Abstract
OBJECTIVE
To evaluate long-term clinical outcomes and survival in young-onset type 2 diabetes (T2DM) compared with type 1 diabetes (T1DM) with a similar age of onset.
RESEARCH DESIGN AND METHODS
Records from the Royal Prince Alfred Hospital Diabetes Clinical Database, established in 1986, were matched with the Australian National Death Index to establish mortality outcomes for all subjects until June 2011. Clinical and mortality outcomes in 354 patients with T2DM, age of onset between 15 and 30 years (T2DM15–30), were compared with T1DM in several ways but primarily with 470 patients with T1DM with a similar age of onset (T1DM15–30) to minimize the confounding effect of age on outcome.
RESULTS
For a median observation period of 21.4 (interquartile range 14–30.7) and 23.4 (15.7–32.4) years for the T2DM and T1DM cohorts, respectively, 71 of 824 patients (8.6%) died. A significant mortality excess was noted in T2DM15–30 (11 vs. 6.8%, P = 0.03), with an increased hazard for death (hazard ratio 2.0 [95% CI 1.2–3.2], P = 0.003). Death for T2DM15–30 occurred after a significantly shorter disease duration (26.9 [18.1–36.0] vs. 36.5 [24.4–45.4] years, P = 0.01) and at a relatively young age. There were more cardiovascular deaths in T2DM15–30 (50 vs. 30%, P < 0.05). Despite equivalent glycemic control and shorter disease duration, the prevalence of albuminuria and less favorable cardiovascular risk factors were greater in the T2DM15–30 cohort, even soon after diabetes onset. Neuropathy scores and macrovascular complications were also increased in T2DM15–30 (P < 0.0001).
CONCLUSIONS
Young-onset T2DM is the more lethal phenotype of diabetes and is associated with a greater mortality, more diabetes complications, and unfavorable cardiovascular disease risk factors when compared with T1DM.
Type 2 diabetes (T2DM) in youth is coming increasingly into focus given its rising incidence and prevalence, tracking together with childhood obesity. For those with young-onset T2DM, the increased lifetime exposure to hyperglycemia predicts a high complications risk over time (1). Moreover, there is evidence for an increased inherent susceptibility to complications, namely retinopathy in diabetes presenting earlier rather than later in life (2). Furthermore, the results from the recent TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study, which examines optimal treatment regimens in young-onset T2DM (3), illustrate the difficulty in achieving and maintaining good glycemic control in youth, highlighting the lifelong metabolic challenges of early onset T2DM. Together, these observations predict a poorer prognosis for young-onset T2DM. Nevertheless, T2DM in youth is a relatively new problem, and there are few data on long-term survival or complications to substantiate this prediction. Such long-term outcomes from this point would take many decades to collect. Therefore, we interrogated a systematically maintained clinical database, with data spanning >20 years, and cross-referenced it to the Australian National Death Index (NDI) to examine the long-term case fatality and cause of death in young-onset T2DM. Long-term complications data were also examined in this group.
In clinical practice, a diagnosis of T2DM as opposed to type 1 diabetes (T1DM) in a young person often is met with relief because T2DM is perceived as the milder form. Again, little exists in the literature to substantiate this assumption. Given that the traditional focus of diabetes in youth has been on T1DM and that established morbidity and mortality data exist for this group (4,5), a comparison was made with T1DM. Accurate comparisons of outcome between T1DM and T2DM of usual onset have always been confounded by either older age of the typical T2DM patient or if age is accounted for, the much longer disease duration of the T1DM patient. By comparing only young-onset groups in this study, we were able to examine the long-term effects T2DM compared with T1DM, minimizing the otherwise unavoidable confounding effects of age differences on morbidity and mortality outcomes.
