Abstract
OBJECTIVE
To assess the efficacy and safety of 32 mg naltrexone sustained-release (SR)/360 mg bupropion SR (NB) in overweight/obese individuals with type 2 diabetes with or without background oral antidiabetes drugs.
RESEARCH DESIGN AND METHODS
This was a 56-week, double-blind, placebo-controlled study in which 505 patients received standardized lifestyle intervention and were randomized 2:1 to NB or placebo. Coprimary end points were percent weight change and achievement of ≥5% weight loss. Secondary end points included achievement of HbA1c <7% (53 mmol/mol), achievement of weight loss ≥10%, and change in HbA1c, waist circumference, fasting blood glucose, and lipids.
RESULTS
In the modified intent-to-treat population (54% female, 80% Caucasian, and mean age 54 years, weight 106 kg, BMI 37 kg/m2, and HbA1c 8.0% [64 mmol/mol]), NB resulted in significantly greater weight reduction (−5.0 vs. −1.8%; P < 0.001) and proportion of patients achieving ≥5% weight loss (44.5 vs. 18.9%, P < 0.001) compared with placebo. NB also resulted in significantly greater HbA1c reduction (−0.6 vs. −0.1% [6.6 vs. 1.1 mmol/mol]; P < 0.001), percent of patients achieving HbA1c <7% (53 mmol/mol) (44.1 vs. 26.3%; P < 0.001), and improvement in triglycerides and HDL cholesterol compared with placebo. NB was associated with higher incidence of nausea (42.3 vs. 7.1%), constipation (17.7 vs. 7.1%), and vomiting (18.3 vs. 3.6%). No difference was observed between groups in the incidence of depression, suicidal ideation, or hypoglycemia.
CONCLUSIONS
NB therapy in overweight/obese patients with type 2 diabetes induced weight loss, which was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.
Type 2 diabetes is a chronic disease characterized by elevated blood glucose and is often associated with a collection of metabolic abnormalities including low HDL cholesterol (HDL-C), elevated triglyceride concentrations, and high blood pressure. Excess body weight, particularly visceral adiposity, may contribute to the development of both diabetes and the associated metabolic abnormalities, perhaps due to pathologic effects of excessive adipose tissue (1). Based on the National Health and Nutrition Examination Survey (NHANES) data from 1999–2010, the combined prevalence of overweight and obesity in the U.S. is 69% (2), while the prevalence of overweight and obesity in people with type 2 diabetes has been estimated to be even higher at ~85% (3). Survey and clinical trial evidence supports a strong association between obesity (characterized by a BMI ≥30 kg/m2 and central adiposity) and the development of insulin resistance, prediabetes, and type 2 diabetes (4).
In patients with type 2 diabetes, the best achievable glycemic control for the individual is recommended to reduce the risk of microvascular and possibly macrovascular disease. While lifestyle modifications can improve glycemic control, most patients with type 2 diabetes will typically require the sequential addition of one or more medications to achieve and sustain glycemic control. Unfortunately, many glucose-lowering medications lead to an increase in body weight (5). This presents challenges in an overweight or obese patient population often already struggling with weight control, particularly given that even modest intentional weight loss can improve cardiovascular risk factors (6,7) and decrease mortality (8).
Bupropion is a dopamine and norepinephrine reuptake inhibitor approved for use in the U.S. as an antidepressant and smoking cessation agent (9,10), with variable and modest effects on body weight as monotherapy (11). Naltrexone is an opioid receptor antagonist approved for use in the management of alcohol and opioid dependence (12). Animal studies suggest that when used together, bupropion and naltrexone increase the firing rate of proopiomelancortin neurons, in part due to naltrexone-induced reduction of an autoinhibitory effect of β-endorphins (13). Increased proopiomelancortin neuronal firing increases secretion of melanocortins such as α-melanocyte stimulating hormone, which is thought to mediate anorectic effects and regulate energy balance (14). Furthermore, naltrexone and bupropion therapy are believed to influence the mesolimbic dopaminergic reward system, which can modulate reward behaviors such as food intake (14,15). Thus, this novel combination treatment is believed to act on the central nervous system to elicit sustained appetite reduction and enhance control of eating behavior.
While the development and clinical use of bupropion and naltrexone have spanned more than two decades, only recently have studies investigated the combined use of these agents in sustained-release (SR) form as a potential therapy for obesity. All three phase 3 trials of naltrexone SR plus bupropion SR (NB) in overweight or obese patients without diabetes demonstrated significant weight loss with an acceptable safety profile (16–18). This report details a 56-week, double-blind, placebo-controlled phase 3 clinical trial of NB for the treatment of overweight or obese patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
This 56-week, randomized, double-blind, placebo-controlled study was conducted at 53 sites in the U.S. between May 2007 and June 2009. The protocol was approved by each site’s Institutional Review Board. All patients provided written informed consent, and the study was conducted according to the guidelines and principles of Good Clinical Practices standards and the Declaration of Helsinki (19).
Participants were smoking or nonsmoking men and women with type 2 diabetes, aged 18–70 years, with a BMI ≥27 and ≤45 kg/m2, HbA1c between 7% (53 mmol/mol) and 10% (86 mmol/mol), and fasting blood glucose <270 mg/dL. Participants were either not taking a diabetes medication or were on stable doses of oral antidiabetes drugs (OADs) for ≥3 months prior to randomization. Systolic and diastolic blood pressure were to be <145 and <95 mmHg, respectively. Most medications for the treatment of dyslipidemia and hypertension were allowed, as long as the doses were stable for at least 4 weeks prior to randomization. A complete list of inclusion and exclusion criteria is provided in the Supplementary Data.
