Abstract
OBJECTIVE
To determine the separate and combined effects of high-protein (HP) and high-fat (HF) meals, with the same carbohydrate content, on postprandial glycemia in children using intensive insulin therapy (IIT).
RESEARCH DESIGN AND METHODS
Thirty-three subjects aged 8–17 years were given 4 test breakfasts with the same carbohydrate amount but varying protein and fat quantities: low fat (LF)/low protein (LP), LF/HP, HF/LP, and HF/HP. LF and HF meals contained 4 g and 35 g fat. LP and HP meals contained 5 g and 40 g protein. An individually standardized insulin dose was given for each meal. Postprandial glycemia was assessed by 5-h continuous glucose monitoring.
RESULTS
Compared with the LF/LP meal, mean glucose excursions were greater from 180 min after the LF/HP meal (2.4 mmol/L [95% CI 1.1–3.7] vs. 0.5 mmol/L [−0.8 to 1.8]; P = 0.02) and from 210 min after the HF/LP meal (1.8 mmol/L [0.3–3.2] vs. −0.5 mmol/L [−1.9 to 0.8]; P = 0.01). The HF/HP meal resulted in higher glucose excursions from 180 min to 300 min (P < 0.04) compared with all other meals. There was a reduction in the risk of hypoglycemia after the HP meals (odds ratio 0.16 [95% CI 0.06–0.41]; P < 0.001).
CONCLUSIONS
Meals high in protein or fat increase glucose excursions in youth using IIT from 3 h to 5 h postmeal. Protein and fat have an additive impact on the delayed postprandial glycemic rise. Protein had a protective effect on the development of hypoglycemia.
Current management of people with type 1 diabetes (T1D) on intensive insulin therapy (IIT) advocates algorithms based on the carbohydrate content of the meal to calculate the prandial insulin dose (1,2). This approach is recommended as a means to improve glycemic control and allow greater dietary flexibility (3,4). Typically, these calculations do not take into account the protein and fat content of the meal.
In recent years, novel algorithms have recommended counting fat and protein units, in addition to carbohydrate, in order to determine a supplementary insulin requirement for high-fat and -protein meals (5). However, increased postprandial hypoglycemia has been observed in children following these recommendations (6). A recent study (7) showed that meals high in fat do require more insulin than lower-fat meals with the same carbohydrate content, supporting the need for alternative insulin dosing algorithms for high-fat (HF) meals. However, there is a general paucity of evidence regarding the impact of protein and fat on postprandial glycemia in patients utilizing IIT, and consistent clinical advice for optimal management of high-protein (HP) and HF meals is lacking.
To date, protein has been considered together with fat in test meal studies, and controlled trials examining the effect of variations in protein content, independent of other macronutrients, on postprandial glucose levels have not been performed in individuals with T1D using insulin pump or multiple daily injection therapies. Therefore, this study was undertaken to examine the separate and combined effects of HP and HF meals, all with the same carbohydrate content, on postprandial glycemia in children and adolescents using IIT.
RESEARCH DESIGN AND METHODS
The study design was a four-by-four randomized crossover trial conducted at two pediatric centers in Australia (Princess Margaret Hospital in Perth and John Hunter Children’s Hospital in Newcastle). Children and adolescents with T1D who had been diagnosed for >1 year and who had been treated with continuous subcutaneous insulin infusion or multiple daily injection (≥4 injections/day) for >6 months were recruited. Inclusion criteria included age between 8 and 17 years, glycated hemoglobin (HbA1c) ≤8.0% (64 mmol/mol), and BMI ≤97th percentile. Exclusion criteria were coexisting medical problems (including celiac disease), evidence of complications of diabetes (including gastroparesis), hyperlipidemia, and dietary restrictions.
Ethics approval was obtained from the ethics committees of the Princess Margaret Children’s Hospital and the John Hunter Children’s Hospital. Written informed consent was gained from all participants and their parents.
In the week leading up to the study, participants and their caregivers were contacted daily by telephone to review the subject’s blood glucose level (BGL). Adjustments were made, if required, to the participant’s insulin therapy to meet a prebreakfast target range of 4–8 mmol/L and to optimize each participant’s insulin-to-carbohydrate ratio. If the subject’s fasting glucose values were high (>12.0 mmol/L) or low (<3.6 mmol/L), participants were instructed to treat as normal, e.g., for hyperglycemia, administer a correction bolus. This study day was then excluded and repeated.
