J Physiol. 1989;257:R1377–R1385 [DOI] [PubMed] [Google Scholar]
- 47. Gong TW, Horwitz BA, Stern JS. The effects of 2-deoxy-D-glucose and sympathetic denervation of brown fat GDP binding in Sprague-Dawley rats. Life Sci. 1990;46:1037–1044 [DOI] [PubMed] [Google Scholar]
- 48. Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R224–R232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agius L, Chowdhury MH, Alberti KG. Regulation of ketogenesis, gluconeogenesis and the mitochondrial redox state by dexamethasone in hepatocyte monolayer cultures. Biochem J. 1986;239:593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dardzinski BJ, Smith SL, Towfighi J, Williams GD, Vannucci RC, Smith MB. Increased plasma β-hydroxybutyrate, preserved cerebral energy metabolism, and amelioration of brain damage during neonatal hypoxia ischemia with dexamethasone pretreatment. Pediatr Res. 2000;48:248–255 [DOI] [PubMed] [Google Scholar]