Abstract
A very old unanswered question in classical cytology is whether chromosomes are arranged randomly in sperm or whether they occupy specific positions. Even with modern methods of chromosome painting, it is difficult to resolve this question for the very condensed and almost spherical sperm head of most mammals. We have taken advantage of the unusual fibrillar sperm head of monotreme mammals (echidna and platypus) to examine the position of chromosome landmarks in a two-dimensional array. We used fluorescence and radioactive in situ hybridization to telomeric, rDNA, and unique sequences to show that chromosomes are arranged tandemly and in a defined order in the sperm nucleus.
Full text
PDF


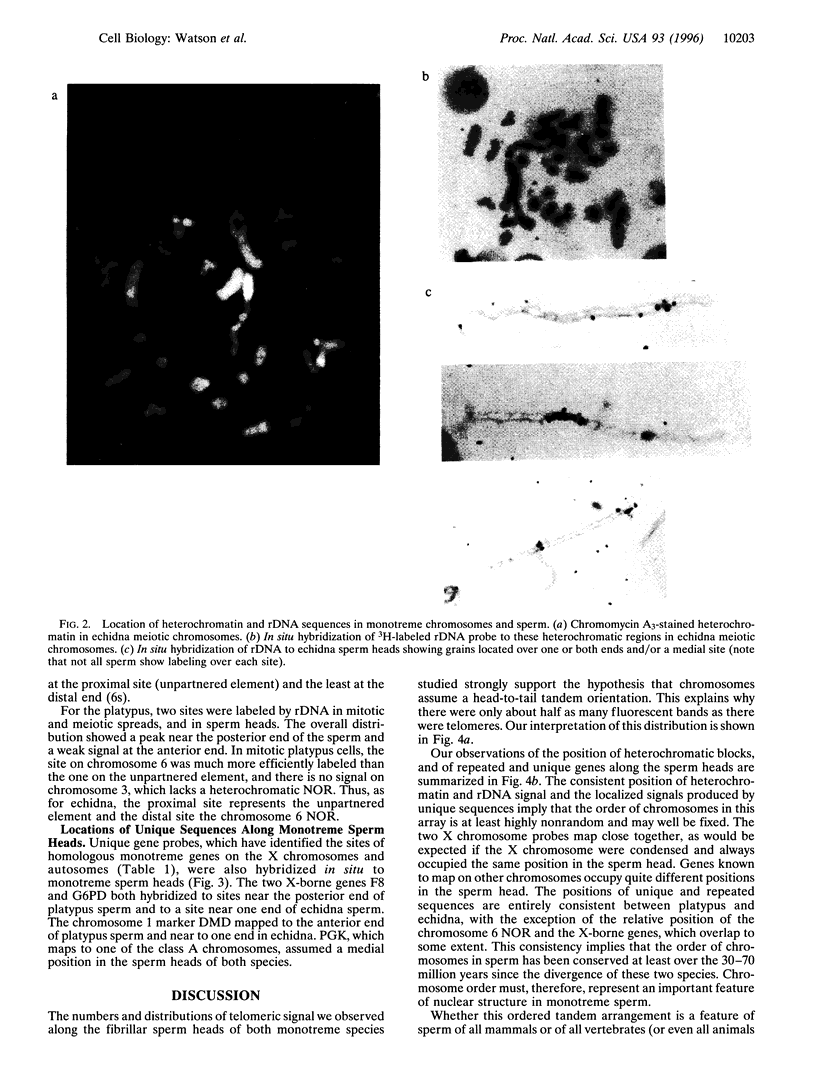
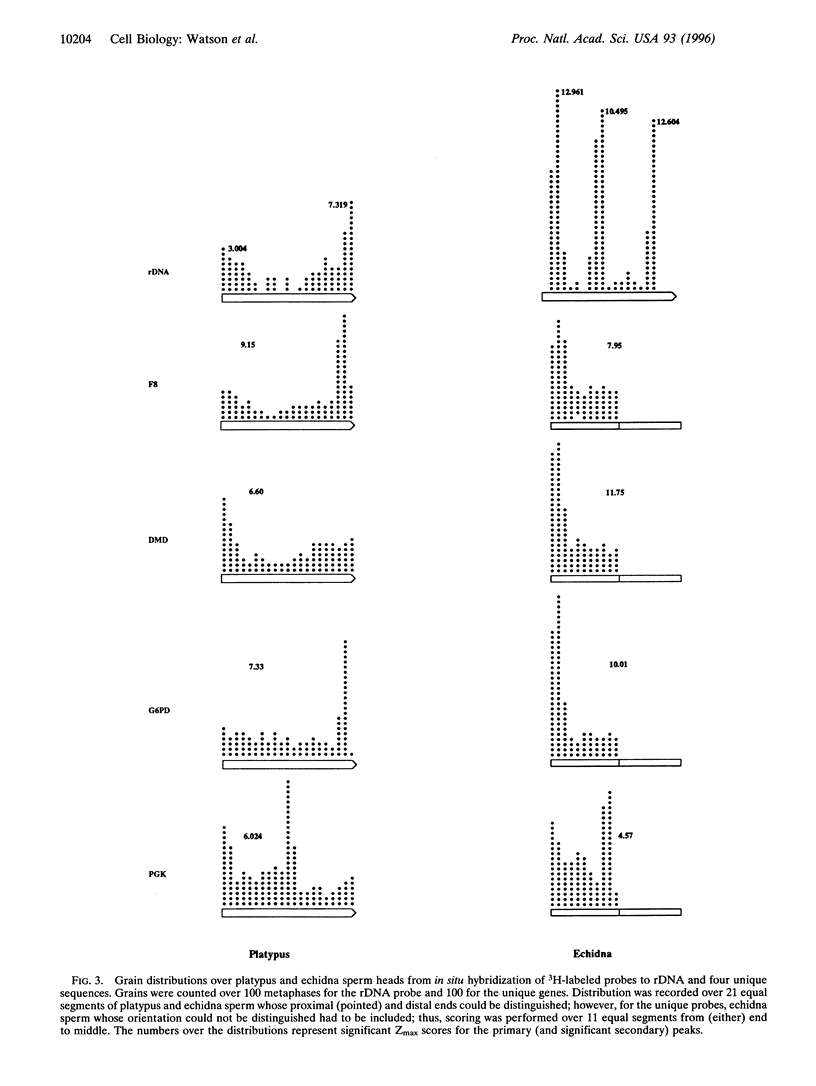

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borden J., Manuelidis L. Movement of the X chromosome in epilepsy. Science. 1988 Dec 23;242(4886):1687–1691. doi: 10.1126/science.3201257. [DOI] [PubMed] [Google Scholar]
- Carrick F. N., Hughes R. L. Aspects of the structure and development of monotreme spermatozoa and their relevance to the evolution of mammalian sperm morphology. Cell Tissue Res. 1982;222(1):127–141. doi: 10.1007/BF00218293. [DOI] [PubMed] [Google Scholar]
- Cooper D. W., Holland E. A., Rudman K., Donald J. A., Zehavi-Feferman R., McKenzie L. M., Sinclair A. H., Spencer J. A., Graves J. A., Poole W. E. Phosphoglycerate kinase pseudogenes in the tammar wallaby and other macropodid marsupials. Mamm Genome. 1994 Sep;5(9):531–537. doi: 10.1007/BF00354925. [DOI] [PubMed] [Google Scholar]
- Costello D. P. Identical linear order of chromosomes in both gametes of the acoel turbellarian Polychoerus carmelensis: a preliminary note. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1951–1958. doi: 10.1073/pnas.67.4.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. E., Pearson P. L., Harper P. S., Murray J. M., O'Brien T., Sarfarazi M., Williamson R. Linkage analysis of two cloned DNA sequences flanking the Duchenne muscular dystrophy locus on the short arm of the human X chromosome. Nucleic Acids Res. 1983 Apr 25;11(8):2303–2312. doi: 10.1093/nar/11.8.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens W. J., Griffiths R. C., Ethier S. N., Wilcox S. A., Graves J. A. Statistical analysis of in situ hybridization data: derivation and use of the zmax test. Genomics. 1992 Apr;12(4):675–682. doi: 10.1016/0888-7543(92)90293-2. [DOI] [PubMed] [Google Scholar]
- Finch R. A., Smith J. B., Bennett M. D. Hordeum and Secale mitotic genomes lie apart in a hybrid. J Cell Sci. 1981 Dec;52:391–403. doi: 10.1242/jcs.52.1.391. [DOI] [PubMed] [Google Scholar]