Participants received their breakfast (test meal) under supervision by one of the two study centers over four consecutive mornings. Four standardized test meals of high- or low-fat and high- or low-protein content, all with the same carbohydrate amount, were given under supervision to each participant in random order over the four study days. Children were required to fast overnight for at least 10 h prior to breakfast, consume the test meal in 20 min, and fast for 5 h after completion of the test meal. Activity was standardized (sedentary) during the 5-h postprandial period for each participant.
The insulin dose for each participant was determined for the carbohydrate content using each participant’s individualized insulin-to-carbohydrate ratio. This dose then remained constant for each of the four test meals. The short-acting insulin bolus was administered 10 min prior to test meal consumption via subcutaneous injection or as a standard bolus via the insulin pump. In the event of hypoglycemia during the 5-h postprandial period, 15 g oral carbohydrate was given and analysis stopped at that point. Participants using continuous subcutaneous insulin infusion changed their infusion site on day 1 and day 3 of the study.
Test meals
Test meals consisted of pancakes varying in protein and fat content but identical in carbohydrate amount. The low-fat (LF) and HF meals contained 4 g fat and 35 g fat, respectively, and the low-protein (LP) meal and HP meals contained 5 g protein and 40 g protein, respectively. All meals contained the same amount of carbohydrate (30 g, 120 kcal). The total energy content of the four test meals was 180 kcal for the LF/LP meal, 330 kcal for the LF/HP meal, 460 kcal for the HF/LF meal, and 615 kcal for the HF/HP meal. Beneprotein (100% whey protein isolate) was used to increase the protein content of the meals without impacting the fat and carbohydrate quantities. See Table 1 for a detailed description of the meals.
Table 1.
Macronutrient composition for LF, HF, LP, and HP test meals
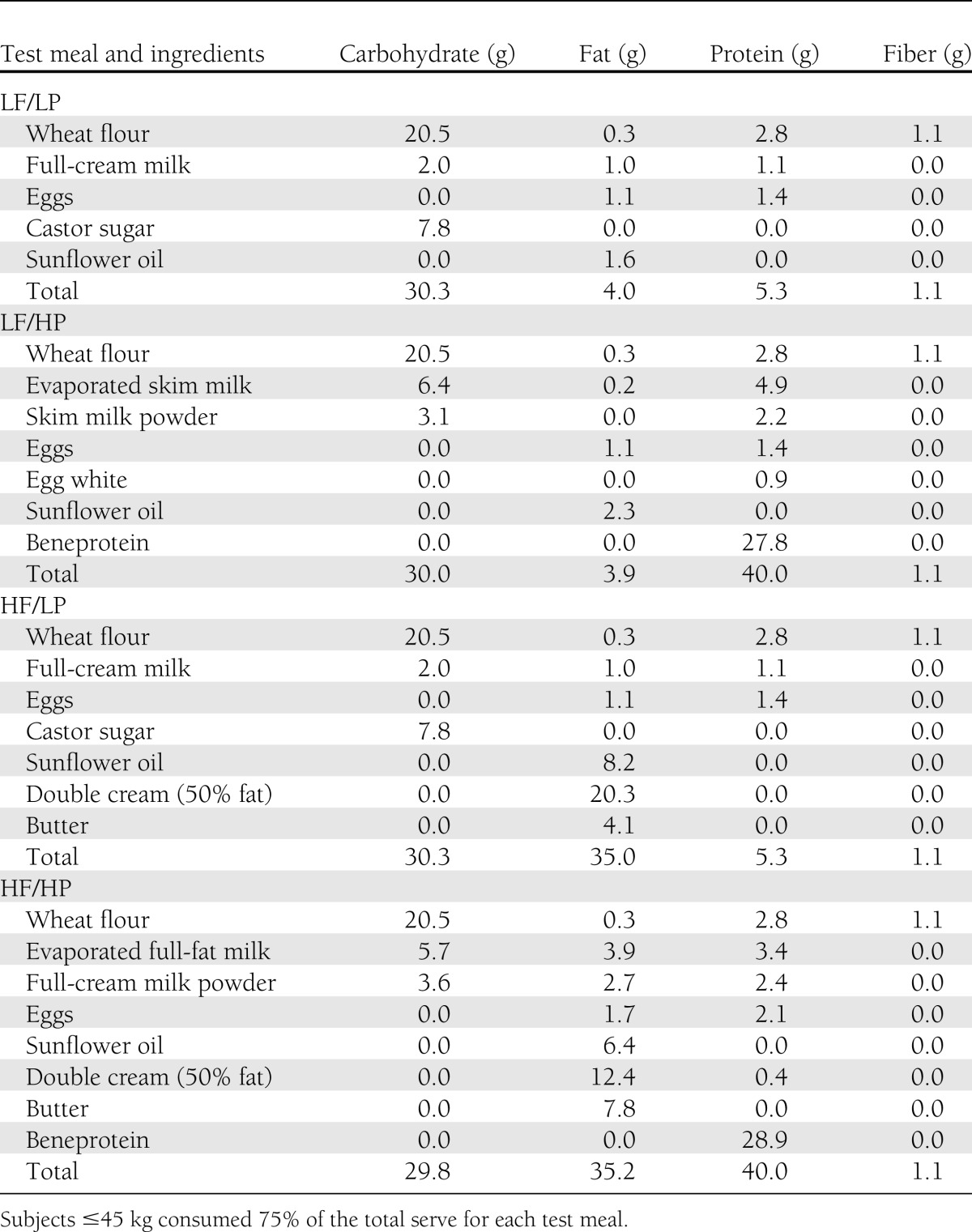
The fat and protein amounts were based on quantities in foods commonly consumed by children and adolescents with diabetes (8). A weight-based cut point for protein was derived from recommendations of upper levels of protein intakes for children (9). To ensure an appropriate protein amount for children ≤45 kg, 75% of the total serving for each pancake was provided. The reduction in the serving size for the smaller children resulted in all macronutrients being altered