Abstract
OBJECTIVE
Foods rich in fiber, such as vegetables and fruits, prevent cardiovascular disease (CVD) among healthy adults, but such data in patients with diabetes are sparse. We investigated this association in a cohort with type 2 diabetes aged 40–70 years whose HbA1c values were ≥ 6.5% in Japan Diabetes Society values.
RESEARCH DESIGN AND METHODS
In this cohort study, 1,414 patients were analyzed after exclusion of patients with history of CVDs and nonresponders to a dietary survey. Primary outcomes were times to stroke and coronary heart disease (CHD). Hazard ratios (HRs) of dietary intake were estimated by Cox regression adjusted for systolic blood pressure, lipids, energy intake, and other confounders.
RESULTS
Mean daily dietary fiber in quartiles ranged from 8.7 to 21.8 g, and mean energy intake ranged from 1,442.3 to 2,058.9 kcal. Mean daily intake of vegetables and fruits in quartiles ranged from 228.7 to 721.4 g. During the follow-up of a median of 8.1 years, 68 strokes and 96 CHDs were observed. HRs for stroke in the fourth quartile vs. the first quartile were 0.39 (95% CI 0.12–1.29, P = 0.12) for dietary fiber and 0.35 (0.13–0.96, P = 0.04) for vegetables and fruits. There were no significant associations with CHD. The HR per 1-g increase was smaller for soluble dietary fiber (0.48 [95% CI 0.30–0.79], P < 0.01) than for total (0.82 [0.73–0.93], P < 0.01) and insoluble (0.79 [0.68–0.93], P < 0.01) dietary fiber.
CONCLUSIONS
Increased dietary fiber, particularly soluble fiber, and vegetables and fruits were associated with lower incident stroke but not CHD in patients with type 2 diabetes.
Type 2 diabetes is a significant cause of premature mortality and morbidity related to cardiovascular disease (CVD), and medical nutritional therapy is an essential component of diabetes care aimed toward prevention of CVD. Current guidelines for diabetes care in many countries encourage consumption of dietary fiber, nondigestible carbohydrates, and lignin that are intrinsic and intact in plants, setting a variety of goals for daily intake of total dietary fiber (14 g/1,000 kcal in the U.S. [1], 40 g in Europe [2], 25–50 g in Canada [3], and 20–25 g in Japan [4]). An increase in dietary fiber can reduce CVD risk through a variety of mechanisms, such as decreasing total and LDL cholesterol (5), reducing postprandial glucose concentration and insulin secretion (6), lowering blood pressure (7), reducing clotting factors (8), and reducing inflammation (9). Lipid-lowering effects were attributable to soluble fiber (5), which reduces absorption of fat and binds bile acids (10). The effects of an unfortified high-fiber (50 g per day) diet on glycemic control and lipids were also demonstrated in a randomized trial in patients with type 2 diabetes (11).
