Abstract
OBJECTIVE
To evaluate the effect of continuous glucose monitoring (CGM) on the frequency of severe hypoglycemia (SH) in patients with established hypoglycemia unawareness.
RESEARCH DESIGN AND METHODS
We conducted a retrospective audit of 35 patients with type 1 diabetes and problematic hypoglycemia unawareness, despite optimized medical therapy (continuous subcutaneous insulin infusion/multiple daily insulin injections), who used CGM for >1 year.
RESULTS
Over a 1-year follow-up period, the median rates of SH were reduced from 4.0 (interquartile range [IQR] 0.75–7.25) episodes/patient-year to 0.0 (0.0–1.25) episodes/patient-year (P < 0.001), and the mean (±SD) rates were reduced from 8.1 ± 13 to 0.6 ± 1.2 episodes/year (P = 0.005). HbA1c was reduced from 8.1 ± 1.2% to 7.6 ± 1.0% over the year (P = 0.005). The mean Gold score, measured in 19 patients, did not change: 5.1 ± 1.5 vs. 5.2 ± 1.9 (P = 0.67).
CONCLUSIONS
In a specialist experienced insulin pump center, in carefully selected patients, CGM reduced SH while improving HbA1c but failed to restore hypoglycemia awareness.
Although real-time continuous glucose monitoring (CGM) has been shown in randomized controlled trials to improve glycemic control and mild-to-moderate hypoglycemia, studies to date have not shown convincing reductions in severe hypoglycemia (SH) (1,2). Clinically, CGM may benefit patients with impaired awareness of hypoglycemia (IAH), who have an increased risk of SH (3), by alerting them to impending hypoglycemia, and thus providing them with “technological” awareness to replace the loss of their “physiological” awareness. In our clinical service, across two associated tertiary hospitals, we have obtained case-specific funding for CGM for 35 patients with type 1 diabetes, IAH, and problematic hypoglycemia limiting daily activities during intensified insulin therapy. This audit evaluates outcomes at 1 year to see whether the use of CGM can reduce SH or improve awareness.
RESEARCH DESIGN AND METHODS
A retrospective case-note audit was carried out in adult type 1 diabetes patients with ongoing problematic hypoglycemia leading to limitation in daily activities and IAH (Gold score [a Likert linear analog scale in which patients are asked to score between 1 and 7, respectively, if they are always aware or never aware of the onset of hypoglycemia] >4) (4) despite structured education with or without continuous subcutaneous insulin infusion (CSII), who then used CGM in addition to CSII or multiple daily injection (MDI) for at least 12 months. Patients were seen in a specialized clinic with expertise in structured education in flexible insulin therapy and CSII (>700 pump patients across two sites). CGM is not routinely funded in the U.K., so a multidisciplinary team evaluated each case, ensured that routine therapy was optimized on CSII or MDI, and sought patient-specific funding from local health boards. We compared HbA1c, SH rates (episodes requiring third-party assistance), and awareness status using the Gold score at baseline and at 1 year.
Twenty-three subjects used the Medtronic (Northridge, CA) Paradigm Veo system, which can suspend basal insulin delivery automatically for up to 2 h if sensor glucose readings fall below a preset threshold and the patient fails to respond (termed low glucose suspend [LGS]) (5); 7 patients used the Medtronic Paradigm RT system, and 3 patients used Dexcom (San Diego, CA) G4 sensors in combination with an Animas Vibe pump (Johnson & Johnson, New Brunswick, NJ). One patient continued receiving MDIs, and one pump user used the Freestyle Navigator CGM system (Abbott Diabetes Care, Alameda, CA).
Results are reported as the mean ± SD or median (interquartile range [IQR]), unless otherwise stated. Groups were compared using the paired t test or Wilcoxon test, as appropriate.
RESULTS
The mean age of the patients was 43.2 ± 12.4 years, the duration of diabetes was 29.6 ± 13.6 years, 24 patients were female, 33 of 35 patients were receiving CSIIs prior to starting CGM, and 1 more patient converted to CSII within 2 months of starting CGM. The median duration of CSII use was 40 months (range 8–352 months) prior to CGM start.