proportionally to provide 75% of the amount in the full serving. The meal types were given to patients in a random order, which was predetermined based on a generalized cyclic block design and was generated using Proc Plan in SAS v9.3, 2010 (SAS Institute, Cary, NC). Food was prepared under controlled conditions and weighed using Salter kitchen scales (accuracy ±1 g, model 323; Salter, Kent, U.K.).
Glucose measurement
The iPro2 Continuous Glucose Monitoring System (CGMS; MedtronicMiniMed, Northbridge, CA) was used to record glucose levels in the participants over the 4 days of the study. Subjects attended the clinic on the day prior to the study commencement for insertion of CGMS. Participants were asked to record at least four capillary blood glucose measurements per day into their study diary to allow for calibration. At the completion of the study, data were downloaded from the CGMS using the Medtronic CareLinkiPro data system (Medtronic MiniMed).
Statistical analysis
The primary outcome measure was the glucose excursion at each 30-min interval from baseline to 300 min after each of the four test meals. This was calculated as the observed postprandial glucose level minus the subject’s glucose level at baseline. Secondary outcomes included hypoglycemic event (defined as a capillary BGL <3.6 mmol/L), peak glucose excursion, and time to peak glucose excursion. Glucose excursion data for children who had a hypoglycemic event were not included after the time of the event.
Differences in mean glucose excursions between meal groups at a single time point were tested using a generalized linear mixed model to account for the repeated measurements on the same children. The outcome in the model was glucose excursion, and the only predictor was meal type, which was included as a four-level factor. A second set of models was fitted to examine the interaction between the effect of fat and protein. This set of models had predictors of fat, protein, and the interaction of fat and protein. Generalized linear mixed models were also used to test for differences in mean peak glucose excursions and mean time to peak glucose excursions. Differences between meal types in the proportion of subjects who had a hypoglycemic event were examined using a logistic regression model within a generalized estimating equation framework.
P values <0.05 were considered statistically significant. Data analysis was conducted using SAS (SAS Institute) and Stata statistical software, 2011, release 12 (StataCorp 2011, College Station, TX).
