Abstract
A variety of fundamental differences have evolved in the physiology of the human and rodent prolactin (PRL) systems. The PRL gene in humans and other primates contains an alternative promoter, 5.8 kbp upstream of the pituitary transcription start site, which drives expression of PRL in “extrapituitary” tissues, where PRL is believed to exert local, or paracrine, actions. Several of these extrapituitary PRL tissues serve a reproductive function (eg, mammary gland, decidua, prostate, etc), consistent with the hypothesis that local PRL production may be involved in, and required for, normal reproductive physiology in primates. Rodent research models have generated significant findings regarding the role of PRL in reproduction. Specifically, disruption (knockout) of either the PRL gene or its receptor causes profound female reproductive defects at several levels (ovaries, preimplantation endometrium, mammary glands). However, the rodent PRL gene differs significantly from the human, most notably lacking the alternative promoter. Understanding of the physiological regulation and function of extrapituitary PRL has been limited by the absence of a readily accessible experimental model, because the rodent PRL gene does not contain the alternative promoter. To overcome these limitations, we have generated mice that have been “humanized” with regard to the structural gene and tissue expression of PRL. Here, we present the characterization of these animals, demonstrating that the human PRL transgene is responsive to known physiological regulators both in vitro and in vivo. More importantly, the expression of the human PRL transgene is able to rescue the reproductive defects observed in mouse PRL knockout (mPRL−) females, validating their usefulness in studying the function or regulation of this hormone in a manner that is relevant to human physiology.
Prolactin (PRL) was discovered in the anterior pituitary (AP) gland as a lactogenic endocrine hormone (1, 2). Subsequent studies, culminating with targeted gene knockouts (KOs) of mouse PRL (mPRL) and its receptor (mPRL receptor [mPRL-R]), have demonstrated that PRL has multiple essential roles in female fertility (3–7).
PRL and GH diverged from a common ancestral gene early in vertebrate evolution (8). In mammals, the PRL gene has undergone amplification and diversification at least twice in rodents and ruminants, whereas in primates, there is a single PRL gene and an amplified GH locus (8). In spite of the apparent simplicity of having a single PRL gene, primates have diversified the physiological potential of PRL by having evolved 2 distinct promoters that differentially regulate its expression (9, 10). Understanding the human PRL (hPRL) locus has been hampered by the limited range of experimentally tractable models.
Expression of PRL in extrapituitary tissues is well documented but poorly understood (11, 12). In primates, including humans, extrapituitary PRL is driven by a distal promoter that is upstream of an extra noncoding exon (exon 1a) (9). Although rodents do not have a distal extrapituitary promoter, PRL transcript has been isolated from endometrial decidua and mammary glands (MGs) (13–15). Potential functions of extrapituitary PRL in vivo have been demonstrated using PRL KO mice. In the decidua of mice, local PRL may suppress expression of genes that are detrimental to pregnancy (6). In mouse MGs, local PRL, induced by Akt signaling, may drive proliferation in the peripartum (16).
In primates, the extrapituitary PRL promoter drives expression in a larger variety of tissues, including several male and female reproductive organs, such as the prostate, uterus, and MGs (17–20). However, ascribing functions to human extrapituitary PRL in vivo has been difficult. Although gene expression has been measured, few studies have measured the PRL protein. The mature peptide is identical to that produced in the AP, making it indistinguishable from circulating PRL and thereby further complicating in vivo studies.
Another fundamental difference between rodent and hPRL is that pregnancy in laboratory rodents is supported by a complex pattern of twice daily PRL surges that are initiated by copulation. Absent the long luteal phase of the human menstrual cycle, the usually short-lived rodent corpus luteum (CL) is rescued by the PRL surges, providing sufficient progesterone for decidualization and development of a hormonally active placenta (21).
To address the multiple differences between the rodent PRL and hPRL systems in vivo, we have developed mice carrying the entire PRL locus from the human genome, including both the proximal and distal promoters, in the absence of mPRL. These mice reveal that the reproductive deficits of mPRL KOs are rescued by hPRL and that hPRL is expressed in a variety of tissues that do not express measurable mPRL.
Materials and Methods
Animals and reagents
Male and female C57Bl/6J mice (Jackson Laboratories) were used to establish breeding colonies with transgenic founder animals. Animals (≤4 mo) were maintained on a 14-hour light, 10-hour dark cycle (lights on 6 am) with food and water available ad libitum. Animals were killed by rapid decapitation after 5 days of acclimation to the guillotine in order to obtain unstressed levels of PRL. Blood was clotted overnight at 4°C, and serum was separated by centrifugation (10 000g, 5 min, 4°C) and then stored at −20°C. All animal handling and procedures were carried out in a facility accredited by the American Association for the Accreditation of Laboratory Animal Care and were approved by the University of Cincinnati's Institutional Animal Care and Use Committee.
Horse serum (HS) and fetal bovine serum (FBS) were purchased from HyClone and Atlas Biologicals, Inc, respectively. RPMI 1640, L-glutamine, and penicillin-streptomycin were from Biofluids, Inc. Molecular biology reagents included: QuantiTect reverse transcription and QuantiFast SYBR Green PCR kits (QIAGEN), AccuPrime Taq PCR kits (Invitrogen), FastStart PCR reagents, and primers from Integrated DNA Technologies. All other reagents, unless otherwise noted below, were purchased from Sigma Chemicals.
Generation of hPRL-expressing mice
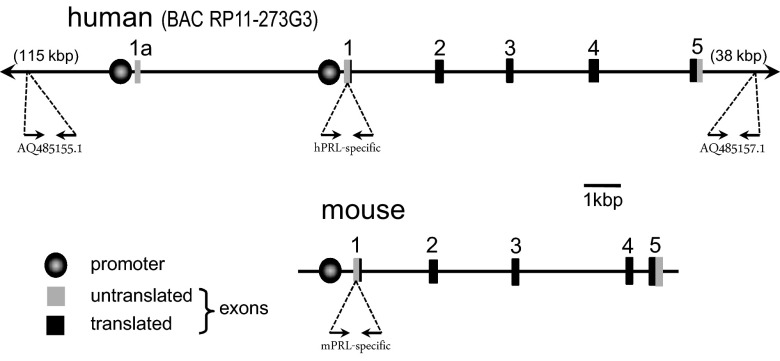
The bacterial artificial chromosome (BAC) RP11–273G3 from human chromosome 6 was purchased from the Children's Hospital Oakland Research Institute. This construct contains the entire hPRL gene, including the pituitary and extrapituitary promoters as well as 115 kbp upstream and 38 kbp downstream of the gene-encoding sequences (Figure 1). Single bacterial colonies were isolated on Luria broth agar plates (12.5 μg/mL chloramphenicol), and 1 was grown in Luria broth (12.5 μg/mL chloramphenicol). DNA was isolated using the QIAGEN EndoFree plasmid kit, and the purified DNA was submitted to the Animal Models Core at the University of North Carolina at Chapel Hill (http://www.med.unc.edu/amc) for pronuclear microinjection into fertilized C57Bl/6J X DBA2 hybrid mouse oocytes and implantation into pseudopregnant dams. Resulting offspring positive for the transgene (hPRL+) were shipped to the University of Cincinnati and put in mating units with wild-type (WT) mice. hPRL+ mice were subsequently crossed with C57Bl/6J mice in which the mPRL gene was deleted (mPRL−) (3).
Figure 1.
Schematics of the hPRL and mPRL loci. The 2-dimensional maps of the promoters and exons of the PRL genes are to scale, based on base pairs. The BAC RP11–273G3 construct used to generate the hPRL transgenic mice contains additional noncoding regions 115 kbp upstream and 38 kbp downstream. Primers for genotyping were designed to recognize unique sequences in the noncoding regions at the 5′ and the 3′ end of the BAC construct (GenBank ID AQ485155.1 and AQ485157.1, respectively). Primers for genotyping and RT-PCR were designed to recognize species-specific sequences in the 5′-untranslated region of exon 1 of the human and mPRL genes. See Supplemental Table 1 for primer sequences.
Genotyping
Founder animals and offspring carrying the BAC transgene were identified by PCR analysis of genomic DNA isolated from tail biopsies (22). Presence of the full BAC construct was verified using primers recognizing unique sequences in the noncoding regions at the 5′ and the 3′ extremes of the BAC construct (GenBank ID AQ485155.1 and AQ485157.1) (see Figure 1). The gene of interest (hPRL; accession number NM_000948) was verified using hPRL-specific primers designed against sequences in exon 1. Amplification protocols used a hot start at 94°C (2 min) followed by 30 sequential cycles (45-s duration each) of the following temperatures: 94°C (denaturing), TA°C (annealing), and 72°C (extending). For animals bred onto the mPRL− background, the mPRL genotype was determined using primers designed to recognize the WT (mPRL+) allele or the KO allele containing the neomycin cassette. Primer sequences and TA°C are presented in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
The nomenclature used within for our animals includes designations for the hPRL and mPRL genes and the allelic genotypes according to the following scheme: hPRL+ = hPRL+/0, hPRL0 = hPRL0/0, mPRL+ = mPRL+/+ or mPRL+/−, mPRL− = mPRL−/−.
