Abstract
OBJECTIVE
Findings on the effect of menopause or age at menopause on the presence of hyperglycemia are controversial, and why women after menopause have a higher probability of having hyperglycemia than men in the same age range remains unknown.
RESEARCH DESIGN AND METHODS
We reviewed data on 29,189 men, 6,308 premenopausal women, and 4,570 postmenopausal women in Japan. Odds ratios (ORs) for diabetes or prediabetes indicated by American Diabetes Association criteria were calculated for men and for pre- and postmenopausal women.
RESULTS
Compared with premenopausal women, women after natural menopause had an age-adjusted OR of 1.40 (95% CI 1.03–1.89) for diabetes, and women after menopause by surgical or other causes had an age-adjusted OR of 1.59 (1.07–2.37). The age-adjusted OR in men was 4.02 (3.15–5.14). Compared with premenopausal nondiabetic women, postmenopausal nondiabetic women had a significantly elevated OR of 1.33 (1.20–1.48) for prediabetes; nondiabetic men had an OR of 1.93 (1.77–2.10) independently of age and demographic and metabolic factors. Even among women aged <50 years, postmenopausal status was significantly associated with an elevated OR (1.50 [1.18–1.91]) for dysglycemia (either diabetes or prediabetes). Postmenopausal women aged ≥50 years had a particularly elevated OR for dysglycemia, regardless of age at menopause.
CONCLUSIONS
The postmenopausal state was significantly associated with the presence of dysglycemia independently of normal aging, although the increased probability in postmenopausal women did not equal that in men. Among women, menopause and older age might additively influence the elevated probability of dysglycemia.
Questions remain about why women after menopause have an increased risk of diabetes compared with men in the same age-group. The prevalence of diabetes was reported to be lower in women than in men aged ≤60 years, whereas women in their 60s and 70s were more likely to have diabetes than men of the same age (1,2), suggesting that the hormonal changes that characterize menopause might be associated with the risk of diabetes in women after menopause (3). Although the association of menopausal status and hyperglycemia has been investigated (4–12), findings about whether the postmenopausal state would influence hyperglycemia independently of normal aging remain controversial. Two cohort studies with a large number of female participants investigated the impact of the postmenopausal state on diabetes compared with the premenopausal state (13,14). A study of 22,426 Japanese women suggested that there is no significant association between the postmenopausal state and diabetes when adjustment is made for chronological age (14). However, the other cross-sectional study of Italian women showed a positive association between spontaneous menopause and diabetes independently of age and demographic factors (13). A review indicated that neither natural nor surgical menopause per se has a strong association with diabetes risk (15).
Weight gain, which commonly occurs during the menopausal transition, seems to be attributable to aging rather than to the menopausal transition itself (3,16). However, menopause is associated with changes in body composition, such as increased total body fat or abdominal fat and a decrease in lean body mass, which in turn are linked to impairments in glucose metabolism and insulin sensitivity (3). The occurrence of dysglycemia may be a direct result of ovarian failure or, alternatively, an indirect result of the metabolic consequences of central fat redistribution with estrogen deficiency (17). A study of Korean women showed that the prevalence rate of individuals with metabolic syndrome is markedly high in those ≥50 years of age and reached a peak in women in their 60s (7). Nonetheless, whether menopause would be associated with hyperglycemia independently of age and these other closely related metabolic factors remains unknown. In a prospective study in Spain that included 475 women, the presence of type 2 diabetes, impaired glucose tolerance, impaired fasting glucose, and other cardiometabolic markers did not differ significantly between women who went from premenopause to postmenopause and those who did not experience menopause during a 6-year follow-up period (18). To date, the joint effect of older age and the postmenopausal state on the presence of dysglycemia has not been clarified. Additionally, a few studies investigated whether a significant association exists between menopause and hyperglycemia among women without diabetes (6,19), and the results were inconsistent.
Controversy also exists about whether early age at menopause would increase diabetes risk. A recent study of postmenopausal women found early menopause to be associated with an increased risk of developing diabetes (20). In another study, on the other hand, age at menopause was not associated with diabetes in Chinese postmenopausal women (21). Cross-sectional studies in Italy (13) and China (11) did not show a significant association of age at menopause with diabetes. It should be noted that the definition of the diagnosis of diabetes differed among these studies (11,13,20,21).
Therefore, in the present cross-sectional study of Japanese individuals, we aimed to investigate whether menopause among women is associated with dysglycemia independently of normal aging and the possible mechanism whereby women after menopause would have a higher probability of having dysglycemia compared with men of a similar age. We also aimed to clarify the effect of age at menopause on the presence of type 2 diabetes and prediabetes in Japanese women.