Participants were randomized via a computer-generated randomization schedule in a 2:1 ratio (stratified by baseline HbA1c [≤8 or >8%; ≤64 or >64 mmol/mol] and sulfonylurea use) to 32 mg/day naltrexone SR combined with 360 mg/day NB or placebo. NB was provided as a single tablet containing 8 mg naltrexone SR and 90 mg bupropion SR. Study medication was initiated at one-quarter of the daily maintenance dose (one tablet) and increased weekly over the first 4 weeks such that the maintenance dose (2 tablets twice daily) was reached at the beginning of the fourth week. At week 2, participants received a telephone call to assess compliance with study medication and to document adverse events. Participant assessments were undertaken at screening, baseline, and every 4 weeks thereafter.
At baseline and weeks 4, 16, 28, and 40, all participants were instructed by study site personnel to follow a hypocaloric diet (500 kcal deficit/day, based on the World Health Organization algorithm for calculating resting metabolic rate). Participants received dietary counseling and the “Exchange Lists for Weight Management” booklets in accordance with the American Diabetes Association and American Dietetic Association guidelines. Participants also received advice on behavioral modification, including written instructions, to increase physical activity (to walking for at least 30 min most days of the week).
Participants were instructed to monitor their blood glucose twice daily for at least the first 4 weeks after randomization and during suspected hypoglycemia. In the case of loss of glucose control, defined as HbA1c >9.5% (80 mmol/mol) at week 16 or subsequent visits, any postbaseline HbA1c ≥10.0% (86 mmol/mol) or two or more successive postbaseline fasting glucose levels ≥270 mg/dL, the participant’s dose of current OADs was to be increased (if not at maximal doses) or an additional OAD was to be added using a protocol-defined algorithm.
Outcome measures
The coprimary end points were 1) percent change in body weight from baseline to week 56 compared with placebo and 2) percentage of participants achieving ≥5% reduction in body weight from baseline to week 56 compared with placebo. Secondary end points included percentage of patients achieving ≥10% reduction in body weight and change in selected cardiovascular risk factors including waist circumference, triglycerides, HDL-C, LDL-C, and high-sensitivity C-reactive protein (hs-CRP). Secondary end points related to glycemic control included change from baseline in HbA1c, fasting blood glucose, fasting insulin, and homeostasis model assessment of insulin resistance (HOMA-IR), as well as the percentage of patients achieving HbA1c <7% (53 mmol/mol) and <6.5% (48 mmol/mol), changes in OADs, percentage of patients requiring rescue medications for their diabetes, and percentage of patients discontinuing from the study owing to poor glycemic control.
Safety assessments included evaluation of treatment-emergent adverse events, vital signs, electrocardiograms, and clinical laboratory measures. Depressive symptoms, evaluated using the Inventory of Depressive Symptomatology–Self Report (IDS-SR) (www.ids-qids.org) and blood pressure were also evaluated for both efficacy and safety.
Statistical analyses
To obtain the targeted number of participant exposures at 1 year, we estimated that 350 participants would need to be randomized to NB, with an assumed 33% attrition rate (20). It was estimated that 525 participants randomized 2:1 (∼350 to NB and ∼175 to placebo) would provide ∼99% power to detect a difference in mean weight loss of ≥5% between NB and placebo (assuming an SD of 5%, comparison between groups using a two-sample t test and two-sided significance level of 0.05).
Unless otherwise specified, primary efficacy analyses were performed using the modified intent-to-treat (mITT) population, prospectively defined as randomized participants with a baseline and one or more postbaseline measurements of body weight while on the study drug. Missing data were imputed by carrying forward the last observation on study drug (last observation carried forward [LOCF]). Analyses were also performed on the completer population, which comprised all mITT patients who had a body weight measurement at week 56 while on the study drug. Safety analyses were performed on all randomized participants who took one or more tablets of the study drug and had at least one contact or assessment with the investigative site at any time after beginning the study.
General linear models (ANCOVA) including terms for treatment, HbA1c strata ≤8 or >8%, pharmacotherapy with or without sulfonylurea, and baseline value as covariate were used to analyze continuous end points. Categorical end points were analyzed with a logistic regression model using the same covariates as the continuous end points. To minimize skewness, values for triglycerides, hs-CRP, insulin, and HOMA-IR were log10 transformed before running the general linear models. The least squares (LS) percent change from baseline was calculated by back-transforming the LS mean in log10 scale.
To control for multiple comparisons, secondary end points were analyzed in a predetermined sequence, beginning with the change from baseline to end point in HbA1c, fasting triglycerides, HDL-C, blood glucose, and waist circumference; proportion of participants with ≥10% decrease in total body weight; proportion of participants with HbA1c <7% (53 mmol/mol); proportion of participants requiring rescue medications for diabetes or changing the doses of their OADs; changes from baseline to end point in HOMA-IR and fasting insulin; proportion of participants with HbA1c <6.5% (48 mmol/mol); changes from baseline to end point in hs-CRP; percentage of participants discontinuing owing to poor glycemic control; and changes from baseline to end point in LDL-C, systolic blood pressure, diastolic blood pressure, and IDS-SR total score. Testing proceeded in a sequential step-down manner until any end point failed to reach P < 0.05, after which nominal P values are reported and findings deemed exploratory. Continuous data are presented as LS mean ± SE unless otherwise indicated. All statistical analyses were performed using Windows SAS, version 9.1 (SAS Institute, Cary, NC).