AP cell dissociation and in vitro studies
AP cells were dissociated as previously described (23) from glands of males or females (random-cycling unless otherwise noted). Cells were plated onto sterile, glass coverslips (5 × 105 cells/slip) and maintained in multiwell dishes in DMEM with 10% heat-inactivated HS at 37°C in 5% CO2. Experiments assessing acute PRL release were performed 2 days after dissociation. Cells were preincubated for 30 minutes in a standard external solution (SES) (145mM NaCl, 5mM KCl, 2mM CaCl2, 1mM MgCl2, 10mM glucose, and 10mM HEPES-HCl; 37°C, 5% CO2) containing 0.01% BSA (SES-BSA). Coverslips were then moved to fresh wells containing 1 mL of SES-BSA with one of the following treatments: vehicle (0.5mM ascorbic acid), DA (dopamine, 300nM), or TRH (100nM). After 60 minutes, treatment media were collected, spun at 1000 rpm (5 min; 4°C), and the supernatants stored at −20°C until analyzed by RIA.
To assess PRL synthesis/storage in vitro, AP cells were plated directly into the wells of 12-well culture plates. Cells were incubated at 37°C in a DMEM modified medium base (no phenol red, 25mM HEPES-HCl, 10 μg/mL insulin, 15% charcoal-stripped, heat-inactivated HS, 2.5% charcoal-stripped, heat-inactivated FBS, 40 μg/mL gentamycin sulfate) containing vehicle (0.003% ethanol) or 17β-estradiol (E2) (100pM) for 6 days (media changed on d 3). On day 6, media were removed and cells lysed by trituration with 200 μL dH2O, followed by addition of 200 μL of 1.8% saline. The lysates were spun (1000 rpm; 5 min), and the supernatants were frozen at −20°C until analyzed by RIA.
In vivo studies
To assess pituitary PRL responsiveness to estrogen, intact male and female mice were given sc implants of 17β-E2 (0.05 mg, 21-d release; Innovative Research of America) or vehicle and killed 14 days later. AP glands were weighed and bisected. One half was flash frozen in liquid nitrogen and stored at −80°C until analyzed for hPRL transcript. The other half was homogenized in ice-cold dH2O. An equal volume of 1.8% saline was added and the homogenate spun at 12 000g for 10 minutes (4°C). Both supernatant and cell pellet were frozen separately at −20°C until analyzed for PRL protein or total protein, respectively. To test pituitary PRL responsiveness to DA, intact female mice received a sc injection of vehicle or bromocriptine mesylate (Brx) (2.5 mg/kg), an agonist of the D2 subtype of the DA receptor (D2R). Injections were administered between 9 and 11 am on diestrus and blood was collected 90 minutes later.
Some animals were subjected to immune challenge by ip injection of lipopolysaccharide (LPS) from Escherichia coli (3 mg/kg) and killed 16 hours later. Serum was harvested, as were spleen, thymus, and AP. Each organ was weighed, immediately homogenized in ice-cold dH2O, and processed as described above.
To assess reproductive development, female mice were monitored for vaginal opening (VO), after which daily lavages were performed to monitor estrous cyclicity. MG development was analyzed on MG whole mounts from 3½- to 4-month-old virgin female mice. To assess fertility, proestrous females (6 wk of age) were housed with proven sires and the time to delivery (days), and litter sizes were recorded. As an index of lactation performance of each dam, pups were weighed daily for the first 12 days of postnatal life, then every few days through weaning. Milk production/let-down was also assessed in weigh-suckle-weigh (WSW) experiments. For WSW, on postpartum day 10 (d 0 = day of delivery), pups were separated from the dam (to another room) for 4 hours. Six pups were weighed individually, returned to the dam, and allowed to suckle for 30 minutes, then weighed again. Pup weight gain (postsuckling weight minus presuckling weight) was calculated as an index of milk production. At the end of the suckling period, a blood sample from each dam was rapidly collected by tail snip and serum harvested as described above for measurement of suckling-induced PRL release.
Analysis of mRNA expression
Expression of hPRL and mPRL was determined with conventional RT-PCR. Whole-tissue samples (AP, MG, uterus, ovaries, testes, prostate, heart, kidney, liver, spleen, thymus, lung, skeletal muscle, small intestine, stomach, and fat) were dissected from hPRL+:mPRL+ mice, flash frozen in liquid nitrogen, and stored at −80°C. Frozen tissues were homogenized in ice-cold TRIzol, and RNA was extracted according to standard protocol guidelines (catalog number 15596–018; Invitrogen). Reverse transcription (RT+) of total RNA was completed following manufacturer's protocol (catalog number 205310; QIAGEN). Negative control reactions (RT−) were handled identically, except that RT enzyme was omitted. Primers corresponding to regions specific for either mPRL or hPRL were designed (Supplemental Table 2) and used in PCR with 1 μL of RT reaction and reagents from Roche FastStart PCR. Reactions were sequentially cycled 35 times for 45-second durations at each of the following temperatures: 94°C (denaturing), TA°C (annealing) (Supplemental Table 2), and 72°C (extending).
The abundance of hPRL mRNA in AP glands of E2-treated mice was determined using quantitative real-time PCR (qRT-PCR) of the hPRL transcript in comparison with mouse glyceraldehyde-3-phosphate dehydrogenase (mGAPDH) transcript. The qRT-PCR was performed using SYBR green (Applied Biosystems) and cycling conditions optimized for each primer set (Supplemental Table 2). Relative quantification ratios were calculated from the PCR efficiencies of the target gene transcripts and the crossing point deviation of the E2-treated vs the vehicle-treated samples, as described by Pfaffl (24).
Immunoassays of PRL protein
Concentrations of mPRL and hPRL in serum, tissue homogenates, and cell lysates were determined by homologous double-antibody RIAs using hormones and primary antibodies purchased from Dr A. Parlow through the National Hormone and Peptide Program (University of California, Los Angeles). The RIAs exhibited no measureable cross-reactivity with the PRL from the other species (see Supplemental Figure 1). Samples were assayed at 2 different doses in duplicate, and all samples from an individual experiment were included in a single assay.
Staining of PRL in AP cells was performed using double-label immunofluorescence. Cells, plated on glass coverslips, were fixed with 4% paraformaldehyde and permeabilized with 0.25% Triton X-100 in PBS. Cells were sequentially stained for mPRL using goat α-mPRL (5 μg/mL, catalog number AF1445; R&D Systems) and visualized with rabbit α-goat IgG-F(ab′)2-Alexa Fluor 488, followed by staining for hPRL with rabbit α-hPRL (1:10 000, AFP-10572105Rb; A. Parlow) visualized with donkey α-rabbit IgG-Alexa Fluor 594.
pSTAT5 in T47D cells
Phospho-specific flow cytometry was performed for analysis of hPRL-R-activated intracellular messenger cascade-phosphorylation of STAT5 (phospho-Signal Transducer and Activator of Transcription 5). (25). Briefly, human breast cancer T47D cells (American Type Culture Collection) were seeded into T25 flasks (∼250 000 cells/flask in RPMI 1640 with 10% FBS) and allowed to plate for 8–9 hours. Cells were then serum deprived (RPMI 1640 with 0.1% BSA) for 16 hours, after which they were treated with one of the following: biologic control (vehicle), recombinant hPRL (10nM), extract from female AP glands (to make 10nM hPRL or mPRL, determined by RIA), and the same total protein from PRL− APs. After 20 minutes, media were removed and cells trypsinized for 2 minutes (0.05% trypsin, 2mM EDTA in PBS; 37°C), then triturated to suspend single cells. Immediately, ice-cold 4% paraformaldehyde was added (final concentration of 2%), and the T47D cells were stored at 4°C. The next day, cells were washed with PBS, resuspended in 1 mL of ice-cold 100% methanol to permeabilize the cell membranes (25), and then stained with α-pSTAT5 Ab. Technical controls for the flow cytometry included vehicle-treated cells in which the primary Ab was omitted (not stained) or substituted with mouse IgG2a κ isotype control. Staining was analyzed on an Accuri C6 Flow Cytometer using the built-in software.
Statistics
One-way ANOVA followed by Bonferroni's or Dunnet's multiple comparison test were used for statistical analysis of the in vitro PRL release data, fertility parameters, and flow cytometry data. Student's t test was used to compare treatment groups in the in vivo studies using E2, Brx, and LPS treatments, as well as individual and litter weight gain assessed in the WSW experiments.