Cohort studies of healthy adults suggest that foods rich in fiber protect against coronary heart disease (CHD) (12) and stroke (Supplementary Table 1) (13–19), but data on patients with type 2 diabetes are sparse (20–22) despite the integral role of medical nutritional therapy. All of the earlier studies in diabetes were conducted in the U.S. and Europe, and the effects of dietary fiber on CVD remain unknown for Asian patients, who account for >60% of the diabetic population worldwide (23). In comparison with type 2 diabetic patients in Western countries, those in East Asian countries, including Japan, are known to have different features regarding cardiovascular complications (24) including a much lower incidence rate of CHD than in Western countries (25) and obesity as a lesser cardiovascular risk factor (20). Therefore, it is still uncertain whether dietary recommendations established by the earlier studies are universally applicable to patients with type 2 diabetes, particularly to Japanese patients. This study therefore aimed to investigate the incidence rates of stroke and CHD in relation to intake of dietary fiber in total, soluble form, and insoluble form and vegetables and fruits in a cohort of Japanese patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
This study is part of the Japan Diabetes Complications Study (JDCS), an open-labeled randomized trial originally designed to evaluate the efficacy of a long-term therapeutic intervention mainly focused on lifestyle education. The original primary end points were CHD, stroke, diabetic retinopathy, and overt nephropathy. The primary results (26) of the JDCS have previously been described. Eligibility criteria were previously diagnosed patients with type 2 diabetes aged 40–70 years whose HbA1c levels were ≥6.5% in Japan Diabetes Society values. From outpatient clinics in 59 university and general hospitals nationwide that specialize in diabetes care, 2,205 patients were initially registered from January 1995 to March 1996. Of the 2,033 patients who met the eligibility criteria and were randomized, 1,588 patients responded to the baseline dietary survey. There was no notable difference in baseline characteristics between responders and nonresponders (27). After exclusion of 174 patients with impaired glucose tolerance, a history of angina pectoris, myocardial infarction, stroke, peripheral artery disease, familial hypercholesterolemia, type III hyperlipidemia (diagnosed by broad β-band on electrophoresis), or nephrotic syndrome (urine protein >3.5 g/day and serum total protein <6.0 mg/dL) or serum creatinine levels >1.3 mg/dL (120 μmol/L) at baseline, 1,414 patients were included in the current analysis. We analyzed follow-up data collected until March 2003. The protocol was approved by the institutional review boards of all of the participating institutes. We obtained written informed consent from all patients.
Outcome measures
A fatal or first nonfatal manifestation of CHD comprised of angina pectoris or myocardial infarction was diagnosed according to criteria defined by the World Health Organization/Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (WHO/MONICA) project, and angina pectoris was defined as typical effort-dependent chest pain or oppression relieved at rest or by use of nitroglycerine as validated by an exercise-positive electrocardiogram or angiography. A patient with a first percutaneous coronary intervention or coronary artery bypass graft was also counted as having a CHD event. Diagnosis of stroke was according to guidelines defined by the Ministry of Health, Labour and Welfare of Japan and WHO criteria. Stroke events were defined as a constellation of focal or global neurological deficits or disturbance of cerebral function that was sudden or rapid in onset and for which there was no apparent cause other than a vascular accident such as epilepsy or brain tumors on the basis of a detailed history, neurological examination, and ancillary diagnostic procedures such as computed tomography, magnetic resonance imaging, cerebral angiography, and lumbar puncture. Stroke events were classified as cerebral infarction (including embolus), intracranial hemorrhage (including subarachnoid hemorrhage), transient ischemic attack, or stroke of undetermined type in accordance with WHO criteria. No cases of asymptomatic lesions detected by brain imaging (i.e., silent infarction) were included. Only first-ever CHD or stroke events during the study period were counted in the analysis and in a patient having both CHD and stroke events; each event was counted separately. Information regarding primary outcome and other clinical variables for each subject was collected through an annual report that included detailed findings at the time of the event from each participating diabetologist who was providing care to those patients. Adjudication of CHD and stroke events was by central committees comprised of experts who were masked to risk factor status and was based on additional data such as a detailed history, sequential changes in ECG and serum cardiac biomarkers, and results of coronary angiography or brain imaging.
Dietary assessment
The Food Frequency Questionnaire based on food groups (FFQg) (28) was administered at baseline. In brief, the FFQg elicited information on the average intake per week of 29 food groups and 10 kinds of cookery in commonly used units or portion sizes. The FFQg was externally validated by comparison with dietary records for seven continuous days of 66 subjects aged 19–60 years (28). The ratios of the estimates obtained by the FFQg against those by the dietary records ranged from 72 to 121%, and the average was 104% (1,666 kcal/1,568 kcal for total energy, 10.0 g/9.5 g for total dietary fiber, 51.0 g/48.0 g for green-yellow vegetables, and 64.8 g/54.7 g for fruits). After patients completed the questionnaire, the dietitian reviewed the answers and in the case of questionable responses interviewed the patient. We use standardized software for population-based surveys and nutrition counseling in Japan to calculate nutrient and food intakes (Excel EIYO-KUN, version 4.5, developed by Shikoku University Nutrition Database; KENPAKUSHA, Tokyo, Japan).