RESULTS
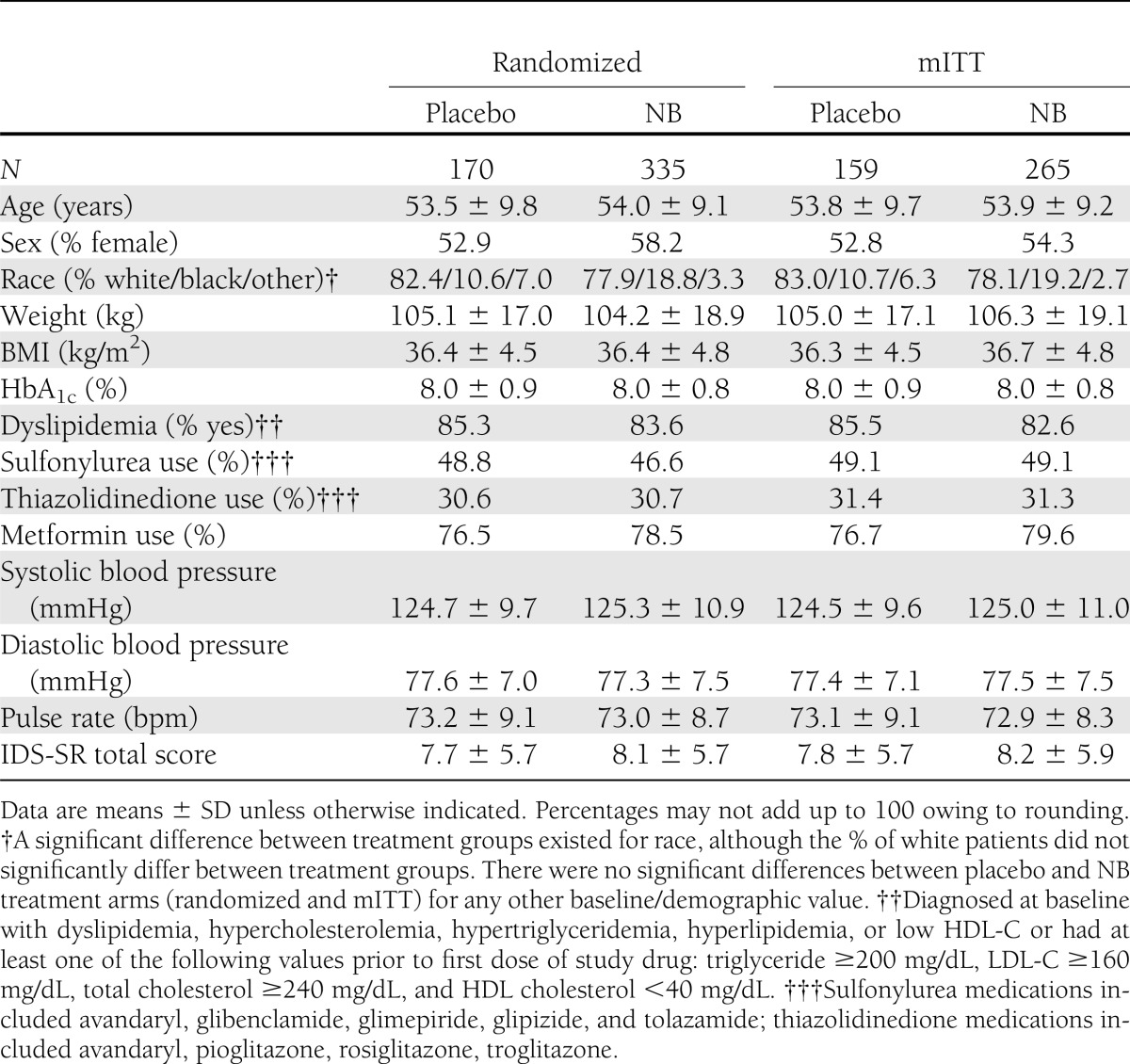
Of 1,625 patients who entered screening, 505 were randomly assigned to receive NB (n = 335) or placebo (n = 170). The most frequent (≥5%) reasons for screen failure were laboratory abnormality (58%, including HbA1c <7 or >10%), concomitant medication (7%), vital sign abnormality (5%), and withdrawal of consent (5%). For NB, the mITT, completer, and safety populations included 265, 175, and 333 patients, respectively. For placebo, the mITT, completer, and safety populations included 159, 100, and 169 patients, respectively (details in Supplementary Fig. 1). Baseline characteristics of the randomized and the mITT populations were similar (Table 1). Over the 56-week trial, 47.8% of participants in the NB group discontinued the study drug compared with 41.2% in the placebo group. A greater percentage of participants who received NB compared with placebo discontinued owing to an adverse event (29.3 vs. 15.3%). Conversely, a greater percentage of placebo than NB participants were lost to follow-up (8.8 vs. 6.6%), withdrew consent (8.8 vs. 6.3%), or withdrew because of self-perceived insufficient weight loss (3.5 vs. 1.5%).