RESEARCH DESIGN AND METHODS
Clinical database
The Royal Prince Alfred Hospital (RPAH) Diabetes Database holds clinical information collected by standardized protocol on patients attending the diabetes service since 1986 (6). Patients are referred from a wide area, with the majority from metropolitan Sydney, Australia, but the catchment also extends rurally. Complications assessments are performed as previously outlined (6), usually on an annual basis. In brief, retinopathy was assessed by direct fundoscopy under mydriasis or, in recent years, by retinal photography. Albuminuria was determined by collection of spot urine samples, and a urine albumin/creatinine ratio (ACR) >2.5 mg/mmol in males and >3.5 mg/mmol in females (or an albumin concentration >30 mg/L if ACR unavailable) was considered abnormal. Peripheral neuropathy assessment involved testing vibration perception threshold by biothesiometer, with results expressed as a Z score adjusting for age. Macrovascular disease and risk factors were assessed by clinical history, symptoms, sitting blood pressure (BP), and lipid profiles. Ischemic heart disease included a history of myocardial infarction or angina or ischemia noted on electrocardiogram or during stress testing. Renal function was assessed by estimated glomerular filtration rate (eGFR) (Modification of Diet in Renal Disease equation) (7). Complications data are available on >80% of subjects for all complications. Glycemic exposure was quantified by the calculation of the updated HbA1c, which accounts for the time between visits and the number of measurements (8,9). All measurements (mean ± SD) of HbA1c up to the last clinic visit were included (4.6 ± 4.4 and 5.4 ± 4.6 for the T2DM and T1DM groups, respectively). HbA1c methodology was not standardized because of the time span over which the data were collected and because different pathology providers were used to analyze samples in an ambulatory clinic setting. Smoking history was ascertained by patient report. Smoking scored as current, ever, or never and pack-year estimates were recorded.
Mortality data
Mortality was ascertained by submitting patient data from the RPAH Diabetes Database to the Australian Institute of Health and Welfare for matching with the NDI, a centralized national mortality registry of all deaths occurring in Australia since 1980. Matching was performed by a standardized probabilistic linkage protocol with the following data items: ID number, surname, first given name, second given name, third given name, sex, date of birth, and date and state of residence at last contact. This linkage protocol is well validated and reported to have a sensitivity and specificity of 94% and 100%, respectively (10). Additionally, matching ambiguities were adjudicated by two authors (M.I.C., A.A.-S.) blinded to age of diabetes onset by cross-referencing with area-wide hospital records or confirmation provided by family members or primary care physicians. Death data were censored to 30 June 2011. The NDI is subject to delays in data acquisition, and as a result, information regarding the primary cause of death was available to 2008 so that cause of death is available for 72% of deaths. Cause of death was classified according to ICD-10 from 1997 onward. For the deaths occurring before 1997, causes of death were converted from ICD-9 to ICD-10 for analysis.