Results
Generation of hPRL transgenic mice
Of the potential founder mice identified by PCR of gDNA, 2 passed the transgene to offspring (BAC-h8 and BAC-h30). The entire BAC construct was incorporated in the germ line of both lines of mice as determined by PCR of noncoding regions at the 5′ and the 3′ ends of the BAC construct as well as exon 1 of the hPRL gene. Fluorescence in situ hybridization analysis of interphase cells revealed a single insertion in the BAC-h8 line and 2 insertions in the BAC-h30 line (Supplemental Figure 2). Both lines were used in all of the studies, with no differences in phenotypes or responses observed.
hPRL rescues fertility in mPRL− females
As previously described (3), VO was delayed in mPRL− females in comparison with WT (mPRL+) controls (P < .001) (Table 1), and mPRL− estrous cycles were irregular, having multiple days of proestrus and/or estrus. Mice expressing the hPRL transgene along with mPRL (hPRL+:mPRL+) were indistinguishable from mPRL+ controls. The hPRL transgene alone (hPRL+:mPRL−) restored the time to VO to control levels (Table 1), and these hPRL+:mPRL− females had regular estrous cycles with single days of proestrus and estrus (data not shown). Thus, hPRL restored the timing of puberty and reproductive cycling to normal patterns.
Table 1.
Fertility Parameters of Female Mice with Varying PRL Genotypes
| Female puberty |
Female fecundity |
||||||
|---|---|---|---|---|---|---|---|
| Genotype | Age of VO (d) | n | Dam genotype | n (dams) | Number of litters | Days to delivery | Pups/litter |
| hPRL0:mPRL+ | 36.3 ± 1.65 | 8 | hPRL0:mPRL+ | 3 | 6 | 25.7 ± 1.3 | 7.8 ± 0.8 |
| hPRL+:mPRL+ | 37.4 ± 0.90 | 14 | hPRL+:mPRL+ | 6 | 11 | 23.5 ± 0.9 | 10 ± 1.0 |
| hPRL0:mPRL− | 49.6 ± 0.87a | 15 | hPRL0:mPRL− | 5 | 0 | NA | NA |
| hPRL+:mPRL− | 38.5 ± 1.07 | 15 | hPRL+:mPRL− | 7 | 15 | 24.5 ± 0.9 | 8.5 ± 0.6 |
P < .001.
NA, not applicable.
Mating units were assembled, in which proven sires were paired with proestrous dams of each of the following types: 1) hPRL0:mPRL+, 2) hPRL+:mPRL+, 3) hPRL0:mPRL−, and 4) hPRL+:mPRL−. The resulting fertility parameters are summarized in Table 1. As expected, females with no PRL (group3, hPRL0:mPRL−) were unable to produce any litters. Dams expressing only hPRL (group 4, hPRL+:mPRL−) produced litters at the same rates and of similar sizes compared with either WT controls (hPRL0:mPRL+) or bigenic controls (hPRL+:mPRL+).
Mammary development and lactation in hPRL+ mice
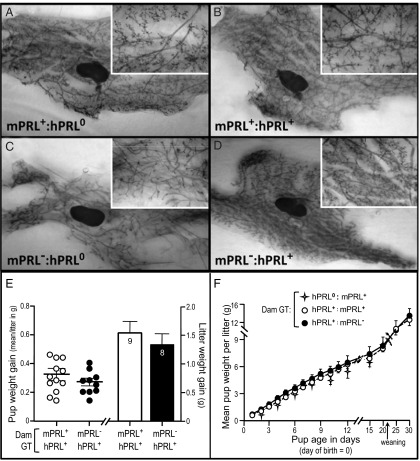
MG development in PRL KO females was arrested in the pubertal state, in which the epithelium consisted of a simply branched ductal tree with persistent terminal endbuds (Figure 2C) (3). The hPRL transgene restored normal MG development in nulliparous (virgin) females, such that the glands differentiated fully into complex epithelial networks of ducts decorated by side branches and alveolar buds, and without persistent terminal endbuds (Figure 2D). Development in the hPRL+:mPRL− females was indistinguishable from the WT and bigenic controls (Figure 2, A and B).
Figure 2.
hPRL rescues MG development and function in mPRLKO mice. (A–D) The right number 4 MG was removed from virgin female mice (3–4 mo of age) of varying genotypes and prepared for whole mount. (A) WT control (mPRL+:hPRL0). (B) Bigenic control (mPRL+:hPRL+). (C) PRL deficient (mPRL−:hPRL0). (D) Mouse expressing hPRL in the absence of mPRL (mPRL−:hPRL+). Images are representative of 4–5 MG whole mounts from each genotype. (E and F) Lactational competence was assessed in dams of varying genotypes. (E) Milk production determined by pup weight gain in a WSW experiment on day 10 of lactation. Pups (culled to 6 per litter) were removed from dams for 4 hours, then weighed immediately before and after a 30-minute suckling period. Numbers in bars indicate sample size. (F) Growth curves of pups through weaning. n = 5 for each group.
Lactational competence of hPRL females was measured acutely using a WSW protocol after a 4-hour fast. Dams expressing only hPRL provided identical amounts of milk to their pups during a 30-minute suckling period, compared with bigenic control animals (Figure 2E). The bigenic dams demonstrated increased circulating levels of both hPRL and mPRL after a 30-minute bout of nursing (Supplemental Figure 3).
Lactational competence over the course of the full lactation cycle was assessed by monitoring the growth rates of pups from birth through weaning. The rate and extent of growth of pups born to hPRL+ (mPRL−) mothers was indistinguishable from that of pups born to WT control (hPRL0:mPRL+) or bigenic control mothers (hPRL+:mPRL+) (Figure 2F).
These data demonstrate that female reproduction in hPRL+:mPRL− female mice (ie, humanized PRL) was indistinguishable from normal from the onset of puberty through gestation and lactation, despite the fundamental differences in the organization and regulation of the human and rodent PRL genes. Additional experiments were done to test specific regulatory mechanisms of hPRL gene expression in the mouse.
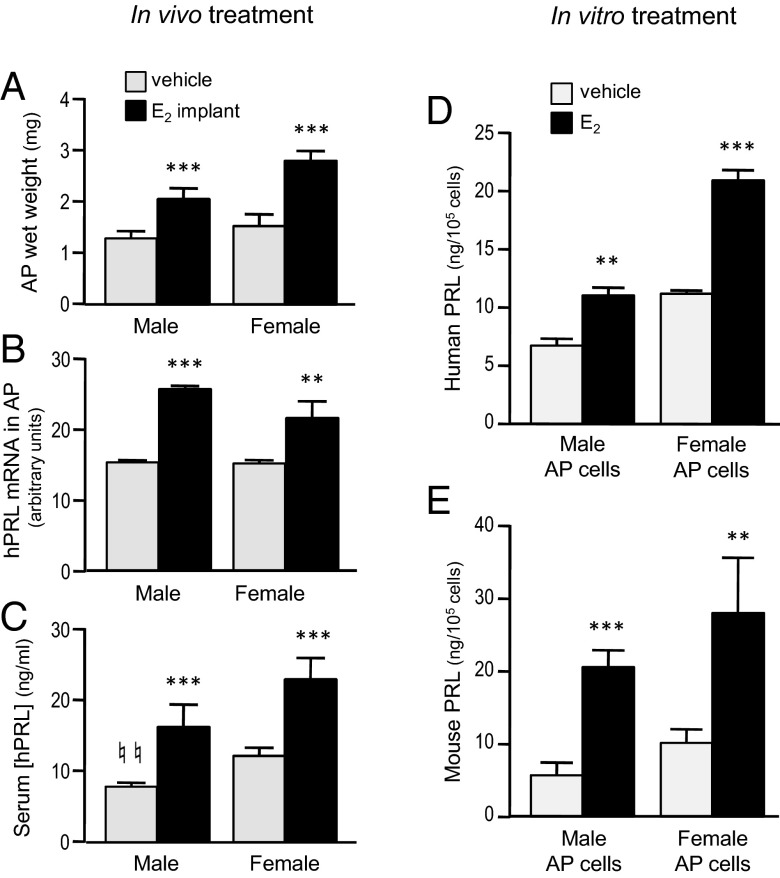
Humanized PRL in pituitary lactotrophs is responsive to E2
Regulation of hPRL in vivo was studied by continuous E2 treatment for 14 days via implanted pellets, which had the expected hypertrophic effect on the AP glands of both male and female hPRL+ mice (>50% increase in wet weight) (Figure 3A). Gene expression of hPRL was induced by in vivo E2 treatment in pituitaries of both males and females (Figure 3B). Basal levels of serum hPRL were significantly lower in male mice compared with females, and E2 increased circulating hPRL by approximately 2-fold in both males and females (Figure 3C).
Figure 3.