RESEARCH DESIGN AND METHODS
The Toranomon Hospital Health Management Center Study included a cohort comprising mainly apparently healthy government employees who underwent an annual health screening in Tokyo, Japan. A total of 41,931 individuals underwent a health examination from 1997 to 2007. Routine health checkups are common in Japan because the Japanese government and companies encourage people to receive periodic examinations. Among the 41,931 individuals, this cross-sectional study included 41,700 individuals for whom data on sex and menopausal status were available (6,458 premenopausal women, 5,701 postmenopausal women, and 29,541 men). Among those 41,700 individuals, we excluded 1,027 women who did not report a cause for menopause (natural, surgical, or other). After the exclusions, 40,673 individuals (6,458 premenopausal women, 3,630 women in natural menopause, 943 women in surgical menopause, 101 postmenopausal women by other causes, and 29,541 men) were available for analysis. We excluded 81 women aged ≥65 years who had been in the premenopausal category because their persistent vaginal bleeding after the age 65 was not likely a result of menses but of pathologic processes (12). We also excluded individuals with missing data on characteristics of lifestyle habits or clinical measures. Subsequently, 40,067 individuals (6,308 premenopausal women, 3,552 women in natural menopause, 1,018 women in surgical menopause or another cause, and 29,189 men) were included in the current analysis. With regard to women with missing data on age at menopause (n = 154), we excluded them only for the analysis of the relationship between age at menopause and dysglycemia. The study protocol followed the Japanese government’s Ethical Guidelines Regarding Epidemiological Studies in accordance with the Declaration of Helsinki and was reviewed by the Institutional Review Board at Toranomon Hospital.
Diagnosis of type 2 diabetes and prediabetes
Diagnosis of type 2 diabetes was made according to American Diabetes Association criteria (22) of a fasting plasma glucose (FPG) level ≥7.0 mmol/L (≥126 mg/dL), self-reported clinician-diagnosed diabetes or the use of hypoglycemic agents or insulin, or HbA1c ≥6.5% (48 mmol/mol). Prediabetes was indicated by an FPG of 5.6–6.9 mmol/L (100–125 mg/dL) or an HbA1c of 5.7–6.4% (39–46 mmol/mol) (22) without type 2 diabetes. Dysglycemia was indicated by the presence of either prediabetes or type 2 diabetes.
Assessment of the menopausal state and other variables
We assessed the menopausal status of women with a self-report questionnaire at the time of the examination. Female participants were asked whether they were in a postmenopausal state. If so, they were asked to indicate the reason for menopause (natural, surgical, or other) and the age at which menopause occurred (≤39, 40–44, 45–49, or ≥50 years). Parental history of diabetes, smoking habit (never, former, or current), physical activity habit (any physical activity for 20–30 min or longer at least once weekly), and self-reported history of medical treatment for hypertension or diabetes were also assessed by the questionnaire for both men and women.
Clinical measurements
Weight and height were measured, and BMI was calculated. Blood samples were collected after an overnight fast (12 h), and measurements were made with an automatic clinical chemistry analyzer. Blood glucose concentrations were measured by enzymatic methods, and HbA1c was assessed by high-performance liquid chromatography. The value for HbA1c (%) was estimated as the National Glycohemoglobin Standardization Program value (%) calculated by Eq. 1: HbA1c (%) = HbA1c (Japan Diabetes Society) (%) × 1.02 + 0.25% (23).
Statistical analysis
Logistic regression analysis was performed to calculate odds ratios (ORs) and 95% CIs. We initially investigated whether there was a difference in the association of dysglycemia between men and women and then calculated ORs for dysglycemia for postmenopausal women (regardless of cause) and for men, with premenopausal women as the reference group. After that, we assessed whether there was a difference in the association according to cause of menopause. Because few women reported an age at menopause of <39 years, we categorized age at menopause into three groups (<45, 45–49, and ≥50 years) for the analysis. To investigate effect modifications, we performed logistic regression analysis with adjustment for age (model 1); age and other demographic factors (BMI, parental history of diabetes, physical activity habit, and smoking habit) (model 2); and age, demographic, and metabolic factors (hypertension indicated by systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or medical treatment and HDL cholesterol and log-transformed triglyceride levels) (model 3). We also examined whether a significant association existed between menopause and prediabetic hyperglycemia among nondiabetic individuals after excluding those with type 2 diabetes.
In an additional analysis, we stratified women according age at the time of examination (<50 or ≥50 years) because the mean age of menopause has been considered to be ∼50 years (9,11,14,21,24,25). A combined effect of older age at the time of the examination and the postmenopausal condition on the presence of dysglycemia (either prediabetes or type 2 diabetes) was assessed, with premenopausal women aged <50 years as the reference group. We then conducted a stratified analysis based on age at the time of examination (<50 or ≥50 years) and calculated ORs for dysglycemia across categories of age at menopause, with the premenopausal state as the reference group for women aged <50 or ≥50 years. Analysis was performed with IBM SPSS Statistics version 19 (IBM, Armonk, NY). Statistical significance was considered for P < 0.05.
RESULTS
Mean (SD) age was 48.4 (9.9) years among the 10,878 women studied and 48.0 (9.7) years among the 29,189 men studied (Table 1). Of the 10,878 women, 2,340 (21.5%) had prediabetes and 246 (2.3%) had type 2 diabetes. Premenopausal women were younger (42.1 [6.6] years) compared with postmenopausal women. We did not observe a marked difference in BMI between premenopausal and postmenopausal women. Among the premenopausal women, only 69 (1.1%) had type 2 diabetes, whereas the prevalence rate was high at 3.8% in women after natural menopause and 4.0% after surgical menopause or menopause from other causes. More than one in three of the postmenopausal women and the men had either prediabetes or type 2 diabetes.