Table 1.
Demographics and baseline characteristics
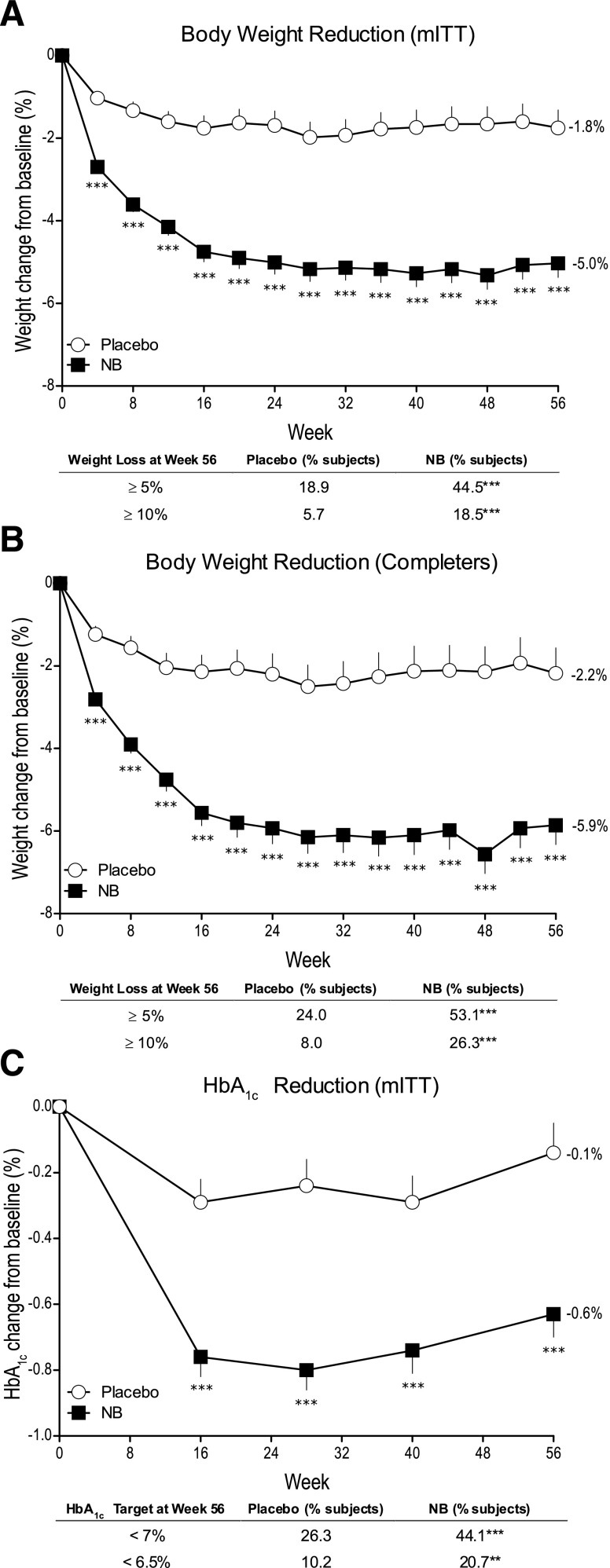
Participants treated with NB lost significantly more weight than placebo-treated participants (mITT analysis: −5.0 ± 0.3 vs. −1.8 ± 0.4%, P < 0.001) (Fig. 1A). The difference between the groups was significant at the first time point assessed (Week 4) and sustained throughout 56 weeks of treatment (P < 0.001 for all visits). A similar pattern was observed in the completer population, with numerically greater weight loss relative to the mITT population seen in both NB and placebo patients (completers: −5.9 ± 0.5 vs. −2.2 ± 0.6%, P < 0.001) (Fig. 1B). Consistent with the mean percent changes in body weight, more patients treated with NB than placebo achieved ≥5 and ≥10% reduction in body weight at week 56 (all P < 0.001) (Fig. 1A and B).
Figure 1.
A: Body weight change from baseline in the mITT LOCF population. Data are LS mean ± SE. Placebo, n = 159; NB, n = 265. ***P < 0.001 vs. placebo; except for change from baseline to week 56 end point for mITT LOCF population, P values are nominal (i.e., not adjusted for multiple comparisons) and associated with exploratory analyses. B: Body weight change from baseline to week 56 end point; placebo, n = 100; NB, n = 175. ***P < 0.001 vs. placebo; except for ≥5% weight loss category for the mITT-LOCF population, P values are nominal (i.e., not adjusted for multiple comparisons) and associated with exploratory analyses. C: Change in HbA1c from baseline to week 56 end point; placebo, n = 137; NB, n = 222. **P < 0.01 vs. placebo; ***P < 0.001 vs. placebo; the mITT LOCF population consists of patients who had a baseline measurement and at least one postbaseline measurement while on study drug. The last observation on study drug was carried forward. The completer population comprised all randomized patients with a baseline measurement and a 56-week measurement while on the study drug.