Identification and comparison of young-onset cohorts
A total of 24,415 records were available in the RPAH Diabetes Database. We identified 354 patients with young-onset T2DM defined as T2DM diagnosed between 15 and 30 years of age (T2DM15–30). We examined the outcome of this early onset T2DM cohort with patients with T1DM in several ways. For the primary analysis, data from the T2DM15–30 subjects were compared with data from all T1DM patients in the database who were diagnosed between 15 and 30 years of age (T1DM15–30) (n = 470) . The two cohorts were compared with respect to clinical characteristics, cardiovascular risk factors, and the presence of complications evident at the last clinical visit. To examine for differences in clinical parameters that may have been present early in the disease, clinical data were also compared for a subset for whom there was the full complement of complications information at a time point of 2–5 years post diagnosis. Long-term survival outcomes between the T2DM15–30 and the T1DM15–30 cohorts were examined.
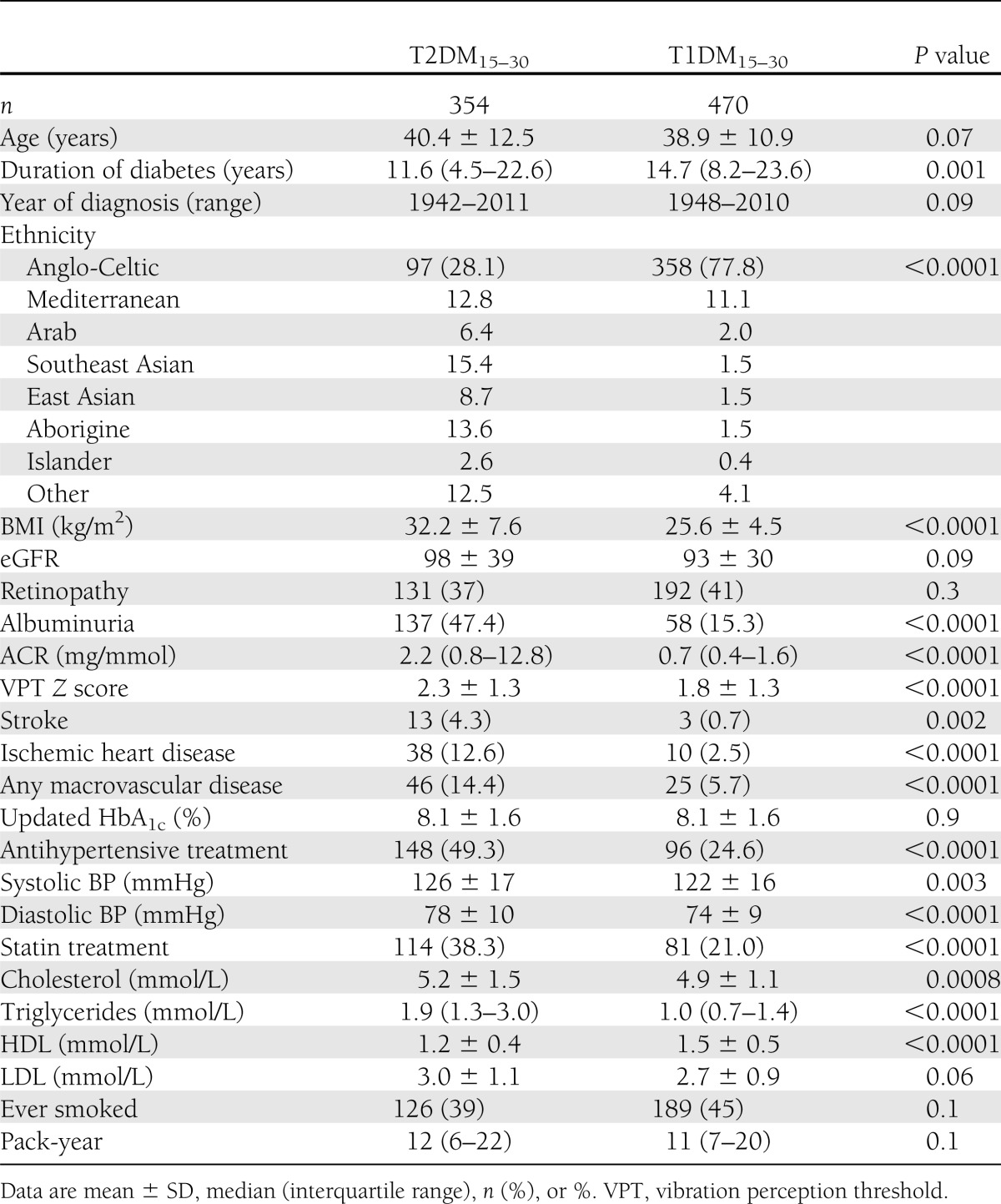
For supplementary analyses, we analyzed the entire T1DM cohort diagnosed before 30 years of age (n = 870) as a comparator. We also compared complications prevalence by 1:1 matching of the T2DM15–30 cohort with the T1DM15–30 cohort (n = 354 each) for age of onset to attenuate the confounding effects of diabetes duration on the presence of complications. The data from the matched cohort are presented in Table 1.
Table 1.
Complications status and risk factor profile at last clinical visit for study cohorts
Statistical methods
Data were analyzed with NCSS 2007 (11) and ACCorD (Analysis of Censored and Correlated Data) (12). Continuous data were checked for normality and presented as mean or median. The two-sample t test or the Mann-Whitney U test were used to compare means or medians. Categorical data were represented as percentages. The χ2 test was used to compare groups. Logistic regression was used to examine the determinants of macrovascular complications. The independent variables were the following: diabetes type, age, systolic BP, diastolic BP, BMI, HbA1c, cholesterol level, triglyceride level, sex, ethnicity, albuminuria, smoking status, and lipid-lowering treatment.
A Kaplan-Meier survival curve was constructed to determine the time-based survival rate between the groups. A Cox regression analysis was performed to examine the relationship between mortality as the dependent variable and duration of diabetes as the time variable. The independent variables used in this analysis were the following: diabetes type, age, systolic BP, diastolic BP, HbA1c, cholesterol level, triglyceride level, sex, ethnicity, albuminuria, smoking status, and lipid-lowering treatment. Significance was accepted at P < 0.05.
For the supplementary analysis involving a matched T1DM cohort, the NCSS Greedy (13) data-matching algorithm based on propensity scores was used. As described previously, patients with T2DM15–30 were matched 1:1 according to age of diagnosis with patients with T1DM15–30. A propensity score was calculated for the matching procedure with the use of logistic regression. Sum of rank distances, including the propensity score, were used to calculate the distance between the groups. Pairwise tests were used for all matched data.