Statistical analysis
Hazard ratios (HRs) and 95% CIs for the incidence of stroke or CHD in relation to dietary intakes were estimated by Cox regression with adjustment for age, sex, BMI, HbA1c, diabetes duration, diabetic retinopathy, treatment by insulin, treatment by oral hypoglycemic agents, systolic blood pressure (SBP), LDL cholesterol, HDL cholesterol, triglycerides (log transformed), current smoking, physical activity, alcohol intake, proportions of total fat, saturated fatty acids, n-6 fatty acids and n-3 fatty acids, cholesterol intake, and sodium intake as confounders. In addition to the multivariate adjustment, we applied the standard multivariate method for energy adjustment. We performed both quartile and linear Cox regression analyses, and the primary analysis was conducted using linear regression. Potential nonlinear relationships between dietary fiber and stroke were explored by a spline function, a smooth curve of incidence rate of stroke depending on dietary fiber. The spline function and 95% CI were estimated by energy-adjusted generalized additive models, and the degree of freedom was determined by generalized cross-validation. Potential effect modification by age ≥60 years, sex, HbA1c ≥9%, duration of diabetes ≥10 years, overweight (BMI ≥25 kg/m2), smoking status, hypertension (SBP ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or treatment by antihypertensive agents), and dyslipidemia (LDL cholesterol ≥120 mg/dL, HDL cholesterol <40 mg/dL, triglycerides ≥150 mg/dL, or treatment by lipid-lowering agents) was explored by subgroup analysis and Wald tests for interaction terms using energy-adjusted Cox regression. All P values are two-sided, and the significance level is 0.05. All statistical analyses and data management were conducted at a central data center using SAS, version 9.2 (SAS Institute, Cary, NC).
RESULTS
The baseline characteristics and daily dietary intake of the 1,414 patients according to quartiles of total dietary fiber are shown in Table 1. Mean total dietary fiber in quartiles ranged from 8.7 to 21.8 g. Mean energy intake in quartiles ranged from 1,442.3 to 2,058.9 kcal. Intake of total dietary fiber was positively associated with proportions of protein and fat intake but not with the proportion of carbohydrate intake. Patients in higher quartiles were significantly older and included more women and had preferable lifestyles such as a lower smoking proportion and increased physical activity. However, there were no significant trends in blood pressure, lipids, and medications, and the difference in HbA1c values was only marginal. Total dietary fiber was positively associated with not only intakes of grain, vegetables, and fruits but also intakes of seafood, meat, and sodium.
Table 1.