RESULTS
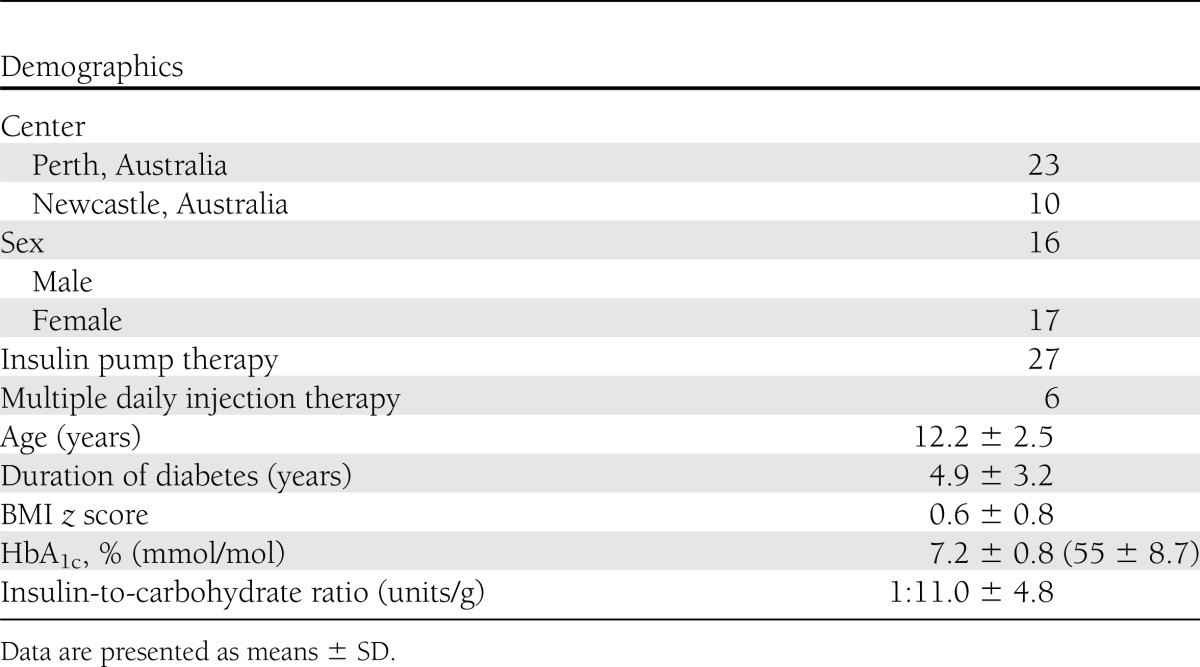
The mean (SD) age of the 33 children who completed the study was 12.2 (2.5) years, and 17 (52%) were female. Other baseline characteristics are presented in Table 2.
Table 2.
Clinical characteristics of participants
The prebreakfast target range of 4–8 mmol/L was achieved on 78 days over the 132 study days. Ninety-seven percent of fasting glucose values (n = 128) were between 3.6 and 12.0 mmol/L, requiring 4 study days to be repeated because of high or low fasting glucose values. On each occasion, the day was successfully repeated.
Nine children had incomplete study days where data from 1 day were excluded from the analysis due to an incomplete sensor reading over the 5-h postprandial period (n = 7) or failure to complete one of the test meals in 20 min (n = 2). Data from all of the other days (n = 123) were included in the analysis. Twelve children weighed ≤45 kg and were given 75% of the total meal serving. The outcome data for these children did not differ significantly from subjects who weighed >45 kg (P > 0.05).