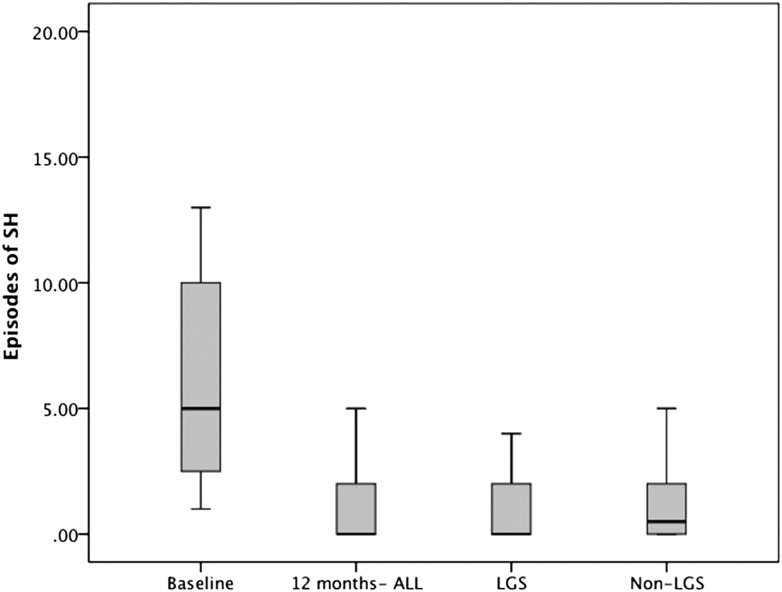
The median rate (IQR) of SH was reduced from 4.0 (0.75–7.25) episodes/patient-year at baseline to 0.0 (0.0–1.25) episodes/patient-year (P < 0.001) (mean 8.1 ± 13 to 0.6 ± 1.2 episodes/year; P = 0.005) after 1 year of CGM (Fig. 1). There was no difference between those patients receiving treatment with or without LGS in final mean HbA1c level (7.7 ± 0.7% vs. 7.8 ± 1.5%; P = 0.9) or median SH rate (0.0 [0–0] vs. 0.0 [0–2.0]; P = 0.3), but three patients were transferred onto LGS pumps after having SH on standard CGM and did not have any further episodes. HbA1c levels for all patients were reduced from 8.1 ± 1.2% to 7.8 ± 1.0% (65 ± 10 to 62 ± 10 mmol/mol) at 1 year (P = 0.007).
Figure 1.
Annual rates of SH, requiring third-party help at baseline and 12 months after starting CGM. Also shown are the 12-month rates divided into those treated with or without LGS.
Nineteen patients (54%) reported subjective improvement in awareness, with 13 reporting no change and 3 reporting a slight worsening in awareness. Paired Gold scores were available in 19 of 34 subjects, showing no change over the year: 5.0 ± 1.5 vs. 5.0 ± 1.9 at 1 year (P = 0.67).
CONCLUSIONS
This study describes the efficacy of CGM in reducing SH in patients with type 1 diabetes and IAH who had problematic hypoglycemia limiting daily activities despite structured education and (predominantly) treatment by CSII. Thirty to 40% of patients with long-duration type 1 diabetes have IAH, and such patients have a threefold to sixfold increase in the risk of SH (3), and experience a negative impact on quality of life through time off work, injury, and fear of hypoglycemia (6), in addition to having an increased risk of mortality (7). Strategies for improving the management of these patients are urgently required.
Structured education in flexible insulin therapy can significantly reduce SH and restore awareness in almost half of those who report unawareness (8). CSII can reduce SH by a mean of about fourfold versus MDI, with the greatest reductions occurring in those with the most hypoglycemia at baseline (9). It was because the subjects in our study had failed to resolve their disabling SH with such measures that a trial of CGM was instigated. Randomized controlled trials have demonstrated a benefit of CGM in reducing mild-to-moderate hypoglycemia but not SH (1,2), perhaps because of the deliberate exclusion of patients with problematic hypoglycemia and low SH rates at baseline in these studies. We hypothesized that SH might be reduced by CGM when used in clinical practice in those patients with problematic SH.
A proportion of our patients used CGM in conjunction with an insulin pump with automatic suspension of basal insulin during hypoglycemia (LGS). Prolonged overnight hypoglycemia still occurs commonly during CGM (10), and patients may sleep through up to 71% of nocturnal alarms (11). Even 30 min of mild hypoglycemia can impair epinephrine responses to subsequent hypoglycemia (12). CSII with LGS is known to reduce CGM-recorded nocturnal hypoglycemia (5) and to increase epinephrine responses to experimental hypoglycemia in adolescents with IAH (13), but its effect on SH has not been documented. We found that both HbA1c and SH were reduced by CGM used with LGS, but to a similar extent to CGM used conventionally. However, we caution that we studied small numbers of patients in a nonrandomized study, and a randomized controlled trial of the two types of CGM pump use would be required to compare the effects on SH.