RESULTS
Subject characteristics
For the primary analysis of 354 T2DM15–30 and 470 T1DM15–30 patients, the age of diabetes onset was 25.6 ± 3.7 and 22.0 ± 4.3 years (P < 0.01), respectively, and duration of diabetes was 11.6 vs. 14.7 years (P = 0.001), respectively. There was an excess of males found in both groups, particularly in the T1DM15–30 cohort (50.6 vs. 60.0%, P = 0.007). Patients in the T1DM15–30 group were mainly of Anglo-Celtic background (77.8%), and by contrast, the T2DM15–30 group was of a more multiethnic background (28.1% Anglo-Celtic) (Table 1). Within 5 years of diagnosis, the majority of T2DM15–30 subjects were treated with diet or oral hypoglycemic agents, and only 7% were being treated with insulin alone. With relevance to the concern of excess myocardial infarction in patients treated with rosiglitazone, only one subject had been treated with this agent. The T2DM15–30 group had a significantly higher BMI; however, both groups were in the overweight to obese range (32.2 ± 7.6 vs. 25.6 ± 4.5 kg/m2 for T2DM15–30 and T1DM15–30, respectively, P < 0.0001). There were no significant differences in the calendar year of diagnosis between the two study groups (P = 0.09), excluding a significant cohort effect. Of note, the updated HbA1c as a measure of glycemic exposure was similar between the groups (8.1 ± 1.6% for both, P = 0.9).
Cardiovascular risk factors
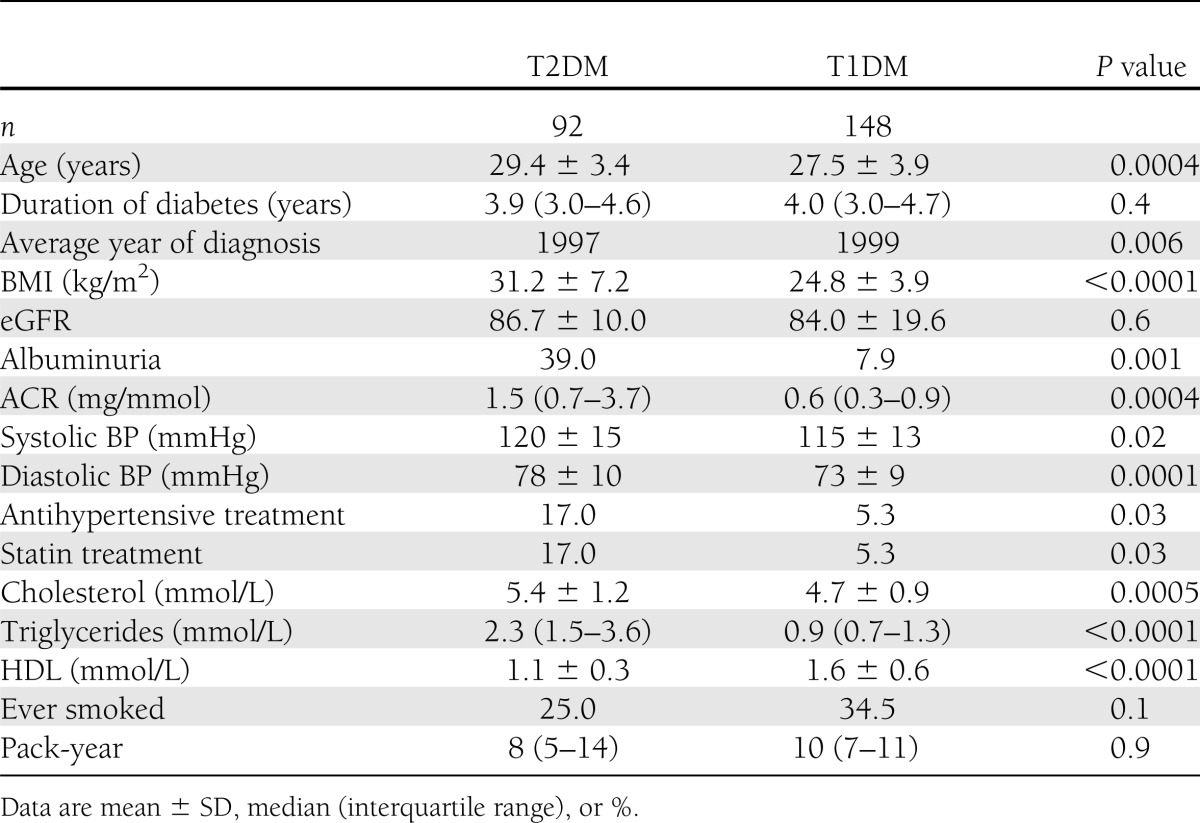
For the primary analysis, at the final clinical visit, less favorable cardiovascular risk factors were found in the T2DM cohort, with significantly higher levels of serum triglyceride levels, lower HDL levels, higher BP readings, and higher use of antihypertensive and statin treatment (Table 1). With respect to mortality, smoking prevalence was not different between the two cohorts. To explore whether these adverse risk factors were present early in the disease process, we examined clinical data within 2–5 years of diagnosis. Clinical data were available for 92 T2DM15–30 subjects and 148 T1DM15–30 subjects (Table 2). Again, we found that the presence of cardiovascular disease (CVD) risk factors, such as BMI, albuminuria, dyslipidemia, systolic and diastolic BP, were significantly more unfavorable in the T2DM15–30 group (P < 0.02 for all). There is already a high prevalence of abnormal albuminuria present at this early stage in T2DM15–30 patients (39 vs. 7.9%, P = 0.001). These less favorable risk factor profiles were found early in the disease (average age of 29 years) before any clinical evidence of macrovascular complications (Table 2).