Table 1.
Characteristics of total women, premenopausal women, postmenopausal women (by cause), and men
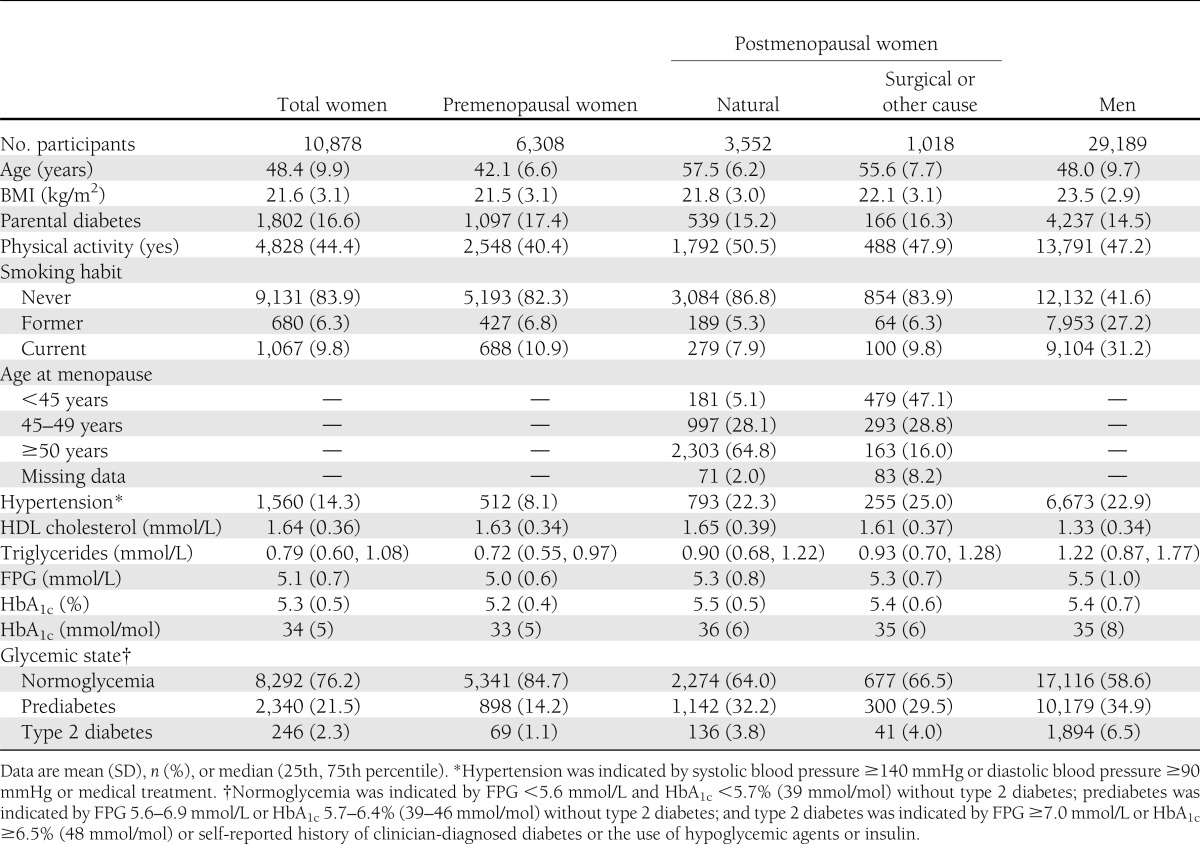
Table 2 shows ORs for type 2 diabetes and prediabetes among men and among women by menopausal status. Men were 2.10 (95% CI 1.81–2.45) times more likely to have type 2 diabetes than the total number of women studied according to multivariate model 3, which included age and demographic and metabolic factors. Postmenopausal women had a significant association with type 2 diabetes (1.36 [1.01–1.82]) compared with premenopausal women that was independent of age, BMI, smoking habit, physical activity habit, and parental history of diabetes (model 2), although the OR was not as high as that in men (2.87 [2.23–3.69]). After adjustment for lipid measurements and hypertension (model 3), the OR for the postmenopausal women was attenuated (1.17 [0.88–1.58]), and a significant association with the presence of type 2 diabetes remained only among the men (2.35 [1.82–3.03]). Among the women, we did not find an obvious difference in the association of type 2 diabetes and menopausal status regardless of the cause of menopause. The association of prediabetes with menopause among individuals without type 2 diabetes showed that postmenopausal women had a significantly elevated OR for prediabetes in model 3 (1.33 [1.20–1.48]) compared with premenopausal women. Additionally, the men had a significantly elevated OR for prediabetes compared with postmenopausal women (1.93 [1.77–2.10]). Regardless of age or demographic or metabolic factors, women with natural menopause or other causes of menopause had similarly elevated ORs for prediabetes (1.36 [1.22–1.52] and 1.22 [1.04–1.43], respectively). We observed that early age at menopause (<45 years) was significantly associated with an elevated OR for diabetes after adjustment for age (1.89 [1.21–2.96]) or for age and demographic factors (1.73 [1.10–2.73]). This association was not significant after adjustment for hypertension and lipid measurements (model 3). We did not observe an association of early menopause with an increased probability of having prediabetes among individuals without type 2 diabetes.