Postprandial glucose excursions
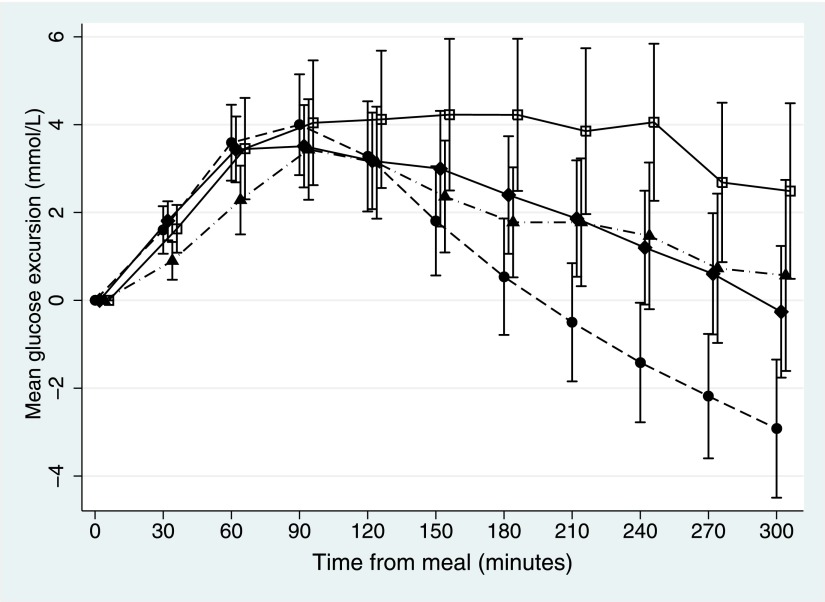
Figure 1 presents the mean postprandial glucose excursions by meal type at each time point (30-min increments from 0 to 300 min). Differences in mean glucose excursions between the test meals became apparent from ~120 min after the meals, with a sustained and attenuated additive effect of the HF/HP meal. The mean glucose excursions after the LF/HP meal were significantly greater than the mean glucose excursions after the LF/LP meal commencing at 180 min (P = 0.02) and continuing to 300 min (P < 0.01) (Table 3). The mean glucose excursions after the HF/LP meal were significantly higher than the glucose excursions after the LF/LP meal at 210 min (P = 0.01) and continuing to 300 min (P < 0.01).
Figure 1.
Mean postprandial glucose excursions from 0 to 300 min for 33 subjects after test meals of LF/LP (●), LF/HP (♦), HF/LP (▲), and HF/HP (□) content. Carbohydrate amount was the same in all meals. There were significant differences in glucose excursions between meal types from 150 to 300 min (P < 0.03). Error bars represent 95% CIs.
Table 3.
Mean postprandial glucose excursions by meal type at 30-min intervals to 300 min
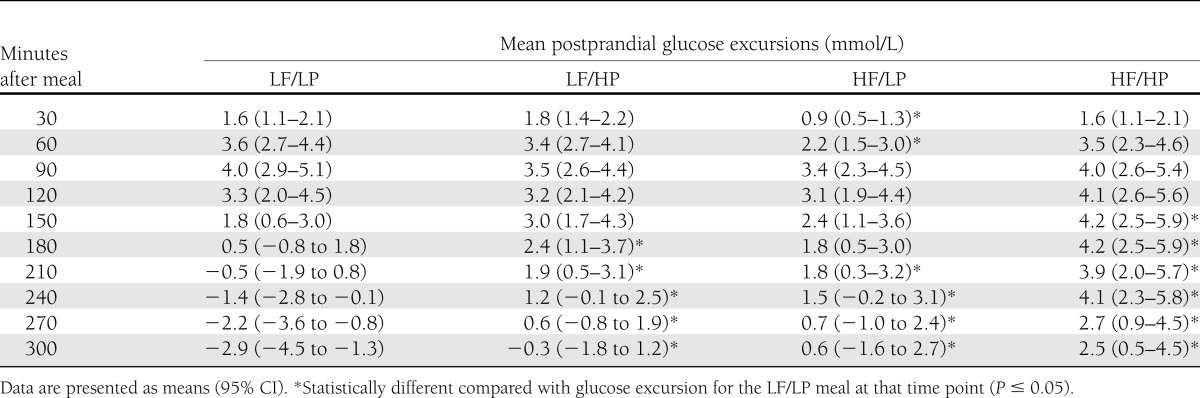
The HF/HP meal resulted in significantly higher glucose excursions from 180 min to 300 min compared with all other meals (P < 0.04) (Fig. 1). Compared with the LF/LP meal, mean glucose excursions were significantly greater from 150 min after the HF/HP meal (P = 0.004) (Table 3). At 300 min, the mean glucose excursion for the HF/HP meal was 5.4 mmol/L higher than for the LF/LP meal (P < 0.001).