Table 2.
Cardiovascular risk factors present after 2–5 years of known diabetes
Diabetes complications
Data comparing the prevalence of diabetes complications are presented in Table 1. Despite a statistically shorter duration of diabetes and remarkably similar glycemic exposure, there was a significant excess of complications in the T2DM15–30 cohort. Specifically, the ACR, prevalence of abnormal albuminuria, and biothesiometer Z scores were significantly increased (P < 0.0001 for all indices). However, there were no differences found between the groups with regard to the prevalence of retinopathy or renal function assessed by eGFR. A marked excess of macrovascular disease was found in the T2DM15–30 cohort, with a higher prevalence of ischemic heart disease (12.6 vs. 2.5%, P < 0.0001), stroke (4.3 vs. 0.7%, P = 0.002), and the composite end point of any macrovascular disease (14.4 vs. 5.7%, P < 0.0001). Findings are similar for the matched cohorts, where duration of diabetes is similar (Supplementary Table 1).
Logistic regression analyses based on data from all available T1DM patients (n = 870) and young T2DM subjects (n = 354) showed a significant independent relationship between a diagnosis of T2DM and the presence of macrovascular disease (odds ratio 5.4 [95% CI 2.7–10.5], P < 0.0001). Other significant independent variables were diabetes duration, albuminuria, male sex, and smoking history. Ethnicity was not a significant variable.
Survival analyses
After a similar median observation period of >20 years for both groups (21.4 [14.0–30.7] vs. 23.4 [15.7–32.4] years for T2DM15–30 and T1DM15–30, respectively, P = 0.002), altogether, 71 of 824 patients (8.6%) died. A significant excess case fatality rate of 39 deaths in 354 T2DM15–30 subjects (11%) compared with 32 deaths in 470 T1DM15–30 patients (6.8%) was noted (P = 0.03). Deaths in the T2DM15–30 cohort occurred after a significantly shorter disease duration (26.9 [18.1–36.0] vs. 36.5 [24.4–45.4] years, P = 0.01), and subjects died at a relatively young age in both groups (52.9 ± 14.7 and 57.4 ± 12 years for T2DM15–30 and T1DM15–30, respectively). The Kaplan-Meier analysis shows that cumulative survival was decreased for a given diabetes duration in the T2DM15–30 compared with the T1DM15–30 cohort (Fig. 1A), with separation of the survival curves appearing after ∼15 years of diabetes duration. The hazard ratio (HR) for death was increased significantly in the T2DM15–30 cohort to 2.0 (95% CI 1.2–3.2, P = 0.003) compared with the T1DM matched cohort. Because ethnicity varied significantly between the two groups, we also examined outcomes for the Anglo-Celtic groups only (n = 97 for T2DM15–30, n = 358 for T1DM15–30). This analysis still showed that the more unfavorable risk factors and a higher mortality rate were seen in the T2DM15–30 cohort (18.6 vs. 7.5%, P = 0.001), and Kaplan-Meier analysis showed that survival was also reduced in this group (Supplementary Fig. 1).
Figure 1.
A: Kaplan-Meier survival curve for T2DM15–30 (n = 357) and T1DM15–30 (n = 470) patients. B: Kaplan-Meier survival curve for T2DM15–30 and all T1DM (age of onset <30 years) (n = 870) patients.