- Grumbach M. M., Morishima A., Taylor J. H. HUMAN SEX CHROMOSOME ABNORMALITIES IN RELATION TO DNA REPLICATION AND HETEROCHROMATINIZATION. Proc Natl Acad Sci U S A. 1963 May;49(5):581–589. doi: 10.1073/pnas.49.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttenbach M., Schakowski R., Schmid M. Incidence of chromosome 3, 7, 10, 11, 17 and X disomy in mature human sperm nuclei as determined by nonradioactive in situ hybridization. Hum Genet. 1994 Jan;93(1):7–12. doi: 10.1007/BF00218904. [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J. S., Bennett M. D. Nuclear architecture in plants. Trends Genet. 1990 Dec;6(12):401–405. doi: 10.1016/0168-9525(90)90300-u. [DOI] [PubMed] [Google Scholar]
- Lichter P., Cremer T., Borden J., Manuelidis L., Ward D. C. Delineation of individual human chromosomes in metaphase and interphase cells by in situ suppression hybridization using recombinant DNA libraries. Hum Genet. 1988 Nov;80(3):224–234. doi: 10.1007/BF01790090. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. Individual interphase chromosome domains revealed by in situ hybridization. Hum Genet. 1985;71(4):288–293. doi: 10.1007/BF00388453. [DOI] [PubMed] [Google Scholar]
- Mathog D., Hochstrasser M., Gruenbaum Y., Saumweber H., Sedat J. Characteristic folding pattern of polytene chromosomes in Drosophila salivary gland nuclei. 1984 Mar 29-Apr 4Nature. 308(5958):414–421. doi: 10.1038/308414a0. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico M. G., Viglietto G., Martini G., Toniolo D., Paonessa G., Moscatelli C., Dono R., Vulliamy T., Luzzatto L., D'Urso M. Isolation of human glucose-6-phosphate dehydrogenase (G6PD) cDNA clones: primary structure of the protein and unusual 5' non-coding region. Nucleic Acids Res. 1986 Mar 25;14(6):2511–2522. doi: 10.1093/nar/14.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D., Cran D. G., Jennings C., Jones R. Spatial organization of repetitive DNA sequences in the bovine sperm nucleus. J Cell Sci. 1990 Sep;97(Pt 1):185–191. doi: 10.1242/jcs.97.1.185. [DOI] [PubMed] [Google Scholar]
- Qumsiyeh M. B. Impact of rearrangements on function and position of chromosomes in the interphase nucleus and on human genetic disorders. Chromosome Res. 1995 Dec;3(8):455–465. doi: 10.1007/BF00713959. [DOI] [PubMed] [Google Scholar]
- Savage J. R. Interchange and intra-nuclear architecture. Environ Mol Mutagen. 1993;22(4):234–244. doi: 10.1002/em.2850220410. [DOI] [PubMed] [Google Scholar]
- Schardin M., Cremer T., Hager H. D., Lang M. Specific staining of human chromosomes in Chinese hamster x man hybrid cell lines demonstrates interphase chromosome territories. Hum Genet. 1985;71(4):281–287. doi: 10.1007/BF00388452. [DOI] [PubMed] [Google Scholar]