Patients treated with NB exhibited a greater improvement in HbA1c (−0.6% [−6.6 mmol/mol]) than in placebo-treated patients (−0.1% [−1.1 mmol/mol]), with a placebo-corrected difference of −0.5% (−5.5 mmol/mol) for the mITT population at the end of 56 weeks (P < 0.001) (Fig. 1C). Treatment with NB also resulted in a greater percentage of patients achieving an HbA1c of <7.0% (53 mmol/mol) compared with placebo (44.1 vs. 26.3%; P < 0.001) and <6.5% (48 mmol/mol) (20.7 vs. 10.2%; P = 0.004) (Table 2). Exploratory analyses demonstrated that HbA1c change was significantly correlated with change in body weight with both treatments (NB mITT: r = 0.509, P < 0.001; placebo: r = 0.168, P < 0.05). Patients with higher baseline HbA1c values also exhibited significantly greater HbA1c reductions with NB vs. placebo: baseline >8% (64 mmol/mol) −1.1 vs. −0.5%; baseline >9% (75 mmol/mol) −1.2 vs. −0.3%; both P < 0.05 (Supplementary Fig. 2). Changes in fasting glucose concentration and HOMA-IR were not significantly different compared with placebo (Table 2).
Table 2.
Changes in secondary end points

Over the course of the study, fewer NB-treated patients required an increase in dose or the addition of another OAD owing to deterioration of glycemic control (22.3% NB vs. 35.2% placebo; P < 0.01). Because alterations in medications for diabetes can obscure the impact of treatment on glycemic-related secondary end points, an exploratory analysis was performed to investigate changes in glycemic parameters accounting for rescue medication by carrying forward the last observation prior to introduction of the rescue medication. In this analysis, larger placebo-corrected improvements in HbA1c (−0.7% [7.7 mmol/mol]; P < 0.001) and fasting blood glucose (−17.5 mg/dL; P < 0.001) were observed; differences in fasting insulin and HOMA-IR were not significant between NB and placebo groups (Supplementary Table 2).
This study also examined the effect of NB treatment on cardiovascular risk factors. Compared with placebo-treated patients, NB-treated patients had significantly greater reductions in waist circumference and serum triglyceride concentration and significant increases in HDL-C concentration (Table 2). No significant differences were observed between the groups in LDL-C or hs-CRP. Systolic and diastolic blood pressure tended to be reduced from baseline in both NB and placebo-treated patients, with numerically but not statistically greater reductions in placebo-treated patients (Table 2).
Safety and tolerability
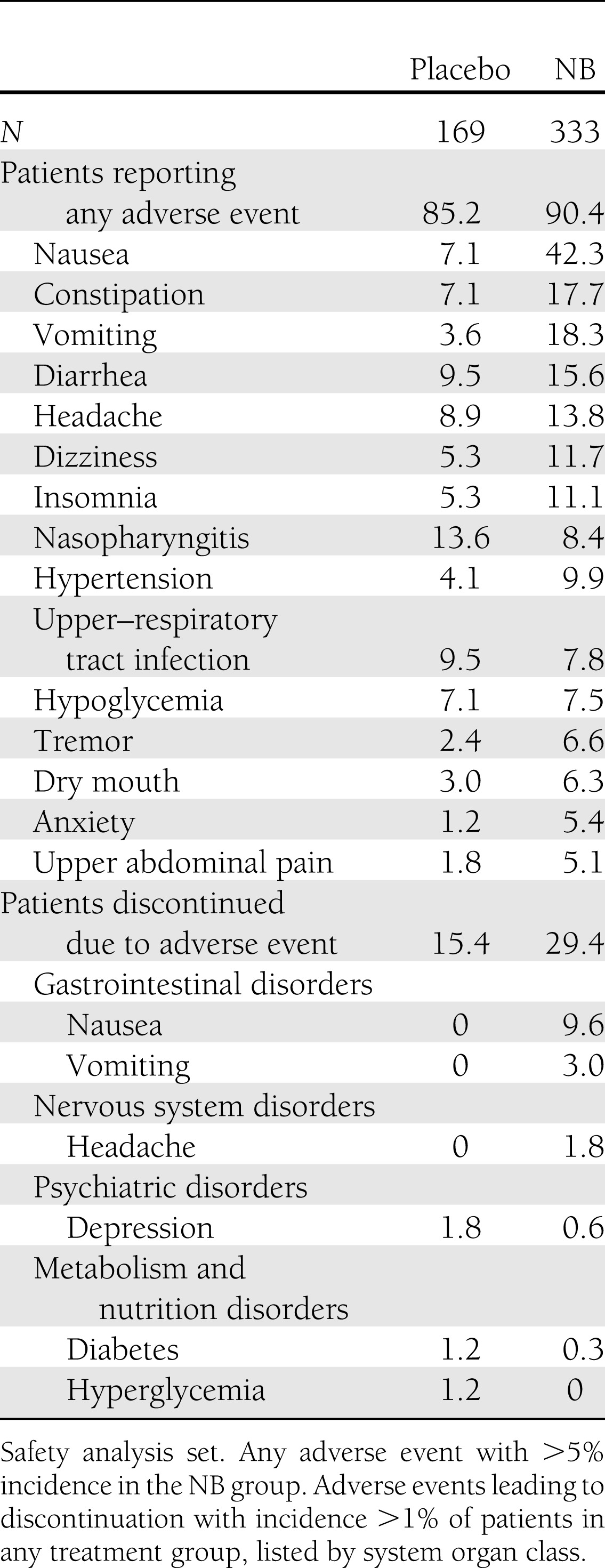
The most common adverse events that were more prevalent in the NB-treated patients were nausea, constipation, vomiting, and diarrhea(Table 3). Nausea led to study withdrawal in 9.6% of NB-treated patients, with the vast majority (28 of 32) of these withdrawals occurring as the result of nausea with an onset during the first 4 weeks of treatment. Adverse events that led to medication discontinuation during the first 4 weeks of treatment were the primary reason that relatively fewer randomized NB patients were included in the mITT population. Nausea occurred at a greater incidence in NB-treated patients taking metformin at baseline (46.2%) compared with those not on metformin (28.2%). The incidence of subjects with serious adverse events was low (3.9% for NB and 4.7% for placebo) and similar to that previously reported for patients without type 2 diabetes (16–18).