This excess risk for death in T2DM15–30 subjects was still seen when the cohort was compared with the larger unmatched T1DM population diagnosed at <30 years of age (n = 870), yielding an HR of 2.7 (95% CI 1.6–4.4, P = 0.0001) (Fig. 1B). Additionally, Cox regression analysis of this larger cohort showed a significantly increased risk of death for the T2DM15–30 cohort (2.1 [1.1–3.8], P = 0.02) together with an independent impact of diastolic BP (1.06 [1.03–1.09], P = 0.0002) and albuminuria (2.0 [1.1–3.7], P = 0.03) on mortality.
The predominant primary causes of death were cardiovascular (ICD-10 code I11-I80) for both cohorts, but there was a notable excess of cardiovascular deaths in the T2DM15–30 cohort (50.0 vs. 30.3%, P < 0.053) (Fig. 2). Kaplan-Meier survival analysis for vascular mortality showed for both cohorts that the first vascular deaths occurred in the third decade of life, with an increased HR for vascular death for the T2DM15–30 cohort of 3.5 (1.4–8.5, P = 0.004). Self-harm, ketoacidosis, and accidents were not listed as a major cause of death for this cohort. Causes of death are listed in Supplementary Table 2.
Figure 2.
Kaplan-Meier curve for cardiovascular deaths.
CONCLUSIONS
This analysis of systematically collected data provides a unique opportunity to examine the future burden of a disease that, until recently, has been a relatively rare phenomenon. Such information on mortality and long-term complications will require several decades of observation to examine prospectively, underscoring the value of the data. We found that case fatality is increased twofold in young-onset T2DM compared with T1DM of a similar age and duration. This increased death rate is driven primarily by cardiovascular deaths occurring in the prime of life, and these results give substance to the notion that young-onset T2DM is an aggressive disease, even more so than T1DM. Because T1DM itself carries an increased mortality risk, with standardized mortality ratios in the order of 4 (14,15) compared with the general population, the present findings give a disquieting perspective on the long-term mortality risk of T2DM15–30 and a sobering glimpse of the future for patients, such as those in the TODAY study cohort.
In recognition of the paucity of data on survival in young-onset T2DM and that it will take decades from this point to understand the long-term mortality risk, Rhodes et al. (16) used a Markov modeling approach to project survival outcomes in this group, predicting that these patients lose ∼15 years from an average remaining life expectancy compared with the average 20-year-old. Only a few previous studies have looked at comparative mortality in T1DM and T2DM onset in patients <30 years of age. In a Swedish study of patients with diabetes aged 15–34 years compared with a general population, the standardized mortality ratio was higher for the T2DM than for the T1DM cohort (2.9 vs. 1.8) (17). A study of mortality in a multiethnic, low-income population with diabetes onset before age 30 found that the excess mortality was greatest for the insulin-treated cohort, but the investigators were unable to differentiate between T1DM and T2DM requiring insulin therapy (18). Recently, Dart et al. (19) examined survival in youth aged 1–18 years with T2DM versus T1DM. Kaplan-Meier analysis revealed a statistically significant lower survival probability for the youth with T2DM, although the number at risk was low after 10 year’s duration. Taken together, these findings are in keeping with the present observations and are supportive evidence for a higher mortality in young-onset T2DM than in T1DM.
The majority of deaths appear to be from cardiovascular causes and significantly more so for young T2DM. This would be predicted from the higher prevalence of macrovascular disease in the T2DM15–30 cohort seen during the last follow-up period. Indeed, other studies have predicted this outcome, with surrogate measures of arterial stiffness found to be higher in young T2DM patients than in T1DM patients (20). However, with the results of the present study, we now have confirmatory evidence of more concrete outcomes of clinically apparent vascular disease and death. The presence of less favorable cardiovascular risk factors and higher prevalence of macrovascular disease evident in the T2DM15–30 cohort is a contributing factor in the survival outcomes. These more adverse risk factors seen at the last visit were also evident even as early as 2–5 years from diagnosis. The constellation of higher BMI, diabetic dyslipidemia, BP, and urine ACR, all seen in the 20-year age-group, is alarming, particularly in the context of the patients’ youth. Others too have found a high incidence of cardiovascular risk factors, including microalbuminuria in young-onset T2DM, particularly in some ethnicities such as Maori, Pima, Japanese, Hispanic, and African American populations (21–24). Most recently, Dart et al. (19) examined renal outcomes in young-onset T2DM subjects compared with T1DM and normal control subjects and found a high burden of kidney disease and a fourfold risk of renal failure over T1DM subjects.