Background characteristics and dietary intake for 1,414 patients with type 2 diabetes according to quartiles of total dietary fiber
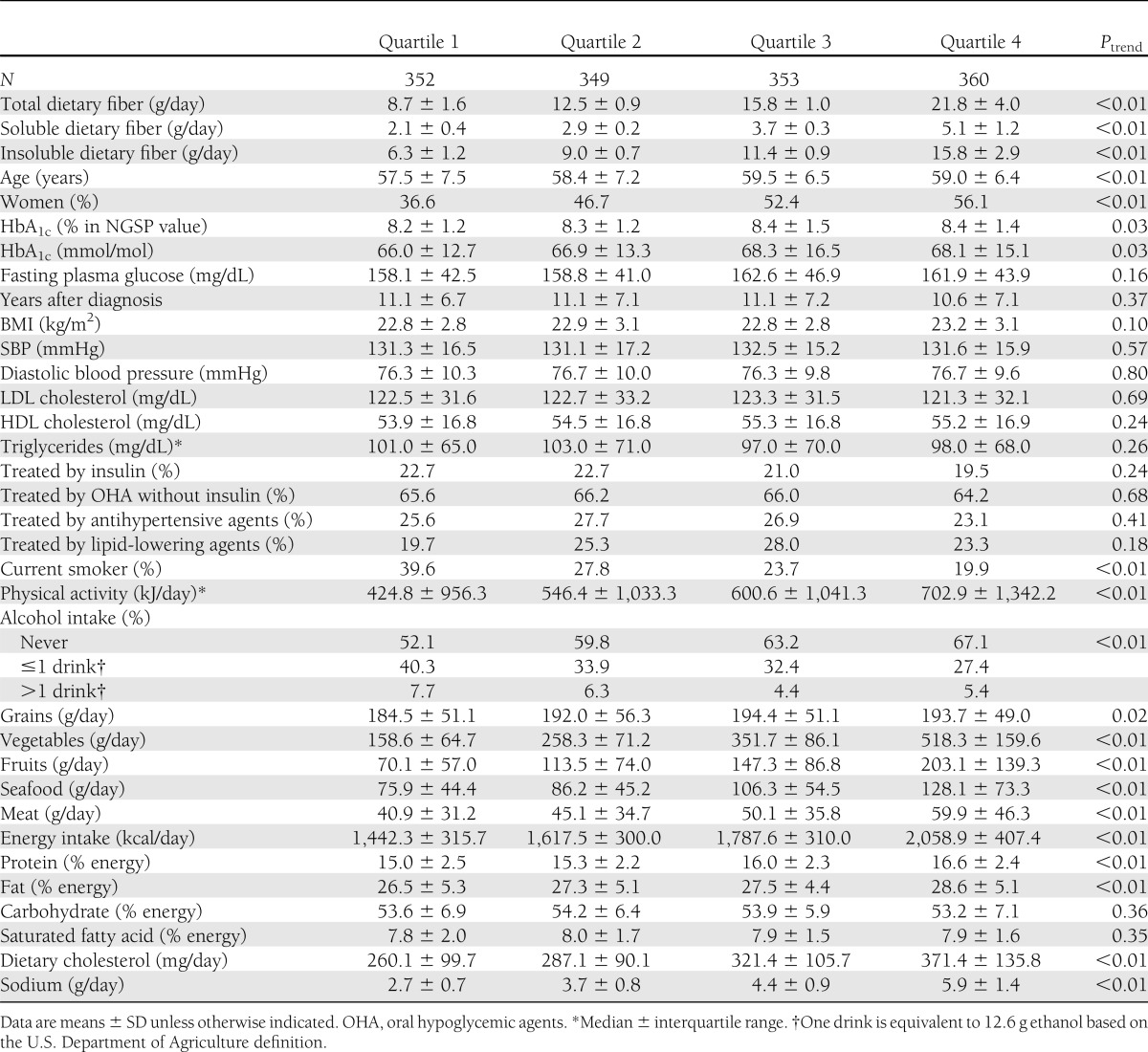
During the follow-up of a median of 8.1 years, the numbers of incident CVD according to quartiles of total dietary fiber were 21, 24, 27, and 24 for CHD; 22, 15, 13, and 18 for stroke; and 19, 12, 11, and 15 for cerebral infarction, respectively. The 68 stroke events included 58 cerebral infarctions, 5 intracranial hemorrhages, 4 transient ischemic attacks, and 1 stroke of undetermined type in accordance with WHO criteria. The crude incidence rates per 1,000 patient-years for CHD, stroke, and cerebral infarction were 9.70, 6.81, and 5.69, respectively, and the follow-up rate at 8 years was 78%. There was no notable difference in baseline characteristics between patients who completed 8-year follow-up and the other patients (27).