Table 2.
ORs (95% CI) for type 2 diabetes or prediabetes between women and men (A), among pre- or postmenopausal women and men (B and C), or among pre- or postmenopausal women according to age at menopause and men (D)
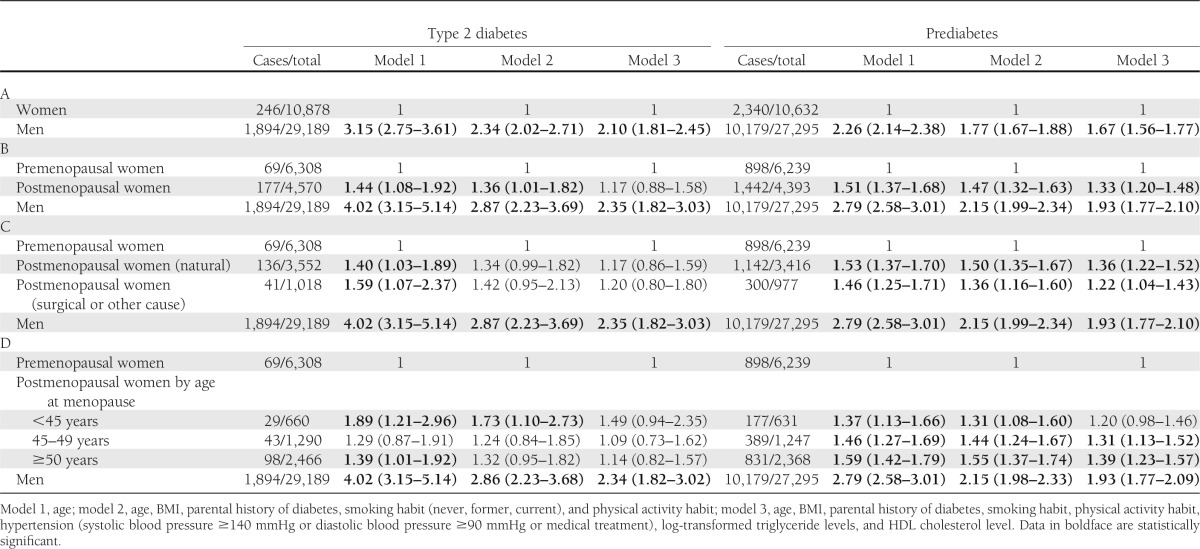
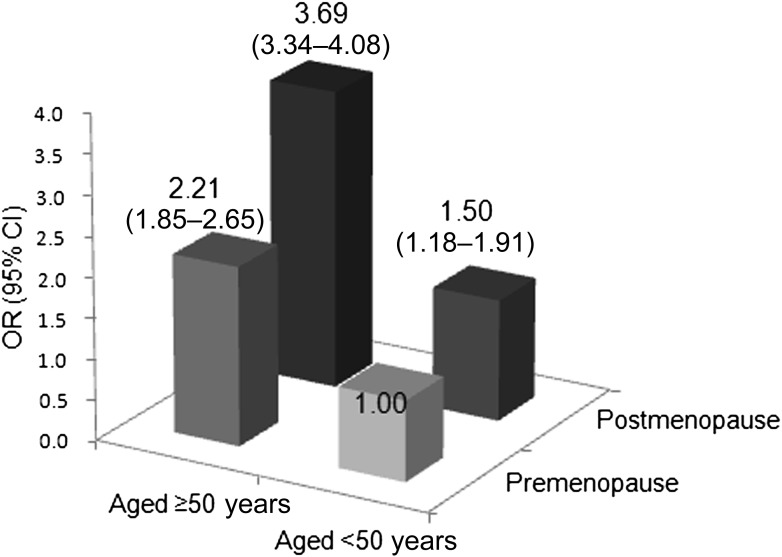
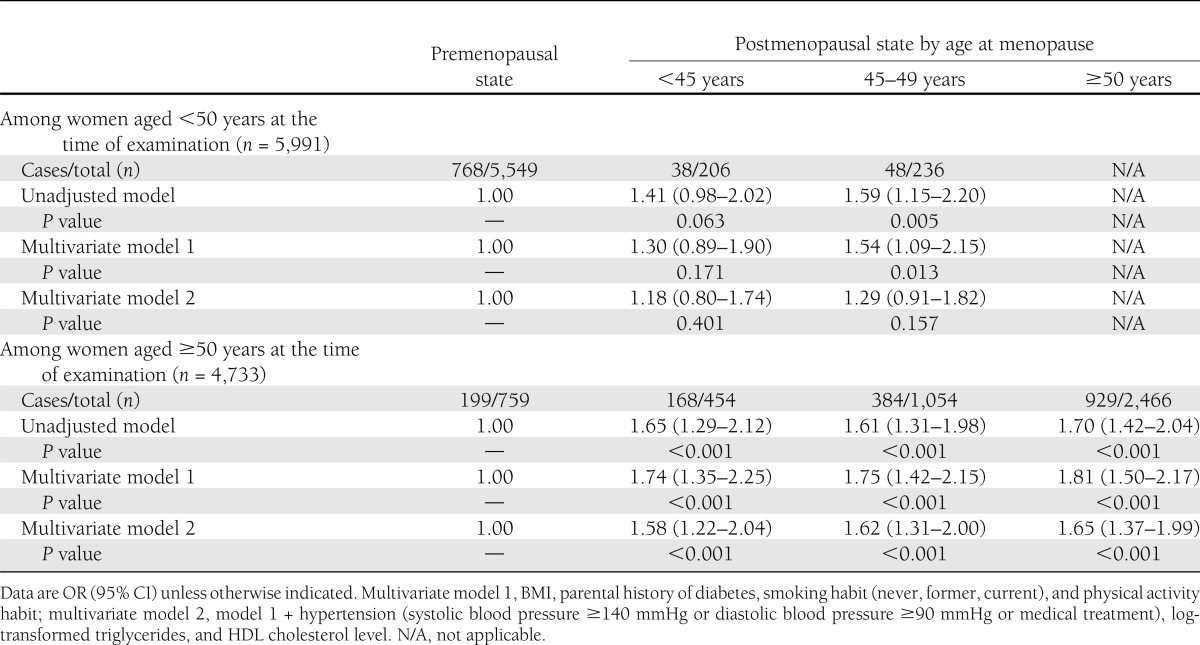
Figure 1 shows the combined effect of age at examination and menopausal status on the presence of dysglycemia (either prediabetes or type 2 diabetes). Although older age alone at the time of the examination (≥50 years) was significantly associated with dysglycemia (OR 2.21 [95% CI 1.85–2.65]), postmenopausal status alone was also significantly associated with an elevated OR for dysglycemia (1.50 [1.18–1.91]). The postmenopausal condition and older age additively influenced an elevated OR because postmenopausal women aged ≥50 years had a markedly elevated OR (3.69 [3.34–4.08]) for dysglycemia. We stratified women by age at the time of the examination and investigated whether there was an association of age at menopause with the presence of dysglycemia (Table 3). Compared with premenopausal women, postmenopausal women who underwent menopause at <45 or 45–49 years had a 1.41 (0.98–2.02) and 1.59 (1.15–2.20) times increased OR for dysglycemia, respectively, even among women aged <50 years at the time of examination (n = 5,991). Adjustment for demographic and metabolic factors (multivariate model 2) attenuated the ORs (1.18 [0.80–1.74] and 