The HF/LP meal reduced the glucose excursion within the first 60–90 min after the meal compared with all other meals (Table 3). The mean excursion at 60 min after the HF/LP meal was significantly lower than the excursion for the LF/LP meal (P = 0.009). This effect was only seen for the LP/HF meal.
Beyond 120 min, the glycemic profiles after the HF/LP meal were similar to the profiles after the LF/HP meal (Fig. 1). There was no statistically significant interaction between the effects of the fat and protein on glucose excursions at all time points (P > 0.05), which is consistent with their effects being additive. The effects of protein and fat on glucose excursions were additive, as indicated by the lack of interaction of the two effects and as seen in Fig. 1. For example, at 180 min the mean glucose excursion for the HF/HP meal (4.2 mmol/L [95% CI 2.5–5.9]) was equivalent to the combined excursions of the LF/HP meal (2.4 mmol/L [1.1–3.7]) and the HF/LP meal (1.8 mmol/L [0.5–3.0]).
Peak glucose excursion and time to peak glucose
There was a significant difference in peak glucose excursions between meal types (P = 0.049), with the highest value recorded after the HF/HP meal. The mean peak glucose excursions from baseline for the LF/LP, LF/HP, HF/LP, and HF/HP meals, respectively, were 4.7 mmol/L (95% CI 3.6–5.8), 4.4 mmol/L (3.5–5.3), 4.3 mmol/L (3.1 to 5.4), and 5.9 mmol/L (4.6–7.3).
There was also a statistically significant difference in the time to mean peak glucose excursion between meal types (P < 0.001), with the longest time being observed after the HF/HP meal. The mean time to peak glucose excursion for the LF/LP, LF/HP, HF/LP, and HF/HP meals, respectively, were 79 min (95% CI 68–89), 96 min (74–119) min, 126 min (97–154) min, and 143 min (112 to 174).
Hypoglycemic events
Twenty-nine symptomatic hypoglycemic events occurred in the 5-h postprandial period during the study. Fourteen occurred in the LF/LP group, 10 in the HF/LP group, 4 in the LF/HP group, and 1 in the HF/HP group. There were no episodes of severe hypoglycemia. The number of hypoglycemic events differed significantly between the four meal types (P = 0.003). There was a statistically significant reduction in the odds of a hypoglycemic event when children consumed the HP meals (odds ratio 0.16 [95% CI 0.06–0.41]; P < 0.001) but not when they consumed the HF meals (odds ratio 0.50 [0.22–1.09]; P = 0.08).
CONCLUSIONS
This study has demonstrated an effect of dietary protein independent of fat on postprandial glycemia in children with T1D. Importantly, the glycemic rise after protein was shown in meals of both HF and LF contents, with identical carbohydrate quantities. In addition, when a meal containing high levels of both protein and fat was eaten, the impact of protein and fat was additive and caused significantly higher glucose excursions between 3 and 5 h postprandially compared with meals of only HF or HP contents.
An important finding of this study was that there were significantly higher glycemic excursions for the HP and HF meals compared with the LP/LF meal from ~180 to 300 min postprandially. This late effect was increased and sustained when the HF and HP loads were combined. As expected, the HF meal initially reduced the glycemic excursion for up to 90 min after the meal. This is most likely due to the effect of fat in delaying gastric emptying (10,11) and is consistent with studies in both adolescents with T1D (10) and patients with type 2 diabetes (12). However in our study, the addition of protein to the HF meal prevented this, suggesting that protein may have a protective effect in the development of early postprandial hypoglycemia.
The cause of the late sustained hyperglycemia noted when meals high in protein and fat are eaten has been postulated but is currently unknown. Protein may lead to delayed hyperglycemia by gluconeogenesis and increased glucagon secretion (13). Proposed mechanisms by which dietary fat and free fatty acids contribute to hyperglycemia are by impairing insulin sensitivity and enhancement of hepatic glucose production, along with delayed gastric emptying, which causes an increase in the peak time and amplitude of the glucose response (11). Further studies are required to fully elucidate the pathways of action.