The strength of our study is that we specifically selected patients who reported problematic hypoglycemia. The study is limited by being a nonrandomized clinical observation, and it is possible that factors other than CGM use (e.g., training, education, and contact with healthcare professionals when starting CGM) contributed to the benefit that was observed. Nevertheless, all subjects had previously undergone structured education, and 33 of 35 subjects had already used CSII for a considerable period. Although more than half of the patients reported subjective improvement in awareness, there was no change in reported Gold scores. Because complete avoidance of hypoglycemia can restore awareness of hypoglycemia (14), we had anticipated an improvement in this measure. It is possible that, although CGM was able to reduce the profound and extended hypoglycemia requiring third-party support, the lag between sensor and capillary glucose concentrations (15) prevented a substantial reduction in the milder, shorter, and more frequent biochemical hypoglycemia that is needed to restore awareness.
This study is the first report of the ability to significantly reduce the incidence of SH in a carefully selected group of patients who were struggling with hypoglycemia despite optimized therapy. These findings serve as proof of principle; they contrast with previous reports that suggested a limited role for CGM in avoiding SH and lay the foundation for further randomized studies in this group of patients.
Acknowledgments
P.C., L.G., G.G., S.P., S.A.A., and J.C.P. have received speaker fees and/or honoraria from companies that produce insulin pumps and/or continuous glucose monitoring devices (Medtronic, Animas, Roche, and Abbott). P.C., S.A.A., and J.C.P. have received funding for clinical trials from Medtronic. No other potential conflicts of interest relevant to this article were reported.
P.C. conceived the study, wrote the manuscript, and researched the data. S.R., L.G., G.G., S.P., and A.B. researched the data. S.A.A. wrote the manuscript. J.C.P. conceived the study and wrote the manuscript. All authors contributed to the clinical care of subjects and to the review and editing of the manuscript. P.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the members of their clinical team who were involved in starting up and maintaining these patients on continuous glucose monitoring, including Helen Rogers, Alison Cox, Jakki Berry, and David Hopkins.
References
- 1.Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhary P, Geddes J, Freeman JV, Emery CJ, Heller SR, Frier BM. Frequency of biochemical hypoglycaemia in adults with type 1 diabetes with and without impaired awareness of hypoglycaemia: no identifiable differences using continuous glucose monitoring. Diabet Med 2010;27:666–672 [DOI] [PubMed] [Google Scholar]
- 4.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 5.Choudhary P, Shin J, Wang Y, et al. Insulin pump therapy with automated insulin suspension in response to hypoglycemia: reduction in nocturnal hypoglycemia in those at greatest risk. Diabetes Care 2011;34:2023–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis RE, Morrissey M, Peters JR, Wittrup-Jensen K, Kennedy-Martin T, Currie CJ. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin 2005;21:1477–1483 [DOI] [PubMed] [Google Scholar]
- 7.Sovik O, Thordarson H. Dead-in-bed syndrome in young diabetic patients. Diabetes Care 1999;22(Suppl. 2):B40–B42 [PubMed] [Google Scholar]
- 8.Hopkins D, Lawrence I, Mansell P, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care 2012;35:1638–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 10.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care 2010;33:1004–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckingham B, Block J, Burdick J, et al. Diabetes Research in Children Network Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther 2005;7:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SN, Mann S, Galassetti P, et al. Effects of differing durations of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia in normal humans. Diabetes 2000;49:1897–1903 [DOI] [PubMed] [Google Scholar]
- 13.Ly TT, Hewitt J, Davey RJ, Lim EM, Davis EA, Jones TW. Improving epinephrine responses in hypoglycemia unawareness with real-time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care 2011;34:50–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cranston I, Lomas J, Maran A, Macdonald I, Amiel SA. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 1994;344:283–287 [DOI] [PubMed] [Google Scholar]
- 15.Kulcu E, Tamada JA, Reach G, Potts RO, Lesho MJ. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care 2003;26:2405–2409 [DOI] [PubMed] [Google Scholar]