The observation of less favorable neuropathy scores in the T2DM cohort is a novel finding. Dyslipidemia has been implicated in the pathogenesis of diabetic neuropathy, and it is possible that less favorable lipid profiles in the T2DM15–30 cohort are contributory (20). In contrast to albuminuria, the prevalence of retinopathy is very similar for T1DM and T2DM. Perhaps for the retinal vasculature, glycemia is the main contributor to the development of retinopathy, whereas in the kidney, obesity and hypertension demonstrably present early in the genesis of young-onset T2DM have a much greater impact on the development of albuminuria in concert with other CVD risk factors.
The evidence presented underscores that metabolic syndrome features are a frequent and early accompaniment in young-onset T2DM and that common factors may be involved in their pathogenesis. The higher prevalence of macrovascular disease in the present patients with T2DM was evident despite a shorter or equivalent duration of disease and glycemic exposure. The implication of this observation is that control of glycemia alone at an early stage would not be enough to attenuate the excess vascular risk in early onset T2DM. Although the uptake of established CVD-protective therapies, such as ACE inhibitor/angiotensin receptor blocker and statin use is higher in the present T2DM15–30 cohort than in the T1DM15–30 cohort, the overall use of these therapies is low given the abnormalities that already exist. It is important to note that CVD risk reduction is largely dictated by results derived from adult populations. For example, in most statin intervention trials, the lower age entry criterion is 40 years. It is assumed that pharmaceuticals such as statins and ACE inhibitors/angiotensin receptor blockers have equivalent benefits in this younger age group because larger intervention trials have not systematically included such young patients with either type of diabetes. However, assuming that such treatments are efficacious in youth, there are issues regarding teratogenicity during childbearing years. Furthermore, in Australia, age is an arbiter for which patients are eligible for a national subsidy of pharmaceuticals such as statins, with older age-groups given priority preference because of their higher absolute risk. Such barriers add to the treatment gap and residual risk in young-onset T2DM patients that need to be addressed more fully.
One of the strengths of this study is the long duration of observation, allowing sufficient events to have accumulated for overall mortality and complications risk to be evaluated. In this context, the study provides a robust platform on which to compare outcomes that have been systematically collated. However, several limitations should be discussed. The subjects were referred to a diabetes center in a large metropolitan teaching hospital. It is possible that only severe cases of T2DM with high cardiovascular risk were referred, resulting in a selection bias toward less favorable outcomes in T2DM patients. Although this could be a confounder, we are reassured by studies that have screened for T2DM in youth in the community that also reported similar risk profiles of obesity, hypertension, and albuminuria, thus arguing against a selection bias as a significant contributor to the present findings (25). Differences in ethnicity and socioeconomic factors could account for poorer outcomes for the T2DM patients in this study. However, access to care for our services in a public hospital is free, so any impact of socioeconomic status resulting in a financial barrier to health care would be minimized. Although differences in complications risk may cosegregate with ethnicity, ethnicity was not a significant independent risk factor for either macrovascular complications or earlier mortality. Of note, even when only Anglo-Celtic groups are compared, T2DM patients still fared worse than their T1DM counterparts. Male sex often is associated with a higher mortality; however, in this study, the slight excess of males is seen in the T1DM cohort and, therefore, unlikely to have negatively biased the results. Although the age of onset of T1DM diabetes is usually in little doubt because of a more abrupt presentation, it is possible that the age of onset of T2DM was in fact earlier than recognized. With a previously published method for estimating time delay until diagnosis of T2DM (26) by plotting the prevalence of retinopathy against duration and extrapolating to a point of zero retinopathy, we found that there is no difference in the slope and intercept of this relationship between the T2DM and the T1DM cohorts (Supplementary Fig. 2). These data are reassuring in that delay in diagnosis is unlikely to be an explanation for the differences in observed outcome. Moreover, the survival analysis that used as a comparator an unmatched cohort of 870 T1DM patients who as a group had an earlier onset of diabetes still showed an excess mortality in those with T2DM. Because of the long time span over which the data were collected, many of the patients studied did not have autoantibodies measured. However, we are reassured by the low prevalence of early insulin use in the young-onset T2DM cohort, so any possible misclassification bias would be expected to be low. Finally, causes of death are derived from death certificates, which have recognized inaccuracies applicable to both types of diabetes.