Pituitary hPRL is responsive to E2 treatment both in vivo and in vitro. (A–C) Intact hPRL-only mice (hPRL+:mPRL0) were implanted with a single 17β-E2 (21-d release, 0.05 mg/pellet; n = 6) or vehicle pellet (n = 6), sc. Blood and tissues were collected 14 days later. AP glands (wet weight, posterior pituitary removed) were weighed (A) and then processed for qRT-PCR (B). Serum was assayed for hPRL (C). **, P < .01 and ***, P < .001, E2 treated vs vehicle treated; ♮♮, P < .01, male vs female; Student's t test. (D and E) Dissociated AP cells from bigenic (mPRL+:hPRL+) male or female mice were treated in vitro with 17β-E2 (100pM) or vehicle (0.0003% ethanol). At the end of 6 days, cells were washed and lysed, and the supernatant was assayed for PRL proteins by RIA. Data represent the means of 3 repeated experiments. **, P < .01; ***, P < .001 compared with vehicle-treated group within same sex; Student's t test.
To analyze direct E2 actions on the pituitary cells, primary cultures of AP cells were cultured in a hormone-depleted medium (26) supplemented with E2 (100pM) or vehicle (0.0003% ethanol). Cells were derived from bigenic mice (hPRL+;mPRL+), and significant increases in both hPRL and mPRL protein were observed after 6 days of E2 treatment of cells collected from either males or females (Figure 3, D and E).
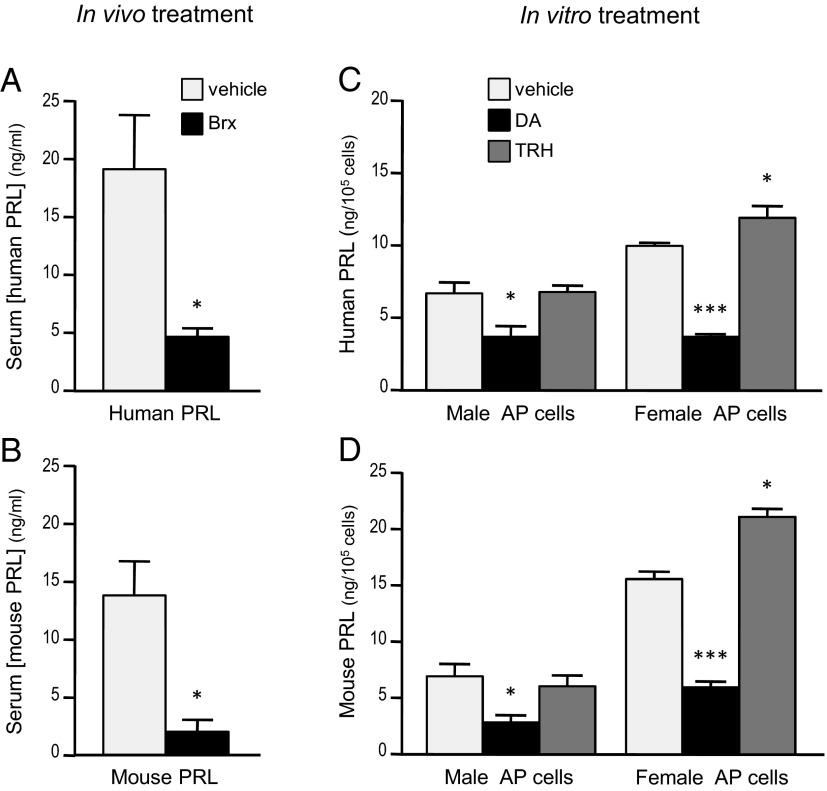
Humanized PRL secretion from AP lactotrophs is regulated by DA and TRH
DA is the predominant acute physiological regulator of PRL secretion (27). Brx (a D2R agonist) injection suppressed serum PRL levels of both hPRL and mPRL in bigenic female mice (Figure 4, A and B). Correspondingly, DA inhibited secretion of both hPRL and mPRL during a 1-hour in vitro release experiment (Figure 4, C and D) of primary cultured AP cells derived from either male or female mice. In addition, TRH, a known pituitary PRL secretagogue, significantly stimulated release of both hPRL and mPRL from female, but not male, AP cells in this experiment (Figure 4, C and D). This sex difference is most likely due to exposure of the female cells to greater levels of estrogen, which increases TRH receptor density (28) and reduces TRH-degrading ectoenzyme (29) in lactotrophs.
Figure 4.
Dopamine agonism suppresses secretion of both pituitary hPRL and mPRL in vivo and in vitro. (A and B) Serum was collected from bigenic (hPRL+;mPRL+) female mice 90 minutes after sc injection of Brx (2.5 mg/kg, n = 5) or vehicle (n = 7), then assayed for hPRL (A) and mPRL (B). *P < .02, Brx vs vehicle; Student's t test. (C and D) Primary cultured AP cells from bigenic male or female mice were incubated for 60 minutes with vehicle, DA (300nM), or TRH (100nM). At the end of incubation, media were collected and centrifuged, and the supernatants were assayed for hPRL (C) and mPRL (D). Data represent the means of 4 repeated experiments. *, P < .05; ***, P < .001 compared with vehicle-treated control group within the same category (sex); 1-way ANOVA followed by Bonferroni's multiple comparison test.
The hPRL gene is expressed in numerous extrapituitary mouse tissues
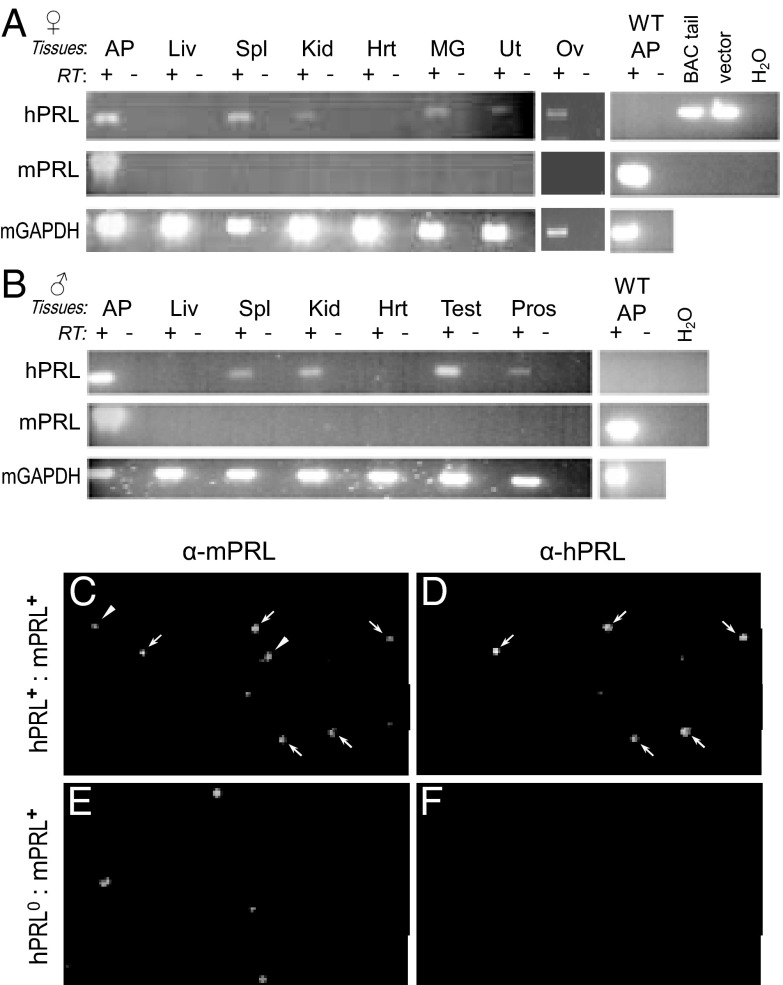
Based on previous studies of extrapituitary PRL expression (17–20), a variety of tissues were harvested from bigenic hPRL+:mPRL+ mice and used to determine the expression patterns of hPRL and mPRL transcript simultaneously. Assays were performed on males and nulliparous (ie, virgin) female mice. Both mPRL and hPRL were expressed in the AP glands (Figure 5, A and B). In contrast, hPRL, but not mPRL, expression was detected in several extrapituitary sites: spleen, thymus, kidney, and reproductive tissues of both males and females, including MG, uterus, ovary, prostate, and testis (Figure 5, A and B, and Supplemental Figure 4). No hPRL expression was detected in liver, heart (Figure 5, A and B), lung, skeletal muscle, small intestine, fat, or stomach (Supplemental Figure 4).
Figure 5.