Table 3.
Adverse events and adverse events leading to discontinuation
Analyses were performed to examine the percentage of patients who had blood pressure and pulse rate increases that met prespecified criteria (Supplementary Table 3). The proportions of patients who had increases meeting these criteria were similar between groups but tended to be numerically higher in the NB treatment group. Importantly, ≤1% of patients in each treatment group had a systolic or diastolic blood pressure value corresponding to stage 2 hypertension (≥160 mmHg or ≥100 mmHg, respectively [21]) for two consecutive visits at any time during the study (or once if it occurred on the last visit).
Effects of NB on depression and mood was examined using the IDS-SR questionnaire and adverse event reports. At baseline, mean IDS-SR total scores in the safety analysis set were similar in the NB and placebo groups (8.1 and 7.7, respectively; scores can range from 0 to 84, with higher scores indicating more severe depression; <14 = nondepressed). At end point, the LS mean ± SE change from baseline in the total score was 0.53 ± 0.37 for NB and −1.41 ± 0.49 for placebo (P = 0.001). IDS-SR scores of ≥25 (indicating moderate depression) at any time during the study were observed in 6.2% of patients receiving placebo and 7.2% of NB-treated patients (NS). There were no differences between NB- and placebo-treated patients in incidence of adverse events of either depression or depressed mood during the study (Table 3).
CONCLUSIONS
Treatment of overweight or obese individuals with type 2 diabetes with NB resulted in a 5.0% reduction in body weight, compared with a reduction of 1.8% with placebo. Patients treated with NB were more than twice as likely to lose ≥5% of their initial body weight as patients treated with lifestyle intervention alone. The weight loss in the NB-treated group was evident as early as the first visit (week 4), and was maintained for the 56-week period, with no evidence of weight regain. This weight loss was accompanied by a significant reduction in HbA1c (placebo-corrected difference −0.5%) along with other favorable glycemic effects. Modest improvements in waist circumference, HDL-C, and triglycerides were also observed. The most frequently reported adverse event with NB was nausea.
Adipose tissue is an active endocrine and immune organ whose dysfunction contributes to metabolic diseases (e.g., diabetes) and increases cardiovascular disease (22). Thus, the objective of weight loss in overweight patients with metabolic disease is not solely cosmetic but also to improve the health of patients. In terms of clinically meaningful weight loss, human studies suggest that 5% weight loss significantly improves glucose control and other cardiovascular parameters and results in reduced mortality in patients with diabetes (6–8). This magnitude of weight loss has also been associated with a delay in the progression to type 2 diabetes in patients with prediabetes (23–25).
The magnitude of weight loss (both absolute and placebo corrected) in the current study of overweight and obese individuals with type 2 diabetes was lower than that observed in NB trials in patients without type 2 diabetes. In the COR-I and COR-II studies (16,17), which used a lifestyle intervention similar to the current study, mean body weight changes (mITT) were −6.1 and −6.4% with NB vs. −1.3 and −1.2% with placebo. In the COR-BMOD study (18), which included a more intensive, group-based lifestyle intervention, mean mITT body weight changes were −9.3% (NB) and −5.1% (placebo). The somewhat more modest weight loss in the current study is not surprising, as it is typical that less weight loss is observed in patients with diabetes taking OADs compared with patients without diabetes (26,27). While the reason for the attenuated magnitude of weight loss in patients with diabetes is not well understood, it may relate to differences in insulin resistance, adipose cell metabolism, concomitant medications for the treatment of diabetes, and glucose metabolism. Nevertheless, of study participants who completed 1 year of treatment, more than one-half of NB patients lost ≥5% of body weight and more than one-quarter achieved ≥10% weight loss.
Consistent with the observed improvement in body weight, NB-treated patients exhibited clinically and statistically significant improvement in HbA1c and generally beneficial changes in other markers related to glycemic control. The extent of reduction in HbA1c (−0.5% [−5.5 mmol/mol] vs. lifestyle intervention alone) compares well with the reported efficacy of several drugs currently used for treating diabetes (28). The magnitude of reduction was even greater in patients with higher baseline HbA1c values. This degree of improvement is meaningful in relation to long-term risk for microvascular complications and may contribute to a favorable environment with regard to macrovascular complications (29–32). The clinical impact of the HbA1c reduction with NB was demonstrated by the fact that patients on placebo were more likely to require an increased dose or number of OADs to maintain glycemic control (which lessened the apparent treatment difference with NB on both HbA1c and fasting glucose) (Supplementary Table 2).