In conclusion, this study highlights young-onset T2DM as a high-risk phenotype requiring intensive intervention directed not only toward the treatment of glycemia, but also toward cardiovascular risk factors that often are concurrent early in the course of diabetes. From the CVD risk management point of view, strategies for this patient group cannot necessarily be extrapolated from the adult situation. Therefore, the benefits, optimal timing, and mode of delivery of risk-lowering interventions in this high-risk group remain to be determined, with teratogenic risk a significant consideration. Additionally, given the severity of the young-onset T2DM phenotype and in the context of burgeoning numbers, if today we are to protect against tomorrow’s outcomes as predicted by this study, there is an urgent need for efforts to be redoubled toward diabetes prevention targeted to youth.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
M.I.C., D.K.Y., and J.W. researched the data and wrote and edited the manuscript. L.M., F.L.-G., A.A.-S., and C.L. researched the data and reviewed the manuscript. T.W. and S.M.T. reviewed and edited the manuscript. M.I.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in an oral session at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Profs. V. Gebski, A. Keech, and S. Colagiuri, University of Sydney, for their expert advice. The authors acknowledge the support of the Endocrinology and Diabetes Research Foundation of the University of Sydney and The NSW Ladies Bowl for Others Association. They also acknowledge the Australian Institute of Health and Welfare for support and expertise with the mortality data linkage.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2455/-/DC1.
See accompanying commentary, p. 3857.
A slide set summarizing this article is available online.
References
- 1.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care 2003;26:2999–3005 [DOI] [PubMed] [Google Scholar]
- 2.Wong J, Molyneaux L, Constantino M, Twigg SM, Yue DK. Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care 2008;31:1985–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeitler P, Hirst K, Pyle L, et al. TODAY Study Group A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laing SP, Swerdlow AJ, Slater SD, et al. The British Diabetic Association Cohort Study, I: all-cause mortality in patients with insulin-treated diabetes mellitus. Diabet Med 1999;16:459–465 [DOI] [PubMed] [Google Scholar]
- 5.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 6.McGill M, Molyneaux LM, Yue DK, Turtle JR. A single visit diabetes complication assessment service: a complement to diabetes management at the primary care level. Diabet Med 1993;10:366–370 [DOI] [PubMed] [Google Scholar]
- 7.Mathew TH, Australasian Creatinine Consensus Working Group Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust 2005;183:138–141 [DOI] [PubMed] [Google Scholar]
- 8.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manley S. Haemoglobin A1c—a marker for complications of type 2 diabetes: the experience from the UK Prospective Diabetes Study (UKPDS). Clin Chem Lab Med 2003;41:1182–1190 [DOI] [PubMed] [Google Scholar]
- 10.Powers J, Ball J, Adamson L, Dobson A. Effectiveness of the National Death Index for establishing the vital status of older women in the Australian Longitudinal Study on Women’s Health. Aust N Z J Public Health 2000;24:526–528 [DOI] [PubMed] [Google Scholar]
- 11.Hintze J. NCSS 2007 Kaysville, UT, NCSS, 2007 [Google Scholar]
- 12.Boffin Software. ACCorD (Analysis of Censored and Correlated Data) (V.2.0.10), 2011
- 13.Hintze J. Data matching – Optimal and Greedy. In NCSS User’s Guide Kaysville, UT, NCSS, 2007, Chapter 123 [Google Scholar]
- 14.Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ 2011;343:d5364 [DOI] [PMC free article] [PubMed]
- 15.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 16.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabet Med 2012;29:453–463 [DOI] [PubMed] [Google Scholar]
- 17.Waernbaum I, Blohmé G, Östman J, et al. Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. Diabetologia 2006;49:653–659 [DOI] [PubMed] [Google Scholar]
- 18.Conway BN, May ME, Signorello LB, Blot WJ. Mortality experience of a low-income population with young-onset diabetes. Diabetes Care 2012;35:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care 2012;35:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadwa RP, Urbina EM, Anderson AM, et al. SEARCH Study Group Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. Diabetes Care 2010;33:881–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 22.Ettinger LM, Freeman K, DiMartino-Nardi JR, Flynn JT. Microalbuminuria and abnormal ambulatory blood pressure in adolescents with type 2 diabetes mellitus. J Pediatr 2005;147:67–73 [DOI] [PubMed] [Google Scholar]
- 23.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr 2000;136:664–672 [DOI] [PubMed] [Google Scholar]
- 24.McGrath NM, Parker GN, Dawson P. Early presentation of type 2 diabetes mellitus in young New Zealand Maori. Diabetes Res Clin Pract 1999;43:205–209 [DOI] [PubMed] [Google Scholar]
- 25.Sillars BA, Davis WA, Kamber N, Davis TM. The epidemiology and characteristics of type 2 diabetes in urban, community-based young people. Intern Med J 2010;40:850–854 [DOI] [PubMed] [Google Scholar]
- 26.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care 1992;15:815–819 [DOI] [PubMed] [Google Scholar]