The results of this study have direct clinical translation. The protein, fat, and carbohydrate contents in this study were based on real meals commonly consumed by children and adolescents. For such meals, patients may be advised that significant hyperglycemia is likely to occur between 3 and 5 h after the meal, particularly accentuated and prolonged for the HF/HP meal. The findings of this study suggest the need for both prolonging insulin delivery by the use of a different wave form, such as a dual-wave bolus in those on pump therapy and that additional insulin is required to match the delayed hyperglycemia. Future studies are needed to determine an alternative insulin dosing algorithm to separately account for the fat and protein in HF/HP meals.
Typically, insulin bolus dosing has been determined using the insulin-to-carbohydrate ratio. Our findings support recent evidence that dietary fat increases insulin requirements (7), but suggest that the additional insulin should be given by an extended insulin bolus or as a split bolus in order to prevent early hypoglycemia. This study also adds new data pointing to the need for additional insulin for HP meals independent of the fat content. A feature of insulin pump therapy that is potentially advantageous is the ability to vary the delivery of a bolus of insulin over time by use of a dual-wave or square-wave bolus. A dual-wave bolus has already been shown to limit glycemic excursions in pizza studies (14,15). The question of the optimal timing and distribution of the bolus in combined HF and HP meals, however, requires more investigation. Some centers have recently reported their experiences in pump patients of calculating fat and protein units and using these to determine insulin bolus dosing for the mixed meals (6,16). These studies provide some data using a normal-wave bolus given for carbohydrate and also a square-wave bolus with supplementary insulin for the fat and protein content. While reduced postprandial hyperglycemia has been noted, the rate of hypoglycemia using these calculations has been unacceptably high (33–35%) (6,16). There has also been a lack of standardization of bolus types between the groups (16), which makes it difficult to compare the effect of additional insulin as opposed to the method of insulin delivery. Clearly, further studies are needed to refine and quantify the extra insulin that is required for HF or HP meals and to determine algorithms for the best dose and rate of insulin delivery over time.
A limitation of the study was that we did not examine the effect of protein and fat beyond 5 h, although previous studies have noted an effect of HF/HP meals after this time period (14,15). During daylight hours, food is typically eaten so frequently that additional bolus doses of insulin within a few hours of the meal may correct the hyperglycemia. We therefore suggest that the composition of the evening meal is particularly important to consider in mealtime insulin calculations, as in the case of fasting overnight no additional insulin is given and prolonged hyperglycemia may result. Furthermore, our data from this and previous studies (17,18) indicate that postprandial testing may be more appropriate at 3 h rather than 2 h after the meal, as the glycemic excursion from even those meals lower in fat and protein did not return to baseline until this time.
In conclusion, this is the first study to demonstrate that the addition of protein and fat to meals containing the same carbohydrate amount results in prolonged postprandial hyperglycemia in children using IIT. When the protein and fat were consumed together, there was an additive effect on postprandial glycemia. Furthermore, the protein appeared to have a protective effect against hypoglycemia. This study provides supportive evidence that protein and fat should both be considered in insulin dosing.
Acknowledgments
A Telethon Foundation Fellowship grant supported S.M.O.’s contribution to this study. A Hunter Children’s Research Foundation grant supported the Newcastle study site.
This project was supported by a Pfizer Australia Paediatric Endocrine Care Research grant. No other potential conflicts of interest relevant to this article were reported.
C.E.M.S. conceived, designed, and conducted the study; recruited subjects; collected data; and wrote the manuscript. M.E. recruited subjects, conducted the study, and collected data. S.M.O. designed and conducted the study, recruited subjects, collected data, and wrote the manuscript. P.M. analyzed data and contributed to the writing of the manuscript. P.E.L. recruited subjects, conducted the study, collected data, and contributed to the writing of the manuscript. T.W.J., E.A.D., and B.R.K. contributed to the discussion and reviewed and edited the manuscript. B.R.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
The authors thank Niru Paramalingam and Adam Retterath, Princess Margaret Hospital, and Virginia McRory, John Hunter Children’s Hospital, for their support with the equipment and preparation of the test meals.
Footnotes
A slide set summarizing this article is available online.