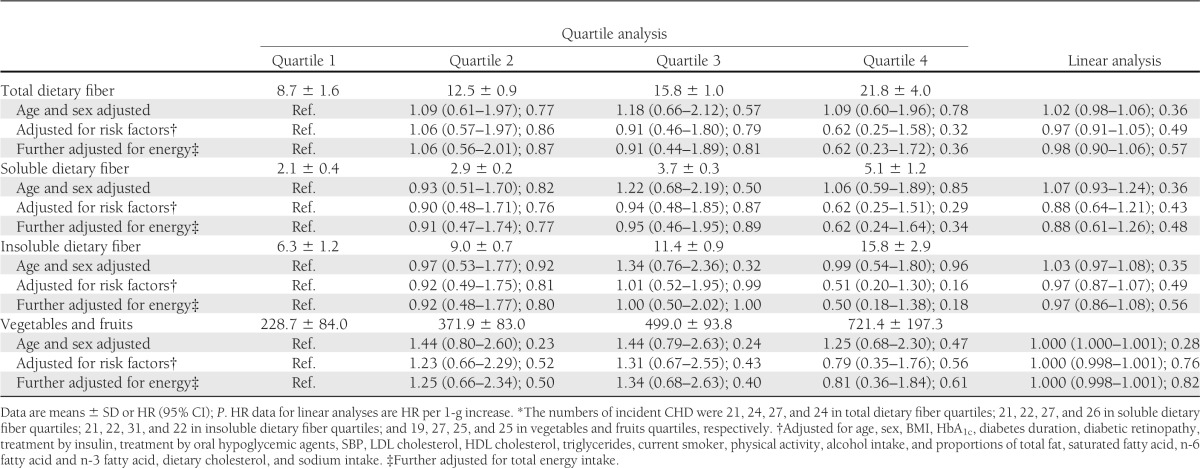
Tables 2 and 3 show HRs for dietary fiber, vegetables, and fruits estimated by Cox regression models unadjusted (top model), adjusted for risk factors (middle model), and further adjusted for total energy intake (bottom model). The energy-adjusted HRs for stroke in the fourth quartile compared with the first quartile were 0.39 (95% CI 0.12–1.29, P = 0.12) for total dietary fiber and 0.35 (95% CI 0.13–0.96, P = 0.04) for vegetables and fruits (Table 2). There were no significant decreasing trends between grain intake, a major source of dietary fiber, and incident stroke (data not shown). The HR per 1-g increase was smaller for soluble dietary fiber (0.48 [95% CI 0.30–0.79], P < 0.01) than for total (0.82 [95% CI 0.73–0.93], P < 0.01) and insoluble (0.79 [95% CI 0.68–0.93], P < 0.01) dietary fiber. The HRs for cerebral infarction were similar to those for stroke (Supplementary Table 2). In contrast, both the quartile and linear analyses showed no significant trends toward a decreased incidence rate of CHD (Table 3). Supplementary Fig. 1 shows results of subgroup analysis according to risk factors for CVD. None of these associations indicated significant interactions, suggesting lack of clear evidence of effect modifications.
Table 2.