1.29 [0.91–1.82], respectively). Among women aged ≥50 years, the postmenopausal state was significantly associated with the presence of dysglycemia, regardless of age at which menopause occurred. In multivariate model 2, postmenopausal women had a similarly elevated OR for dysglycemia to premenopausal women, regardless of age at menopause (<45 years of age 1.58 [1.22–2.04], 45–49 years of age 1.62 [1.31–2.00], ≥50 years of age 1.65 [1.37–1.99]).
Figure 1.
Probability of having dysglycemia (either prediabetes or type 2 diabetes) through a combination of age at the time of examination and menopausal status. Data are crude OR and 95% CI.
Table 3.
Association of dysglycemia (either prediabetes or type 2 diabetes) and age at menopause among women aged <50 or ≥50 years at the time of examination
CONCLUSIONS
We found that older age and a postmenopausal state independently and additively influenced the high prevalence of dysglycemia in Japanese women. Even among women aged <50 years at the time of examination, menopause was associated with the presence of dysglycemia. The study is unique because it compared the probability of dysglycemia among women across menopausal states with that among mainly middle-aged men who underwent health screening in Japan. Although the OR for postmenopausal women was not high compared with that of the men, their ORs for type 2 diabetes and prediabetes were significantly elevated independently of age compared with those in premenopausal women.