NB treatment was also accompanied by improvements in a number of additional markers of cardiovascular risk, such as waist circumference, triglycerides, and HDL-C. In normoglycemic obese patients, weight loss with NB treatment has been demonstrated to be largely due to reductions in subcutaneous and visceral adipose tissue (33), the latter of which is associated with improvements in glycemia and lipidemia (34–36). Thus, treatment with NB in overweight/obese patients with (and without) diabetes was associated with an improvement in the cardiovascular risk profile. However, bupropion has a documented pressor effect (37,38) that was associated with a numerically, but not statistically, greater reduction in blood pressure in the placebo group compared with NB. Although the hemodynamic effects of NB were demonstrated to be small and transient, the overall cardiovascular effects of NB could not be adequately assessed in the NB phase 3 clinical program because the patient population had an extremely low CV event rate (~0.2/100 patient-years). In order to assess the clinical effect of the NB-related improvements in weight and other cardiovascular risk factors, along with the documented pressor effect of buproprion (a transient elevation in pulse rate and blood pressure), a cardiovascular outcomes trial of NB is currently ongoing (clinical trial reg. no. NCT01601704, clinicaltrials.gov). This outcomes trial is designed to assess the incidence of major cardiovascular events in patients receiving NB compared with placebo.
The safety profile of NB in this population was generally consistent with the phase 3 trials conducted in patients without type 2 diabetes (16–18). Importantly, NB was not associated with an increase in hypoglycemia. The safety profile of the two agents used in combination is also similar to the postmarketing surveillance data available for the two agents used individually. The most common adverse event reported with NB was nausea, which led to withdrawal in ∼10% of NB patients. Much of the patient withdrawal occurred within the first 4 weeks of treatment, during which patients started treatment at a lower initial dose and were required to escalate weekly. Patients unable to tolerate the study medication were discontinued from study drug treatment. The rate of nausea in NB-treated patients in the current study (42%) tended to be higher than previous phase 3 studies (29–34%) (16–18). This may be related to interaction with the background medications used by patients with diabetes in the current study, as NB-treated patients taking metformin (∼75% of the population) reported a higher incidence of nausea than those not on metformin. This result was not unexpected, as gastrointestinal side effects are commonly associated with metformin treatment (39).
Limitations of this study include the exclusion of patients with type 2 diabetes who were receiving insulin therapy (which can promote weight gain) or glucagon-like peptide-1 receptor agonist therapy (which is associated with mild weight loss). Thus, these results may not be applicable to all patients with type 2 diabetes. Furthermore, a relatively high dropout rate was observed in this study. Although the dropout rate was similar to the other NB phase 3 trials (16–18) and does not differ from other reported studies in obesity drug development (40), it should be considered when evaluating the treatment effect. Finally, the 1-year study duration, while common, is not adequate to fully assess the consequences of improved body weight on long-term outcomes.
The current epidemic of obesity and obesity-related diabetes continues to be of increasing concern. Unfortunately, a number of the drugs used to treat diabetes are associated with weight gain, and new approaches to prevention and treatment are needed. In this study of overweight/obese patients with type 2 diabetes, NB treatment, in conjunction with a standardized lifestyle intervention, resulted in a clinically meaningful improvement in body weight, which was associated with improvement in HbA1c and other cardiovascular risk factors. Thus, a combination drug such as NB has the potential to become a useful agent for the treatment of overweight/obese patients diagnosed with prediabetes or type 2 diabetes.
Acknowledgments
P.H., R.P., and H.B. received research funding from Orexigen Therapeutics, Inc. F.G. is a member of the Orexigen Therapeutics, Inc., Advisory Board and is a consultant for Orexigen Therapeutics, Inc. C.B. and P.K. are employees and shareholders of Orexigen Therapeutics, Inc. K.F. is a member of the Orexigen Therapeutics, Inc. Advisory Board and is a consultant for Orexigen Therapeutics, Inc. Brandon Walsh, PhD, and Amy Halseth, PhD, both employees of Orexigen Therapeutics, Inc., assisted in the writing of the manuscript. No other potential conflicts of interest relevant to this article were reported.
P.H. participated in data interpretation and writing and reviewing of the manuscript, approved the final version of the manuscript, participated in the study design, and was an investigator of the study. A.K.G., R.P., and H.B. participated in data interpretation and writing and reviewing of the manuscript, approved the final version of the manuscript, and were investigators of the study. F.G. participated in data interpretation and writing and reviewing of the manuscript, approved the final version of the manuscript, and participated in study design. C.B. participated in data interpretation and writing and reviewing of the manuscript, approved the final version of the manuscript, and performed statistical analysis of data. P.K. participated in data interpretation and writing and reviewing of the manuscript and approved the final version of the manuscript. K.F. participated in data interpretation and writing and reviewing of the manuscript, approved the final version of the manuscript, and participated in study design. P.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010; the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011; and the 11th International Congress on Obesity, Stockholm, Sweden, 11–15 July 2010.