Expression of hPRL mirrors mPRL in AP cells of bigenic mice but not in extrapituitary tissues. (A and B) PRL transcripts in various tissues from bigenic (hPRL+:mPRL+) female (A) and male (B) mice, as analyzed by RT-PCR. Shown are gels from mice representative of the analyses of multiple mice (see Supplemental Figure 3 for additional tissues and summary table). Both hPRL and mPRL transcripts were present in the AP gland. Several nonpituitary tissues also expressed hPRL, indicating the activation of the 1a-hPRL promoter in these tissues. Transcript for mPRL was not detectable in these extrapituitary tissues (35 PCR cycles, equivalent to hPRL amplification). For every tissue, mRNA integrity was confirmed by RT-PCR of mGAPDH. No product was produced if RT enzyme was omitted (RT−), demonstrating the absence of genomic contamination. Panels on the right demonstrate species specificity of the primers. Liv, liver; Spl, spleen; Kid, kidney; Hrt, heart; Ut, uterus; Ov, ovary; Test, testis; Pros, prostate; BAC tail, gDNA from transgenic mouse; vector, purified BAC vector. (C–F) AP cells from bigenic mice stain for both hPRL and mPRL proteins. Dissociated AP cells from mice carrying both the hPRL and mPRL genes (C and D) or from WT controls (E and F) were plated and fixed onto slides then subjected to double immunofluorescence cytochemistry (ICC). Cells were first stained for mPRL (C and E) then hPRL (D and F) and visualized with species selective secondary antibodies conjugated with Alexa Fluor 488 or 568, respectively. Arrows identify some of the cells staining for both hPRL and mPRL in the bigenic AP cells; arrowheads point out cells staining for only mPRL. No staining was observed when primary antibodies were omitted (data not shown). Double ICC was performed on cells derived from 3 mice of each genotype.
Double immunocytochemistry showed staining for both mPRL and hPRL in the same AP cells from bigenic mice (Figure 5, C and D). AP cells from WT mice only stained for mPRL (Figure 5, E and F).
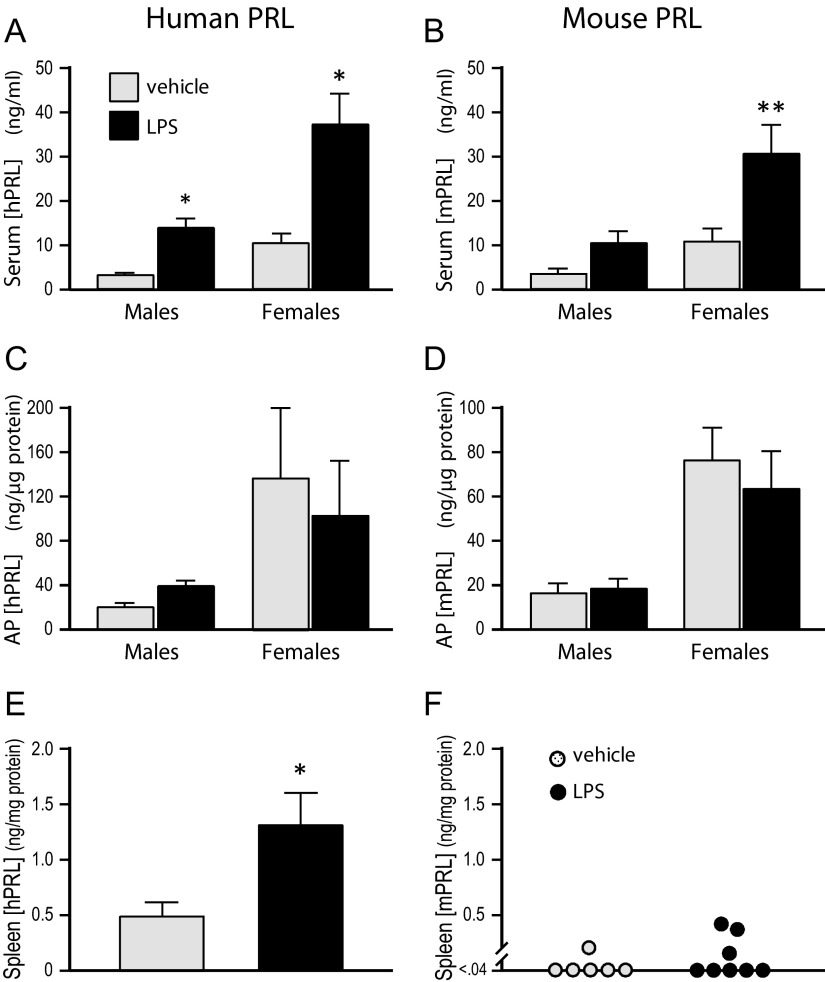
LPS increases hPRL, but not mPRL, in the spleen
To address the regulation of humanized PRL in immune-related cells of mice, we used LPS treatment (30). Serum levels of both hPRL and mPRL were elevated by LPS treatment of hPRL+:mPRL+ mice (Figure 6), consistent with stress-induced release of pituitary PRL after immune challenge, as has been reported before (31). Pituitary contents of both forms of PRL were not different between the 2 treatment groups despite the enhanced secretion into the circulation, reflecting the high storage pools of PRL in the AP. Serum and AP levels of both hPRL and mPRL were higher in female mice as compared with males, regardless of treatment. In the spleens, hPRL content was significantly elevated in response to LPS treatment (Figure 6E), whereas mPRL content was generally below the detection limit and not stimulated by LPS (Figure 6F). No sex differences were seen in spleen PRL content within treatment groups, so values from males and females were pooled for presentation in Figure 6, E and F.
Figure 6.
LPS treatment increased hPRL, but not mPRL, in spleens of bigenic mice. Males and females were injected ip with either vehicle (n = 6) or LPS (3 mg/kg; n = 8), then killed 16 hours later. Sera and homogenates of AP glands and spleens were assayed for hPRL and mPRL by RIA. *, P < .05; **, P < .01 compared with vehicle-treated controls; Student's t test.
Mouse-derived hPRL activates STAT5 in human breast cancer cells
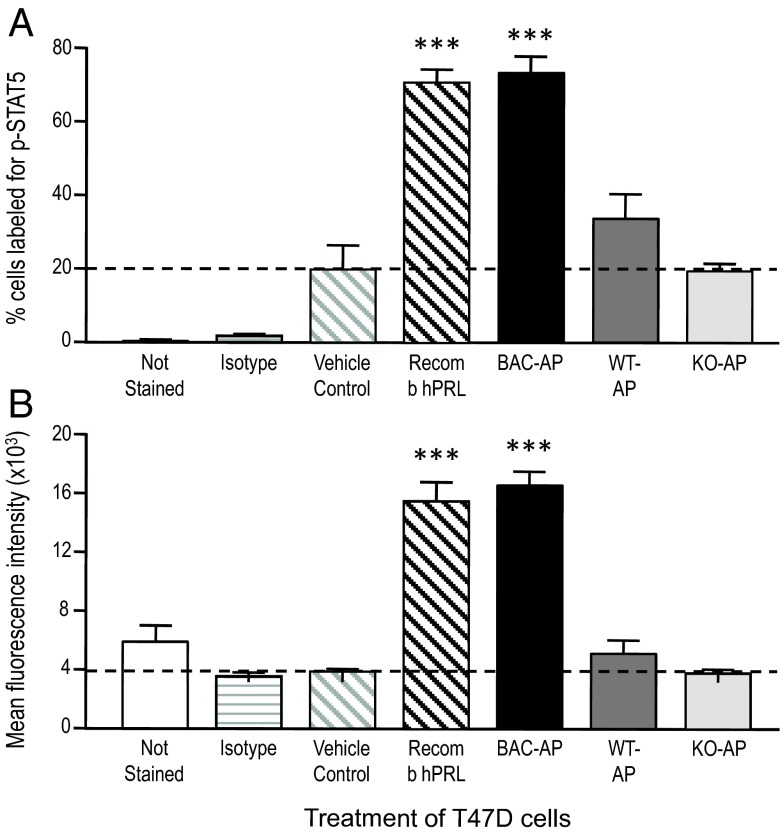
Given that rodent PRL is a poor agonist for hPRL-Rs (32, 33), it was important to establish whether mouse-expressed hPRL retained the ability to activate PRL-Rs in human cells. To this end, human breast cancer T47D cells were treated with AP protein extracts from hPRL+:mPRL−, hPRL0:mPRL+ (WT), or hPRL0:mPRL− (PRL KO) mice. To estimate the potency of mouse-expressed hPRL, we used recombinant hPRL (10nM), and the concentrations of hPRL or mPRL in the AP extracts (measured by RIA) were adjusted so that the treatment doses were also 10nM. For the PRL KO AP extracts, total protein was adjusted to be equivalent to the WT extract. STAT5 activation was measured by immunostaining and flow cytometry.
As shown in Figure 7 (and Supplemental Figure 5), hPRL extracted from AP glands of transgenic humanized mice, activated STAT5 signaling (phospho-STAT5) in human T47D cells. In contrast, extracts from either WT (hPRL0:mPRL+) or PRL KO (hPRL0:mPRL−) AP glands were inactive in T47D cells. The similar STAT5 activation by recombinant hPRL and mouse-derived hPRL indicates that their potencies are roughly equivalent.