References
- 1.Laurenzi A, Bolla AM, Panigoni G, et al. Effects of carbohydrate counting on glucose control and quality of life over 24 weeks in adult patients with type 1 diabetes on continuous subcutaneous insulin infusion: a randomized, prospective clinical trial (GIOCAR). Diabetes Care 2011;34:823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DAFNE Study Group Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scavone G, Manto A, Pitocco D, et al. Effect of carbohydrate counting and medical nutritional therapy on glycaemic control in Type 1 diabetic subjects: a pilot study. Diabet Med 2010;27:477–479 [DOI] [PubMed] [Google Scholar]
- 4.Lowe J, Linjawi S, Mensch M, James K, Attia J. Flexible eating and flexible insulin dosing in patients with diabetes: Results of an intensive self-management course. Diabetes Res Clin Pract 2008;80:439–443 [DOI] [PubMed] [Google Scholar]
- 5.Pańkowska E, Szypowska A, Lipka M, Szpotańska M, Błazik M, Groele L. Application of novel dual wave meal bolus and its impact on glycated hemoglobin A1c level in children with type 1 diabetes. Pediatr Diabetes 2009;10:298–303 [DOI] [PubMed] [Google Scholar]
- 6.Kordonouri O, Hartmann R, Remus K, Bläsig S, Sadeghian E, Danne T. Benefit of supplementary fat plus protein counting as compared with conventional carbohydrate counting for insulin bolus calculation in children with pump therapy. Pediatr Diabetes 2012;13:540–544 [DOI] [PubMed] [Google Scholar]
- 7.Wolpert HA, Atakov-Castillo A, Smith SA, Steil GM. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care 2013;36:810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Øverby NC, Flaaten V, Veierød MB, et al. Children and adolescents with type 1 diabetes eat a more atherosclerosis-prone diet than healthy control subjects. Diabetologia 2007;50:307–316 [DOI] [PubMed] [Google Scholar]
- 9.National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Executive Summary Canberra, Australia, Department of Health and Ageing, 2005 [Google Scholar]
- 10.Lodefalk MAJ, Aman J, Bang P. Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with Type 1 diabetes. Diabet Med 2008;25:1030–1035 [DOI] [PubMed] [Google Scholar]
- 11.Wolever TMMY, Mullan YM. Sugars and fat have different effects on postprandial glucose responses in normal and type 1 diabetic subjects. Nutr Metab Cardiovasc Dis 2011;21:719–725 [DOI] [PubMed] [Google Scholar]
- 12.Gentilcore D, Chaikomin R, Jones KL, et al. Effects of fat on gastric emptying of and the glycemic, insulin, and incretin responses to a carbohydrate meal in type 2 diabetes. J Clin Endocrinol Metab 2006;91:2062–2067 [DOI] [PubMed] [Google Scholar]
- 13.Peters AL, Davidson MB. Protein and fat effects on glucose responses and insulin requirements in subjects with insulin-dependent diabetes mellitus. Am J Clin Nutr 1993;58:555–560 [DOI] [PubMed] [Google Scholar]
- 14.Jones SM, Quarry JL, Caldwell-McMillan M, Mauger DT, Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose montoring system. Diabetes Technol Ther 2005;7:233–240 [DOI] [PubMed] [Google Scholar]
- 15.Lee SW, Cao M, Sajid S, et al. The dual-wave bolus feature in continuous subcutaneous insulin infusion pumps controls prolonged post-prandial hyperglycaemia better than standard bolus in Type 1 diabetes. Diabetes Nutr Metab 2004;17:211–216 [PubMed] [Google Scholar]
- 16.Pańkowska E, Blazik M, Groele L. Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: the conclusion of a randomized study. Diabetes Technol Ther 2012;14:16–22 [DOI] [PubMed] [Google Scholar]
- 17.Smart CE, Ross K, Edge JA, Collins CE, Colyvas K, King BR. Children and adolescents on intensive insulin therapy maintain postprandial glycaemic control without precise carbohydrate counting. Diabet Med 2009;26:279–285 [DOI] [PubMed] [Google Scholar]
- 18.Smart CE, King BR, McElduff P, Collins CE. In children using intensive insulin therapy, a 20-g variation in carbohydrate amount significantly impacts on postprandial glycaemia. Diabet Med 2012;29:e21–e24 [DOI] [PubMed] [Google Scholar]