Appendix
COR-Diabetes Study Group participating investigators
Laura Akright, Mira Baron, Harold Bays, Jolene Berg, Bruce Berwald, James Borders, Robert Buynak, Marc Capobianco, Harold Cathcart, Joseph Cleaver, Milissa Cooper, Martin Conway, Adnan Dahdul, Matthew Davis, Steven Duckor, John Ervin, David Fitz-Patrick, Larry Gilderman, Gopalakrishna Gollapudi, Alok Gupta, Forrest Hanke, Howard Harris, Lydie Hazan, Priscilla Hollander, Adesh Jain, Boris Kerzner, Lawrence Koehler, Ildiko Kovacs, Richard A. Krause, Diane Krieger, Judy Loper, Norman Lunde, Barbara McGuire, Richard Mills, Sunder Mudaliar, Troy Oxner, Naynesh Patel, Angel Pietri, Sanford N. Plevin, Raymond Plodkowski, George Raad, Eric Ross, Robert Schrieman, Sherwyn Schwartz, Stan Self, Stephan Sharp, Diane Smith, Timothy Smith, Philip Snell, Joseph Soufer, Louise Taber, Andrew Thieneman, Joseph Tibaldi, Paul Tung, James Vogt, Claire Waltman, Mervyn Weerasinghe, Daniel Weiss, Troy Williams, Kevin Wingert, Douglas Young, and Douglas Zmolek.
Footnotes
Clinical trial reg. no. NCD00474630, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0234/-/DC1.
A full list of members of the COR-Diabetes Study Group can be found in the appendix.
References
- 1.Bays HE, González-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343–368 [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–497 [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988-1994 and 1999-2002. MMWR Morb Mortal Wkly Rep 2004;53:1066–1068 [PubMed] [Google Scholar]
- 4.Niswender K. Diabetes and obesity: therapeutic targeting and risk reduction - a complex interplay. Diabetes Obes Metab 2010;12:267–287 [DOI] [PubMed] [Google Scholar]
- 5.Hollander PA, Kushner P. Type 2 diabetes comorbidities and treatment challenges: rationale for DPP-4 inhibitors. Postgrad Med 2010;122:71–80 [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 1992;16:397–415 [PubMed] [Google Scholar]
- 7.Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000;23:1499–1504 [DOI] [PubMed] [Google Scholar]
- 9.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 1995;56:395–401 [PubMed] [Google Scholar]
- 10.Foley KF, DeSanty KP, Kast RE. Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother 2006;6:1249–1265 [DOI] [PubMed] [Google Scholar]
- 11.Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial. Obes Res 2002;10:633–641 [DOI] [PubMed] [Google Scholar]
- 12.Bouza C, Angeles M, Muñoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction 2004;99:811–828 [DOI] [PubMed] [Google Scholar]
- 13.Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 2009;17:30–39 [DOI] [PubMed] [Google Scholar]
- 14.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 15.Sinnayah P, Wallingford NM, Evans AE, Cowley MA. Bupropion and naltrexone interact synergistically to decrease food intake in mice (Abstract). Obesity (Silver Spring) 2007;15:A179 [Google Scholar]
- 16.Apovian CM, Aronne LJ, Rubino DM, et al. COR-II Study Group A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013;21:935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenway FL, Fujioka K, Plodkowski RA, et al. COR-I Study Group Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376:595–605 [DOI] [PubMed] [Google Scholar]
- 18.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Medical Association General Assembly World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Int Bioethique 2004;15:124–129 [PubMed] [Google Scholar]
- 20.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF, RIO-Diabetes Study Group Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006;368:1660–1672 [DOI] [PubMed] [Google Scholar]
- 21.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 22.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol 2011;57:2461–2473 [DOI] [PubMed] [Google Scholar]
- 23.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowler WC, Fowler SE, Hamman RF, et al. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindström J, Louheranta A, Mannelin M, et al. Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 26.Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care 1998;21:1288–1294 [DOI] [PubMed] [Google Scholar]
- 27.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–161 [DOI] [PubMed] [Google Scholar]
- 28.Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med 2011;154:602–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 30.Turnbull FM, Abraira C, Anderson RJ, et al. Control Group Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 31.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765–1772 [DOI] [PubMed] [Google Scholar]
- 32.Mannucci E, Monami M, Lamanna C, Gori F, Marchionni N. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2009;19:604–612 [DOI] [PubMed] [Google Scholar]
- 33.Smith SR, Fujioka K, Gupta AK, et al. Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adipostiy: effects of the naltrexone/bupropion combination on body composition. Diabetes Obes Metab. 12 March 2013 [Epub ahead of print] [DOI] [PubMed]
- 34.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000;133:92–103 [DOI] [PubMed] [Google Scholar]
- 35.Katsoulis K, Blaudeau TE, Roy JP, Hunter GR. Diet-induced changes in intra-abdominal adipose tissue and CVD risk in American women. Obesity (Silver Spring) 2009;17:2169–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887 [DOI] [PubMed] [Google Scholar]
- 37.Thase ME, Haight BR, Johnson MC, et al. A randomized, double-blind, placebo-controlled study of the effect of sustained-release bupropion on blood pressure in individuals with mild untreated hypertension. J Clin Psychopharmacol 2008;28:302–307 [DOI] [PubMed] [Google Scholar]
- 38.Settle EC, Stahl SM, Batey SR, Johnston JA, Ascher JA. Safety profile of sustained-release bupropion in depression: results of three clinical trials. Clin Ther 1999;21:454–463 [DOI] [PubMed] [Google Scholar]
- 39.Glucophage (metformin hydrochloride) tablets [package insert]. Bristol-Meyers Squibb, 2009 [Google Scholar]
- 40.Smith SR, Weissman NJ, Anderson CM, et al. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010;363:245–256 [DOI] [PubMed] [Google Scholar]