Figure 7.
Summary of flow cytometric analysis of STAT5 activation in human breast cancer T47D cells. See Supplemental materials for additional information. Flow cytometric analysis of fluorescent labeling measured the percent of T47D cells labeled (A) and the mean fluorescence intensity (degree of labeling) of those positive cells (B). The cells not stained or stained for κ isotype served as technical controls (2 bars on the left of each graph), whereas the remaining groups were all stained for phosphorylated STAT5. Vehicle-treated cells served as the biological control. Extracts of WT or mPRL−/− (PRL KO) APs did not increase either the percent of cells labeled or the mean fluorescence above vehicle treatment. Recombinant hPRL (10nM) increase both parameters approximately 4-fold over vehicle treatment and extracts of APs from hPRL+ mice (10nM hPRL, determined by RIA) were equipotent. Data represent the means of 4 repeated experiments. ***, P < .001 compared with vehicle control; 1-way ANOVA and Bonferroni's multiple comparison test.
Discussion
There are numerous and well-documented differences in the regulation and biological activities of primate (eg, human) and rodent (eg, mouse and rat) PRL. With the ultimate intention of understanding the biology and pathology of hPRL, we were motivated to use molecular genetic approaches to engineer a new mouse model, in which we inserted a large region of human DNA that includes both the structural gene and all of its known regulatory elements (34).
Rescue of fertility in female PRL-deficient mice
Given the profound and multifaceted fertility defects in female PRL KO mice, we had a primary interest in determining whether the hPRL transgene, controlled by human regulatory elements, would rescue these reproductive deficits. Our results show that the hPRL transgene restored the full range of female reproductive deficits seen in mPRL-null mice during puberty, the ovarian cycle, embryonic development, and late gestation in the absence of a biologically active mPRL gene. This result is unsurprising at a superficial level. Deeper consideration must take into account that the rescue of female fertility was effected by the hPRL structural gene, controlled by hPRL regulatory elements. The various patterns of PRL expression in female reproduction, from puberty through lactation, are the culmination of a complex chain of developmental and physiological events (35). The rescue of mouse fertility by hPRL demonstrates that this chain of events can be replicated in a heterologous system. Interestingly, animals expressing both mPRL and hPRL exhibited no phenotypes of hyperprolactinemia in that fertility parameters of bigenic females were not different from WT mice.
The most profound interspecies difference in mammalian fertility is the hormonal regulation of the ovarian cycle, the extremes being embodied by rodents and humans. As noted earlier, the luteal phase of rodents is atypical of mammals, being very brief and producing insufficient quantities of progesterone to either support a decidual reaction of the uterine endometrium or prevent the hypothalamic-pituitary axis from initiating another estrous cycle. The structural and functional survival of the rodent CL absolutely requires pituitary PRL (36), which is secreted in twice daily surges if mating (or cervical stimulation) occurs during the periovulatory period. These surges of PRL (one diurnal, the other nocturnal) continue for approximately 10 days if the mating is fertile and results in pregnancy (37) or 12 days if the mating is not fertile (21). In both cases, the rodent CL is functionally maintained by these PRL surges, and progesterone secretion is enhanced during that period.
In contrast, the human CL is not dependent on pituitary PRL. In normally cycling women, suppression of pituitary PRL with Brx, beginning at ovulation and continuing throughout the luteal phase, does not alter serum progesterone (38). Moreover, only a small percentage of human CL expresses PRL-R (39), and direct treatment of dispersed human luteal cells with hPRL does not alter progesterone secretion (40). PRL transcript has been isolated from human luteinized granulosa cells obtained during oocyte retrieval (41). Whether locally produced PRL impacts CL development and/or function in humans remains to be determined. Ovaries from our mice were positive for hPRL transcript and will provide a model for cell-specific ovarian expression during various reproductive states.
PRL and PRL-R KO female mice are completely infertile (3, 4). In contrast, the absence of PRL does not alter fertility in male mice. PRL deficiency in humans most often occurs as one component of a combined pituitary hormone deficiency. However, a few cases of PRL deficiency without evidence of other pituitary defects have been reported in women. This isolated PRL deficiency results in lactational failure and reproductive difficulty in women with no other obvious problems (42–44). No cases of isolated pituitary PRL deficiency have been reported in men, even in a family with apparent genetically transmitted isolated PRL deficiency in which the women did present with reduced fertility and alactogenesis (44). These observations in a few humans show remarkable concordance with the phenotypes of mice with disruptions of either PRL or its receptor genes: MG development and function is defective, females fail to reproduce, but males lack any overt problems (3, 4). The species-specific differences in luteal control, discussed above, may explain why women with isolated pituitary PRL deficiency are merely subfertile (achieving successful pregnancies after fertility treatment), whereas PRL-deficient female mice are completely infertile.
The progesterone produced by the CL in all mammals is required for functional receptivity of the uterus and also promotes early blastocyst survival in the oviducts. However, it is not sufficient for complete implantation and embryo survival in the mouse. Progesterone treatment of PRL- or PRL-R-null mice does not prevent fetal loss, and local production of decidual PRL has been shown to suppress the expression of several factors that contribute to fetal loss, including inflammatory cytokines (6). PRL produced by human decidualized endometrium may also be required for successful implantation and embryo survival. A recent study has demonstrated that PRL expression (but not PRL-R) was impaired or absent in decidual specimens from women who had undergone spontaneous miscarriage. Again, in extraordinary agreement with the mouse, these tissues expressed inflammatory cytokines that were absent in samples obtained from control subjects undergoing voluntary pregnancy terminations (45).
Regulation of the hPRL transgene
The pituitary hPRL gene, in the mouse context, was induced by E2 and was inhibited by a D2R agonist. These regulatory effects were seen both in vivo, where the hypothalamic control mechanisms are intact, and in vitro, showing that the effects of E2 and D2R activation on the lactotrophs are direct. Because these 2 signals are the primary direct regulators of PRL during the female reproductive cycle (27), the estrogenic and dopaminergic control of hPRL must account, in large measure, for the normalization of reproductive development, fertility, and lactation in these animals. There are other important PRL regulatory mechanisms, such as opioid, and serotonergic signaling, which act indirectly at the hypothalamic level (27, 46–48), and may or may not be affected by hPRL. The neuroendocrine reflex in rodents that activates the postcoital surges of PRL discussed above certainly involves patterned stimulatory input coincident with reduced dopaminergic tone (48). Whether these same neuroendocrine inputs are driving the rodent pattern of PRL release in our hPRL transgenic mice remains to be investigated.
Extrapituitary expression of hPRL
Although PRL expression outside the AP gland has been most extensively described in humans, species other than primates express extrapituitary PRL. In the mouse, PRL is expressed in the endometrial stroma, promoting fetal survival (6), and in the lactating MG, where it stimulates epithelial expansion in the peripartum (16). However, extrapituitary PRL expression is not a consistent feature in mammals. For example, rabbits, dogs, and armadillos do not express PRL in the endometrium (49). The molecular evolution of extrapituitary PRL expression in mammals is complex, with multiple species (rodents, elephants, primates, and possibly others) having independently evolved genomic mechanisms to drive PRL expression in extrapituitary tissues (49). In primates, expression of extrapituitary PRL is a consequence of regulatory sequences derived from insertion of transposable elements (MER39/MER20) approximately 6 kbp upstream of the structural gene (50, 51). The independent evolution of extrapituitary PRL expression in multiple phylogenetic clades of mammals strongly indicates that this system has been useful for adaptation to particular selective pressures, even though it is not essential for basic physiological functions. A related case-in-point is murine PRL-like protein A, which is expressed in the mouse placenta. Although not essential under conventional conditions, PRL-like protein A is required for reproduction under the physiological stress of reduced oxygen tension, such as occurs at high elevations (52). This is an important example of how genes in the PRL family could evolve under selection pressure.
The convergent evolution of extrapituitary PRL expression in rodents and primates poses a significant challenge for understanding hPRL biology. Although extrapituitary PRL in mice appears to be expressed at biologically meaningful levels in lactating MG and in the uterus during pregnancy (6, 16), hPRL is expressed in a much larger variety of tissues and physiological states (10, 17, 31, 53–55). Interestingly, evidence of both distal and proximal promoter usage has been documented in human breast and prostate cancer cells (56, 57). Further study will be required to determine whether dual promoter usage is evidenced in our mice.