Whether menopausal status would influence the occurrence of diabetes independently of age and other confounding factors remains controversial because it is difficult to conduct studies to separate the effects of normal aging from the menopausal transition. A few studies showed a significant positive association of hyperglycemia with menopausal status after adjustment for age and other risk factors for diabetes (7,13,19), but multivariate analyses in other studies showed no such associations (4,11,14,18,26). In a cross-sectional multicenter study of Italian women from outpatient menopausal clinics, those with natural menopause had a 1.38 times higher multivariate-adjusted OR for diabetes than premenopausal women (13). On the other hand, the researchers did not find a significant association in women with surgical menopause and diabetes (13). A cross-sectional study of Korean women suggested that in postmenopausal women, there is a significant association with the presence of hyperglycemia (fasting glucose level ≥110 mg/dL or antidiabetes medications) compared with premenopausal women that is independent of age and BMI (7). In women at high risk for diabetes who participated in the Diabetes Prevention Program, no association was found between natural menopause or bilateral oophorectomy and increased risk of developing diabetes after adjustment for age (12). Another influence of the controversy might be that assessment of the menopausal state usually is based on self-reported responses or interviews and that participant characteristics vary among studies. The present study shows a significant positive association between postmenopause (regardless of cause) and the presence of type 2 diabetes independently of normal aging compared with premenopause. However, we did not include oral glucose tolerance test (OGTT) data in the diagnosis of diabetes. Diabetes and impaired fasting glycemia are reported to be more common in men than in women 30–69 years of age, whereas the prevalence of isolated postload hyperglycemia, particularly impaired glucose tolerance, is reportedly higher in women than in men, especially in individuals >70 years of age (27). Additionally, impaired glucose tolerance is more prevalent than impaired fasting glycemia in Asian populations for all age-groups (28). The lack of data on OGTT for the diagnosis of dysglycemia might lead to an underestimation of the associations between menopause and the prevalence of diabetes. Further prospective studies that include OGTT data are needed to confirm the current findings.
The significant association of type 2 diabetes and postmenopausal status was particularly attenuated after adjustments for hypertension and blood lipid measurements, suggesting that when we consider the association of menopause with diabetes, we also should consider the influence of related metabolic factors. Because the transition from the premenopausal to the postmenopausal state is associated with changes in body composition (increased body fat mass, increased abdominal fat, and decreased lean body mass) (3) and substantial metabolic changes, features of metabolic syndrome would occur in many women (17). More recent research, however, suggested that postmenopausal women have higher levels of adiposity, but the association was predominantly a result of aging (29). Another recent study raised the possibility that even if body composition changes with the menopausal transition, these changes are not accompanied by cardiometabolic deterioration during a relatively short follow-up period (30). Because data on body composition or visceral fat were not available for the current study, we could not assess whether differences in body composition across the menopausal state might have influenced the presence of dysglycemia, even if BMIs across the menopausal state were relatively low. Asians are more likely to have a higher percentage of fat or visceral adipose tissue at a given BMI than Europeans (31). This ethnic difference regarding the obese phenotype might increase insulin resistance, leading to impaired glucose metabolism. Although we did not have data on reproductive hormone concentrations and cannot explain the mechanism for the current observations, it was shown that natural menopause is characterized by increased relative androgenicity, which was reported to be associated with glucose metabolism (15); furthermore, reproductive hormone concentrations can vary by ethnicity and were shown to be confounded by ethnic disparities in body mass (32). Further studies should investigate mechanisms that link menopause and diabetes with detailed assessments of body composition, insulin sensitivity, insulin secretion, and reproductive hormone concentrations in perimenopausal women across various ethnic groups to confirm the current findings.
The current results show that among individuals without diabetes, prediabetic hyperglycemia is significantly associated with postmenopausal status independently of age and demographic and metabolic parameters. In a cross-sectional study of Japanese women without diabetes, stepwise regression analysis showed natural menopause rather than age as a significant determinant of FPG concentrations (19). On the other hand, among middle-aged women living in North Taiwan, no significant difference in FPG, insulin levels, homeostasis model assessment of insulin resistance, and prevalence of hyperglycemia between premenopausal and postmenopausal women was shown (6). The current results show that prediabetic hyperglycemia and the postmenopausal state are positively associated, suggesting that postmenopausal women might be at high risk for diabetes.
In a prospective case-cohort study that included only postmenopausal women, earlier age at menopause was associated with a greater risk of type 2 diabetes (20). The hazard ratio for diabetes was 32% higher in women who entered menopause before 40 years of age compared with those experiencing menopause at age 50–54 (20). A study in Chinese postmenopausal women, however, showed no association between age at menopause and diabetes (21). The results of the current cross-sectional investigation suggest that early age at menopause (<45 years) might be more strongly associated with type 2 diabetes than menopause at ≥50 years. Nonetheless, regardless of age at menopause, postmenopausal women aged ≥50 years at the time of the examination had an ∼1.5 times increased probability of having dysglycemia compared with premenopausal women. Some differences exist in age at menopause onset across ethnic groups (25). Additionally, age at menopause can be affected by various social and environmental factors (25,33), which might be one possible explanation for the mixed results of the association of age at menopause and diabetes compared with existing studies. It has been established that a smoking habit is significantly associated with an early age at natural menopause (34). Factors of lower educational attainment; being separated, widowed, or divorced; and nonemployment have been associated with early natural menopause, whereas Japanese ethnicity is associated with late age at natural menopause (33). Furthermore, BMI has been associated with age at menopause (35). On the other hand, a recent study of women from five racial and ethnic groups indicated that there is no significant racial/ethnic difference in age at the final natural menstrual cycle after controlling for sociodemographic, lifestyle, and health factors (36). Further prospective investigations are needed to assess whether early menopause would increase the risk of developing diabetes across various ethnic groups while considering differences in demographic factors among study participants.