- Sinclair A. H., Wrigley J. M., Marshall Graves J. A. Autosomal assignment of OTC in marsupials and monotremes: implications for the evolution of sex chromosomes. Genet Res. 1987 Oct;50(2):131–136. doi: 10.1017/s0016672300023533. [DOI] [PubMed] [Google Scholar]
- TAYLOR J. H. THE ARRANGEMENT OF CHROMOSOMES IN THE MATURE SPERM OF THE GRASSHOPPER. J Cell Biol. 1964 May;21:286–289. doi: 10.1083/jcb.21.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB W. B., AGNEW H. W., Jr Sleep deprivation, age, and exhaustion time in the rat. Science. 1962 Jun 29;136(3522):1122–1122. doi: 10.1126/science.136.3522.1122. [DOI] [PubMed] [Google Scholar]
- Watson J. M., Riggs A., Graves J. A. Gene mapping studies confirm the homology between the platypus X and echidna X1 chromosomes and identify a conserved ancestral monotreme X chromosome. Chromosoma. 1992 Oct;101(10):596–601. doi: 10.1007/BF00360536. [DOI] [PubMed] [Google Scholar]
- Watson J. M., Spencer J. A., Riggs A. D., Graves J. A. Sex chromosome evolution: platypus gene mapping suggests that part of the human X chromosome was originally autosomal. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11256–11260. doi: 10.1073/pnas.88.24.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. M., Spencer J. A., Riggs A. D., Graves J. A. The X chromosome of monotremes shares a highly conserved region with the eutherian and marsupial X chromosomes despite the absence of X chromosome inactivation. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7125–7129. doi: 10.1073/pnas.87.18.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Brown D. D., Reeder R. H. The molecular basis for length heterogeneity in ribosomal DNA from Xenopus laevis. J Mol Biol. 1976 Aug 25;105(4):461–486. doi: 10.1016/0022-2836(76)90229-1. [DOI] [PubMed] [Google Scholar]
- Wrigley J. M., Graves J. A. Karyotypic conservation in the mammalian order monotremata (subclass Prototheria). Chromosoma. 1988;96(3):231–247. doi: 10.1007/BF00302363. [DOI] [PubMed] [Google Scholar]
- Wrigley J. M., Graves J. A. Two monotreme cell lines, derived from female platypuses (Ornithorhynchus anatinus; Monotremata, Mammalia). In Vitro. 1984 Apr;20(4):321–328. doi: 10.1007/BF02618595. [DOI] [PubMed] [Google Scholar]
- Zalensky A. O., Breneman J. W., Zalenskaya I. A., Brinkley B. R., Bradbury E. M. Organization of centromeres in the decondensed nuclei of mature human sperm. Chromosoma. 1993 Sep;102(8):509–518. doi: 10.1007/BF00368344. [DOI] [PubMed] [Google Scholar]
- Zelesco P. A., Graves J. A. Chromosome segregation from cell hybrids. IV. Movement and position of segregant set chromosomes in early-phase interspecific cell hybrids. J Cell Sci. 1988 Jan;89(Pt 1):49–56. doi: 10.1242/jcs.89.1.49. [DOI] [PubMed] [Google Scholar]