Cox regression analysis of incidence of stroke* and intake of total, soluble, and insoluble dietary fiber and vegetables and fruits
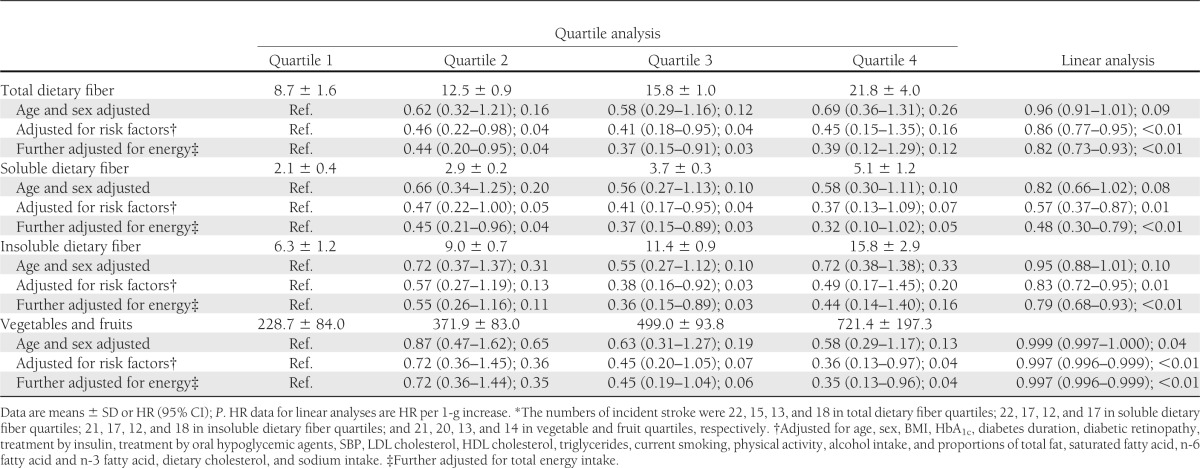
Table 3.
Cox regression analysis of incidence of CHD* and intake of total, soluble, and insoluble dietary fiber and vegetables and fruits
To explore potentially nonlinear relationships between total dietary fiber and the incidence of stroke, we fitted the energy-adjusted generalized additive models (Fig. 1). As shown graphically, decreasing trends according to higher values for dietary fiber were clearly shown, with the relationships appearing to be nonlinear. Notably, the estimated incidence rate was very low, i.e., <0.90/1,000 patient-years, among patients consuming total dietary fiber >25 g. Indeed, the maximum total dietary fiber in the 68 cases of stroke was 24 g.
Figure 1.
Incidence rate (solid curve) and 95% CI (broken curve) of stroke in relation to total dietary fiber intake estimated by the generalized additive model.
CONCLUSIONS
This 8-year follow-up study of Japanese patients with type 2 diabetes revealed an ~60% risk reduction of stroke in the fourth quartile of total dietary fiber and vegetables/fruits compared with the first quartile. The estimated incidence rate of stroke was very low in patients consuming >25 g/day of total dietary fiber, suggesting a potential threshold of ~20–25 g. The association in relation to soluble fiber seemed to be stronger, but there were no significant associations between CHD and any types of dietary fiber. Our findings are in line with results of earlier cohort studies on the incidence of stroke among healthy adults, as summarized in Supplementary Table 1.
In comparison with people in Western countries, diabetic patients in East Asian countries, including Japan, are known to have quite different features such as the much lower incidence rate of CHD than in Western countries (25) and the low prevalence of obesity (20). As expected, the incidence rate of stroke among patients in this cohort, 6.81/1,000 patients-years, was 2–10 times higher than those in earlier studies (14–19) (Supplementary Table 1). The “metabolically obese” phenotype (20) characterized by normal body weight with increased abdominal adiposity was also common (Table 1). Furthermore, most patients typically had a “low-fat energy-restricted diet,” i.e., the proportions of protein, fat, and carbohydrate consumption met the Western guidelines (1–3), which recommended carbohydrate intake ranging from 45 to 65%, fat intake <30–35%, and protein intake from 10 to 20%. On the other hand, despite the possible difference in dietary habits, the distribution of dietary fiber intake substantially overlaps with those in populations of healthy adults except for cohorts in Finland and Sweden (Supplementary Table 1). The current goals for daily intake of total dietary fiber in guidelines are similar between Japan (20–25 g [4]) and the U.S. (14 g/1,000 kcal [1]). We observed a lower incidence of stroke around intake of 20–25 g (Fig. 1), supporting these dietary goals. Achieving such intake would be realistic given the national average in Japanese adults, i.e., 14.6 g (29).