Recent reports indicated that early age at natural menopause is associated with an increased risk of ischemic stroke (37) and mortality (38). Nonetheless, both menopause and aging are nonmodifiable factors. Regular physical activity may help to mitigate the tendency for weight gain and adverse changes in body composition and fat distribution that accompany aging and the menopausal transition (39). High levels of habitual physical activity, such as walking, have been associated with a favorable cardiovascular risk profile in postmenopausal women (40). Further investigations are needed on whether different interventions for older postmenopausal women could control modifiable factors such as metabolically unhealthy obesity, dyslipidemia, hypertension, and lifestyle.
We recognize several limitations in this study. The study participants were relatively lean, apparently healthy Japanese government employees who underwent a routine health examination. Thus, these individuals were more likely to pay attention to healthy lifestyle habits than those who did not have such an examination. The characteristics of the study participants, such as BMI and cardiometabolic factors, would influence the generalizability of the findings, although we analyzed these factors in multivariate models. The generalizability of the results should be validated in various other populations. Additionally, limitations of the available data prevented a more in-depth analysis of factors that could influence an increased risk of developing diabetes; therefore, we cannot rule out the possibility that residual confounding influenced the results. Because we did not include data on nutritional intake or other known risk factors for diabetes, such as sleep disturbances and depression, which are commonly observed in women at midlife, we could not adjust the results for the influence of such factors. We did not have data on visceral fat or hormone replacement therapy, so that the ORs might be over- or underestimated. Nonetheless, the prevalence of women receiving hormone replacement therapy is considered to be low in Japan. Because the assessment of menopausal status is based on self-report, we cannot deny the possibility of misclassification of menopausal status among the women studied.
In conclusion, in this study of a large number of female and male Japanese individuals, the postmenopausal state in women was significantly associated with the presence of type 2 diabetes and prediabetes, although the increased probability did not equal that in the men. The postmenopausal state was also associated with prediabetic hyperglycemia independently of age and demographic and metabolic factors among women without diabetes. Menopause and older age might additively influence the elevated probability of dysglycemia in Japanese women.
Acknowledgments
This work was financially supported in part by the Ministry of Health, Labour and Welfare, Japan. Y.H. is a research fellow of the Japan Society for the Promotion of Science (JSPS). Y.H. and H.Sh. are recipients of a Grant-in-Aid for Scientific Research from JSPS.
No potential conflicts of interest relevant to this article were reported.
The sponsor had no role in the design and conduct of the study.
Y.H. contributed to the study concept and design and data acquisition, analysis, and interpretation; statistical analysis; study supervision; critical revision of the manuscript; the writing of the manuscript; and approved the final version. Y.A. contributed to the writing of the manuscript and data acquisition and technical or material support and approved the final version. S.K. contributed to the writing of the manuscript and the data acquisition, analysis, interpretation, and statistical analysis and approved the final version. S.D.H., H.T., and S.H. contributed to the writing of the manuscript and data acquisition and technical or material support and approved the final version. K.S. contributed to the writing of the manuscript and data acquisition, analysis, and interpretation and statistical analysis and approved the final version. H.Sh. contributed to the writing of the manuscript and approved the final version. H.So. contributed to the study concept and design and data acquisition, analysis, and interpretation; statistical analysis; study supervision; critical revision of the manuscript; the writing of the manuscript; the data interpretation; acquired funding; and approved the final version. H.So. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge the late Professor Kinori Kosaka, MD, director of the Health Management Center, Toranomon Hospital, who established the foundation and framework of this project and was the pillar of spiritual support of the TOPICS project. The authors also thank Mami Haga and Natsuko Tada, Niigata University Faculty of Medicine, for excellent secretarial assistance.
References
- 1.Yang W, Lu J, Weng J, et al. China National Diabetes and Metabolic Disorders Study Group Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation Diabetes Atlas. 5th ed. Brussels, International Diabetes Federation, 2011 [Google Scholar]
- 3.Szmuilowicz ED, Stuenkel CA, Seely EW. Influence of menopause on diabetes and diabetes risk. Nat Rev Endocrinol 2009;5:553–558 [DOI] [PubMed] [Google Scholar]
- 4.Kim HM, Park J, Ryu SY, Kim J. The effect of menopause on the metabolic syndrome among Korean women: the Korean National Health and Nutrition Examination Survey, 2001. Diabetes Care 2007;30:701–706 [DOI] [PubMed] [Google Scholar]
- 5.Wu SI, Chou P, Tsai ST. The impact of years since menopause on the development of impaired glucose tolerance. J Clin Epidemiol 2001;54:117–120 [DOI] [PubMed] [Google Scholar]
- 6.Lin WY, Yang WS, Lee LT, et al. Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int J Obes (Lond) 2006;30:912–917 [DOI] [PubMed] [Google Scholar]