We studied the expression of extrapituitary hPRL under baseline conditions in males and in females that were nonpregnant and nonlactating. Among the tissues studied, hPRL was expressed in the spleen, thymus, kidney, MG, uterus, prostate gland, and testes. Another possible site of PRL synthesis is the brain, but the complexity of studying brain PRL expression demanded that we defer consideration of the brain to future studies. In general, the tissues that express hPRL in the mouse correspond to those that previously have been documented to express PRL in the human. The apparent fidelity of hPRL expression in this mouse model is illustrated by its expression in the male testis, where hPRL mRNA was expressed robustly. Similarly, hPRL is expressed at the mRNA level, and levels of hPRL protein in human testis were substantial (>50 ng/g wet weight) (58). We did not observe an obvious difference in male reproductive physiology in the hPRL mice under standard laboratory conditions. Therefore, it will be important to study testicular responses to physiological stresses in order to understand whether the substantial testicular hPRL expression has important roles in adaptation. These questions would be impossible to address adequately in humans but can readily be studied in the humanized mouse model. In addition, we studied female tissues only from virgin female mice. Because hPRL in female reproductive tissues is dynamically regulated by changing reproductive status, this new model provides avenues for future in vivo studies of extrapituitary hPRL in females.
We did not detect hPRL mRNA in abdominal fat from our mice, although others have shown PRL transcript in human visceral fat (59). Mouse visceral adipose tissue expresses mPRL-R, but not mPRL, and receptor expression is induced during lactation (60). Moreover, we did not observe any abnormal phenotypes associated with those extrapituitary tissues that did express hPRL. As with the case of detectable expression levels, phenotypes associated with extrapituitary tissues that do express hPRL may be dependent upon physiological states or stresses.
Induction of hPRL by LPS
We also examined PRL expression in the spleen after a physiological challenge (LPS stimulation). In this experiment, we used bigenic mice (WT for mPRL and transgenic for hPRL). Serum levels of both hPRL and mPRL were increased in response to LPS stimulation. However, because LPS elicits a nonspecific systemic stress, the observed change of serum PRL likely reflects stress-induced secretion from the AP but not de novo synthesis. This interpretation is supported by a recent report by Semprini et al (61). Using transgenic rats expressing a hPRL-luciferase reporter gene, they found no activation of the transgene in the pituitary gland after experimentally induced peritonitis. In contrast, luciferase expression dramatically increased in peritoneal neutrophils and monocytes/macrophages. Corresponding to the expression of mRNAs for mPRL and hPRL, the hPRL protein, but not mPRL, was detectable in unstimulated mouse spleens, and LPS stimulated greater than a 2-fold increase in spleen hPRL. In the mouse spleens, 4 of the 14 animals tested showed very low, but detectable, mPRL protein, presumably derived from the blood, not local synthesis. The induction of splenic hPRL synthesis in the mouse is consistent with induction of reporter gene activity observed in transgenic rats, in which enhanced green fluorescent protein is under the control of the hPRL promoter (34, 61).
Bioactivity of transgenic hPRL at the human receptor
Our PRL-humanized mouse model may also subserve studies of certain human pathologies, in particular, PRL-dependent cancers. The gold standard of preclinical models for studying human cancers, xenografting human cancer cells or tissues into immunodeficient mice, presumes that the mouse replicates the human environment. However, as elegantly demonstrated by Utama et al (32), mPRL is a poor agonist of the hPRL-R and, in fact, can antagonize hPRL (33). As evidenced by induction of STAT5 phosphorylation in the human T47D cells, we found that the hPRL produced by our mice is active at the hPRL-R and apparently equipotent to recombinant hPRL. Thus, these mice will enable a comprehensive study of human breast cancer in vivo, because they will produce, in physiological patterns, all of the principal hormones that direct human MG growth and differentiation.
Conclusions
hPRL physiology is poorly understood at many levels because of evolutionary adaptations that have led to major differences in the biological activity and regulation of PRL in humans and typical rodent models. We have developed a mouse model that expresses hPRL under the control of its entire genomic context and showed that hPRL rescues the physiological defects caused by disrupting the mPRL gene. In this model, hPRL is expressed in tissues that do not typically express mPRL and, at least in the spleen, can be elevated by immune challenge.
Acknowledgments
Present address for H.R.C.: Division of Behavioral and Natural Sciences, College of Mount St Joseph, 5701 Delhi Road, Cincinnati, OH 45233.
This work was supported by the National Institutes of Health Grant DK-54966 (to K.A.G.), the Department of Defense Grant BC095713 (to K.A.G.), and the Predoctoral Fellowship HD007463 (to H.R.C.). The project was initiated with a pilot grant from the University of Cincinnati Cancer Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP
- anterior pituitary
- BAC
- bacterial artificial chromosome
- Brx
- bromocriptine mesylate
- CL
- corpus luteum
- DA
- dopamine
- D2R
- D2 subtype of the dopamine receptor
- E2
- estradiol
- FBS
- fetal bovine serum
- hPRL
- human PRL
- HS
- horse serum
- KO
- knockout
- LPS
- lipopolysaccharide
- MG
- mammary gland
- mPRL
- mouse PRL
- mPRL-R
- mPRL receptor
- PRL
- prolactin
- pSTAT5
- phospho-STAT5
- qRT-PCR
- quantitative real-time PCR
- SES
- standard external solution
- STAT5
- Signal Transducer and Activator of Transcription 5
- VO
- vaginal opening
- WSW
- weigh-suckle-weigh
- WT
- wild type.
References
- 1. Riddle O, Bates RW, Dykshorn SW. The preparation, identification and assay of prolactin: a hormone of the anterior pituitary. Am J Physiol. 1933;105:191–216 [Google Scholar]
- 2. Stricker P, Grueter F. Action du lobe antérior de l'hypophyse sur la montée laiteuse. C R Seances Soc Biol Fil. 1928;99:1978–1980 [Google Scholar]
- 3. Horseman ND, Zhao W, Montecino-Rodriguez E, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16:6926–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ormandy CJ, Camus A, Barra J, et al. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997;11:167–178 [DOI] [PubMed] [Google Scholar]
- 5. Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107 [DOI] [PubMed] [Google Scholar]
- 6. Bao L, Tessier C, Prigent-Tessier A, et al. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;148:2326–2334 [DOI] [PubMed] [Google Scholar]
- 7. Bachelot A, Binart N. Reproductive role of prolactin. Reproduction. 2007;133:361–369 [DOI] [PubMed] [Google Scholar]
- 8. Cooke NE, Liebhaber SA. Molecular biology of the growth hormone-prolactin gene system. Vitam Horm. 1995;50:385–459 [DOI] [PubMed] [Google Scholar]
- 9. Berwaer M, Martial JA, Davis JR. Characterization of an up-stream promoter directing extrapituitary expression of the human prolactin gene. Mol Endocrinol. 1994;8:635–642 [DOI] [PubMed] [Google Scholar]
- 10. Gellersen B, Kempf R, Telgmann R, DiMattia GE. Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol. 1994;8:356–373 [DOI] [PubMed] [Google Scholar]
- 11. Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–669 [DOI] [PubMed] [Google Scholar]
- 12. Featherstone K, White MR, Davis JR. The prolactin gene: a paradigm of tissue-specific gene regulation with complex temporal transcription dynamics. J Neuroendocrinol. 2012;24:977–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prigent-Tessier A, Tessier C, Hirosawa-Takamori M, Boyer C, Ferguson-Gottschall S, Gibori G. Rat decidual prolactin. Identification, molecular cloning, and characterization. J Biol Chem. 1999;274:37982–37989 [DOI] [PubMed] [Google Scholar]
- 14. Kurtz A, Bristol LA, Tóth BE, Lazar-Wesley E, Takács L, Kacsóh B. Mammary epithelial cells of lactating rats express prolactin messenger ribonucleic acid. Biol Reprod. 1993;48:1095–1103 [DOI] [PubMed] [Google Scholar]
- 15. Steinmetz RW, Grant AL, Malven PV. Transcription of prolactin gene in milk secretory cells of the rat mammary gland. J Endocrinol. 1993;136:271–276 [DOI] [PubMed] [Google Scholar]
- 16. Chen CC, Stairs DB, Boxer RB, et al. Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways. Genes Dev. 2012;26:2154–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL. Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest. 1997;99:618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Golander A, Hurley T, Barrett J, Handwerger S. Synthesis of prolactin by human decidua in vitro. J Endocrinol. 1979;82:263–267 [DOI] [PubMed] [Google Scholar]