- 7.Cho GJ, Lee JH, Park HT, et al. Postmenopausal status according to years since menopause as an independent risk factor for the metabolic syndrome. Menopause 2008;15:524–529 [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, Hong X, Wilker E, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis 2008;196:590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park HA, Park JK, Park SA, Lee JS. Age, menopause, and cardiovascular risk factors among Korean middle-aged women: the 2005 Korea National Health and Nutrition Examination Survey. J Womens Health (Larchmt) 2010;19:869–876 [DOI] [PubMed] [Google Scholar]
- 10.Zivkovic TB, Vuksanovic M, Jelic MA, et al. Obesity and metabolic syndrome during the menopause transition in Serbian women. Climacteric 2011;14:643–648 [DOI] [PubMed] [Google Scholar]
- 11.He L, Tang X, Li N, et al. Menopause with cardiovascular disease and its risk factors among rural Chinese women in Beijing: a population-based study. Maturitas 2012;72:132–138 [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Edelstein SL, Crandall JP, et al. Diabetes Prevention Program Research Group Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause 2011;18:857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Donato P, Giulini NA, Bacchi Modena A, et al. Gruppo di Studio Progetto Menopausa Italia Risk factors for type 2 diabetes in women attending menopause clinics in Italy: a cross-sectional study. Climacteric 2005;8:287–293 [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Hayashi K, Mishra G, Yasui T, Kubota T, Mizunuma H. Independent association between age at natural menopause and hypercholesterolemia, hypertension, and diabetes mellitus: Japan nurses’ health study. J Atheroscler Thromb 2013;20:161–169 [DOI] [PubMed] [Google Scholar]
- 15.Kim C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Womens Health (Lond Engl) 2012;8:155–167 [DOI] [PubMed] [Google Scholar]
- 16.Davis SR, Castelo-Branco C, Chedraui P, et al. Writing Group of the International Menopause Society for World Menopause Day 2012 Understanding weight gain at menopause. Climacteric 2012;15:419–429 [DOI] [PubMed] [Google Scholar]
- 17.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003;88:2404–2411 [DOI] [PubMed] [Google Scholar]
- 18.Soriguer F, Morcillo S, Hernando V, et al. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: longitudinal study. Menopause 2009;16:817–821 [DOI] [PubMed] [Google Scholar]
- 19.Otsuki M, Kasayama S, Morita S, et al. Menopause, but not age, is an independent risk factor for fasting plasma glucose levels in nondiabetic women. Menopause 2007;14:404–407 [DOI] [PubMed] [Google Scholar]
- 20.Brand JS, van der Schouw YT, Onland-Moret NC, et al. InterAct Consortium Age at menopause, reproductive life span, and type 2 diabetes risk: results from the EPIC-InterAct study. Diabetes Care 2013;36:1012–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu C, Chen H, Wen J, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab 2013;98:1612–1621 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashiwagi A, Kasuga M, Araki E, et al. ; Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society (JDS). International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. Diabetology Int 2012;3:8–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson HD. Menopause. Lancet 2008;371:760–770 [DOI] [PubMed] [Google Scholar]
- 25.Palacios S, Henderson VW, Siseles N, Tan D, Villaseca P. Age of menopause and impact of climacteric symptoms by geographical region. Climacteric 2010;13:419–428 [DOI] [PubMed] [Google Scholar]
- 26.Monterrosa-Castro A, Blümel JE, Portela-Buelvas K, et al. Collaborative Group for Research of the Climacteric in Latin America (REDLINC) Type II diabetes mellitus and menopause: a multinational study. Climacteric. 6 June 2013 [Epub ahead of print]23617887 [Google Scholar]
- 27.DECODE Study Group Age- and sex-specific prevalences of diabetes and impaired glucose regulation in 13 European cohorts. Diabetes Care 2003;26:61–69 [DOI] [PubMed] [Google Scholar]
- 28.Qiao Q, Hu G, Tuomilehto J, et al. DECODA Study Group Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 2003;26:1770–1780 [DOI] [PubMed] [Google Scholar]
- 29.Trikudanathan S, Pedley A, Massaro JM, et al. Association of female reproductive factors with body composition: the Framingham Heart Study. J Clin Endocrinol Metab 2013;98:236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdulnour J, Doucet E, Brochu M, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause 2012;19:760–767 [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 2012;8:228–236 [DOI] [PubMed] [Google Scholar]
- 32.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 2003;88:1516–1522 [DOI] [PubMed] [Google Scholar]
- 33.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874 [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Tan L, Yang F, et al. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause 2012;19:126–132 [DOI] [PubMed] [Google Scholar]
- 35.Akahoshi M, Soda M, Nakashima E, et al. The effects of body mass index on age at menopause. Int J Obes Relat Metab Disord 2002;26:961–968 [DOI] [PubMed] [Google Scholar]
- 36.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol 2013;178:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke 2009;40:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E. Age at menopause and mortality in Japan: the Jichi Medical School Cohort Study. J Epidemiol 2006;16:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sternfeld B, Bhat AK, Wang H, Sharp T, Quesenberry CP., Jr Menopause, physical activity, and body composition/fat distribution in midlife women. Med Sci Sports Exerc 2005;37:1195–1202 [DOI] [PubMed] [Google Scholar]
- 40.Colpani V, Oppermann K, Spritzer PM. Association between habitual physical activity and lower cardiovascular risk in premenopausal, perimenopausal, and postmenopausal women: a population-based study. Menopause 2013;20:525–531 [DOI] [PubMed] [Google Scholar]