- 19. Brown NA, Bethea CL. Cloning of decidual prolactin from rhesus macaque. Biol Reprod. 1994;50:543–552 [DOI] [PubMed] [Google Scholar]
- 20. Tseng L, Mazella J. Prolactin and its receptor in human endometrium. Semin Reprod Endocrinol. 1999;17:23–27 [DOI] [PubMed] [Google Scholar]
- 21. Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226 [DOI] [PubMed] [Google Scholar]
- 22. Wang Z, Storm DR. Extraction of DNA from mouse tails. BioTechniques. 2006;41:410:412 [DOI] [PubMed] [Google Scholar]
- 23. Gregerson KA, Flagg TP, O'Neill TJ, et al. Identification of G protein-coupled, inward rectifier potassium channel gene products from the rat anterior pituitary gland. Endocrinology. 2001;142:2820–2832 [DOI] [PubMed] [Google Scholar]
- 24. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70 [DOI] [PubMed] [Google Scholar]
- 26. Lieberman ME, Maurer RA, Claude P, Gorski J. Prolactin synthesis in primary cultures of pituitary cells: regulation by estradiol. Mol Cell Endocrinol. 1982;25:277–294 [DOI] [PubMed] [Google Scholar]
- 27. Horseman ND, Gregerson KA. Prolactin. In: Jameson JL, DeGroot LJ, eds. Endocrinology, Adult and Pediatric. 6th ed Philadelphia, PA: Saunders Elsevier; 2010:165–178 [Google Scholar]
- 28. Léan AD, Ferland L, Drouin J, Kelly PA, Labrie F. Modulation of pituitary thyrotropin releasing hormone receptor levels by estrogens and thyroid hormones. Endocrinology. 1977;100:1496–1504 [DOI] [PubMed] [Google Scholar]
- 29. Schomburg L, Bauer K. Regulation of the adenohypophyseal thyrotropin-releasing hormone-degrading ectoenzyme by estradiol. Endocrinology. 1997;138:3587–3593 [DOI] [PubMed] [Google Scholar]
- 30. Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225 [DOI] [PubMed] [Google Scholar]
- 31. Matera L. Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci. 1996;59:599–614 [DOI] [PubMed] [Google Scholar]
- 32. Utama FE, LeBaron MJ, Neilson LM, et al. Human prolactin receptors are insensitive to mouse prolactin: implications for xenotransplant modeling of human breast cancer in mice. J Endocrinol. 2006;188:589–601 [DOI] [PubMed] [Google Scholar]
- 33. Utama FE, Tran TH, Ryder A, LeBaron MJ, Parlow AF, Rui H. Insensitivity of human prolactin receptors to nonhuman prolactins: relevance for experimental modeling of prolactin receptor-expressing human cells. Endocrinology. 2009;150:1782–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Semprini S, Friedrichsen S, Harper CV, et al. Real-time visualization of human prolactin alternate promoter usage in vivo using a double-transgenic rat model. Mol Endocrinol. 2009;23:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quentien MH, Barlier A, Franc JL, Pellegrini I, Brue T, Enjalbert A. Pituitary transcription factors: from congenital deficiencies to gene therapy. J Neuroendocrinol. 2006;18:633–642 [DOI] [PubMed] [Google Scholar]
- 36. Döhler KD, Wuttke W. Total blockade of phasic pituitary prolactin release in rats: effect on serum LH and progesterone during the estrous cycle and pregnancy. Endocrinology. 1974;94:1595–1600 [DOI] [PubMed] [Google Scholar]
- 37. Smith MS, Neill JD. Termination at midpregnancy of the two daily surges of plasma prolactin initiated by mating in the rat. Endocrinology. 1976;98:696–701 [DOI] [PubMed] [Google Scholar]
- 38. Schulz KD, Geiger W, del Pozo E, Künzig HJ. Pattern of sexual steroids, prolactin, and gonadotropic hormones during prolactin inhibition in normally cycling women. Am J Obstet Gynecol. 1978;132:561–566 [DOI] [PubMed] [Google Scholar]
- 39. Bramley TA, Stirling D, Swanston IA, Menzies GS, McNeilly AS, Baird DT. Specific binding sites for gonadotrophin-releasing hormone, LH/chorionic gonadotrophin, low-density lipoprotein, prolactin and FSH in homogenates of human corpus luteum. II: concentrations throughout the luteal phase of the menstrual cycle and early pregnancy. J Endocrinol. 1987;113:317–327 [DOI] [PubMed] [Google Scholar]
- 40. Tan GJ, Biggs JS. Effects of prolactin on steroid production by human luteal cells in vitro. J Endocrinol. 1983;96:499–503 [DOI] [PubMed] [Google Scholar]
- 41. Phelps JY, Bugg EM, Shamblott MJ, Vlahos NP, Whelan J, Zacur HA. Prolactin gene expression in human ovarian follicular cells. Fertil Steril. 2003;79:182–185 [DOI] [PubMed] [Google Scholar]
- 42. Kauppila A, Chatelain P, Kirkinen P, Kivinen S, Ruokonen A. Isolated prolactin deficiency in a woman with puerperal alactogenesis. J Clin Endocrinol Metab. 1987;64:309–312 [DOI] [PubMed] [Google Scholar]
- 43. Falk RJ. Isolated prolactin deficiency: a case report. Fertil Steril. 1992;58:1060–1062 [DOI] [PubMed] [Google Scholar]
- 44. Zargar AH, Masoodi SR, Laway BA, Shah NA, Salahudin M. Familial puerperal alactogenesis: possibility of a genetically transmitted isolated prolactin deficiency. Br J Obstet Gynaecol. 1997;104:629–631 [DOI] [PubMed] [Google Scholar]
- 45. Garzia E, Clauser R, Persani L, et al. Prolactin and proinflammatory cytokine expression at the fetomaternal interface in first trimester miscarriage. Fertil Steril. 2013;100(1):108–115 [DOI] [PubMed] [Google Scholar]
- 46. Tavakoli-Nezhad M, Arbogast LA. μ and κ opioid receptor expression in the mediobasal hypothalamus and effectiveness of selective antagonists on prolactin release during lactation. Neuroscience. 2010;166:359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arey BJ, Freeman ME. Oxytocin, vasoactive-intestinal peptide, and serotonin regulate the mating-induced surges of prolactin secretion in the rat. Endocrinology. 1990;126:279–284 [DOI] [PubMed] [Google Scholar]
- 48. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631 [DOI] [PubMed] [Google Scholar]
- 49. Emera D, Casola C, Lynch VJ, Wildman DE, Agnew D, Wagner GP. Convergent evolution of endometrial prolactin expression in primates, mice, and elephants through the independent recruitment of transposable elements. Mol Biol Evol. 2012;29:239–247 [DOI] [PubMed] [Google Scholar]
- 50. Gerlo S, Davis JR, Mager DL, Kooijman R. Prolactin in man: a tale of two promoters. Bioessays. 2006;28:1051–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Emera D, Wagner GP. Transformation of a transposon into a derived prolactin promoter with function during human pregnancy. Proc Natl Acad Sci USA. 2012;109:11246–11251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ain R, Dai G, Dunmore JH, Godwin AR, Soares MJ. A prolactin family paralog regulates reproductive adaptations to a physiological stressor. Proc Natl Acad Sci USA. 2004;101:16543–16548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vlahos NP, Bugg EM, Shamblott MJ, Phelps JY, Gearhart JD, Zacur HA. Prolactin receptor gene expression and immunolocalization of the prolactin receptor in human luteinized granulosa cells. Mol Hum Reprod. 2001;7:1033–1038 [DOI] [PubMed] [Google Scholar]
- 54. Foitzik K, Krause K, Conrad F, Nakamura M, Funk W, Paus R. Human scalp hair follicles are both a target and a source of prolactin, which serves as an autocrine and/or paracrine promoter of apoptosis-driven hair follicle regression. Am J Pathol. 2006;168:748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Emanuele NV, Jurgens JK, Halloran MM, Tentler JJ, Lawrence AM, Kelley MR. The rat prolactin gene is expressed in brain tissue: detection of normal and alternatively spliced prolactin messenger RNA. Mol Endocrinol. 1992;6:35–42 [DOI] [PubMed] [Google Scholar]
- 56. Shaw-Bruha CM, Pirrucello SJ, Shull JD. Expression of the prolactin gene in normal and neoplastic human breast tissues and human mammary cell lines: promoter usage and alternative mRNA splicing. Breast Cancer Res Treat. 1997;44:243–253 [DOI] [PubMed] [Google Scholar]
- 57. Dagvadorj A, Collins S, Jomain JB, et al. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology. 2007;148:3089–3101 [DOI] [PubMed] [Google Scholar]
- 58. Untergasser G, Kranewitter W, Schwärzler P, Madersbacher S, Dirnhofer S, Berger P. Organ-specific expression pattern of the human growth hormone/placental lactogen gene-cluster in the testis. Mol Cell Endocrinol. 1997;130:53–60 [DOI] [PubMed] [Google Scholar]
- 59. Hugo ER, Borcherding DC, Gersin KS, Loftus J, Ben-Jonathan N. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J Clin Endocrinol Metab. 2008;93:4006–4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ling C, Hellgren G, Gebre-Medhin M, et al. Prolactin (PRL) receptor gene expression in mouse adipose tissue: increases during lactation and in PRL-transgenic mice. Endocrinology. 2000;141:3564–3572 [DOI] [PubMed] [Google Scholar]
- 61. Semprini S, McNamara AV, Awais R, et al. Peritonitis activates transcription of the human prolactin locus in myeloid cells in a humanized transgenic rat model. Endocrinology. 2012;153:2724–2734 [DOI] [PubMed] [Google Scholar]