The estimated risk reduction by dietary fiber in this cohort was seemingly stronger than those in the earlier cohort studies (Supplementary Table 1). The HRs of the fifth quintile of total dietary fiber compared with the first quintile ranged from 0.64 (95% CI 0.46–0.88) to 1.05 (0.73–1.51), showing moderate heterogeneity across studies. Data on the effects of dietary fiber in diabetic patients are limited (20–22). The Nurses’ Health Study reported that whole-grain and bran intakes were associated with reduced all-cause and cardiovascular mortality among U.S. women with type 2 diabetes (20). Inverse associations with all-cause and cardiovascular mortality were also observed in a study of self-reported diabetes nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) study (21). The EURODIAB Prospective Complications Study reported that higher dietary fiber consumption, especially that of soluble fiber, was associated with CVD in type 1 diabetic patients (22). Taken together, high intake of dietary fiber would reduce the incidence of stroke not only in healthy adults but also in patients with type 2 diabetes. However, it is unclear whether dietary fiber is more beneficial for diabetic patients.
The precise mechanisms for our findings cannot be clarified merely from epidemiological studies, but it is important to note that the energy-adjusted HR was smaller for soluble dietary fiber (0.48/g) than for total (0.82/g) and insoluble (0.79/g) dietary fiber. Soluble fiber is mainly derived from fruits, vegetables, and legumes in typical Japanese diets. This type of fiber specifically decreases LDL cholesterol by −2.2 mg/dL per 1 g (5) and SBP by −1.32 mmHg (7) if given as supplements. Furthermore, lipids and blood pressure are the leading risk factors for CHD and stroke in Japanese patients, respectively (24). Our observations therefore support the hypothesis that the effects of dietary fiber on some types of stroke are mediated by lipids and blood pressure, but given that the degree of improvement in LDL cholesterol and SBP by dietary fiber is small, the entire risk reduction for stroke does not seem to be attributable to merely the effect on lipids and blood pressure. Other possibilities include reducing postprandial glucose concentration and insulin secretion (6), reducing clotting factors (8), and decreasing inflammation (9). These protective factors against CVD are biologically interrelated, so it may be possible that synergism among them results in the 60% risk reduction of stroke.
Another important finding of this study was that only stroke but not CHD was significantly reduced by dietary fiber. The Japan Public Health Center Study (18) also reported a significant association for only stroke, while dietary fiber was correlated with only CHD—not stroke—in the Japan Collaborative Cohort Study (17) (Supplementary Table 1). However, these three cohorts in Japan consistently reported HRs for CHD of <1 in higher quartiles of dietary fiber, showing weak decreasing trends in CHD (though statistically nonsignificant). Furthermore, our post hoc power calculation suggested that the power of our study may not be sufficient. For example, the observed HR for CHD between the first and fourth quartiles was 0.62, and P = 0.36 (Table 3), but the power to detect a true HR of 0.62 was only 11%, given the 45 CHD incidents in the first and fourth quartiles. Therefore, it is possible that the association between CHD and dietary fiber would become significant by conducting pooled analysis of cohorts.
To the best of our knowledge, this is the first study on dietary fiber and CVD in which patients with type 2 diabetes are prospectively registered based on their HbA1c levels—not retrospectively selected based on self-reported diabetes status. Other strengths include treatment and follow-up plans that were conducted in institutes specializing in diabetes care and adjudication of cardiovascular events by an independent central committee. Our study has several limitations. First, the potential for bias, such as measurement errors in dietary assessments, confounding factors, and informative censoring, cannot be ruled out entirely. However, we found no notable difference in baseline characteristics between patients who completed 8-year follow-up and the other patients (27). Second, in an observational study rather than a randomized trial, it is impossible to conclude whether medical nutritional treatment encouraging dietary fiber or intake of vegetables and fruits would reduce incident stroke in clinical practice. The apparent preferable effects of dietary fiber might be due to a generally healthy lifestyle among high dietary fiber consumers. This possibility cannot be not entirely excluded; patients in higher quartiles of dietary fiber had a relatively low smoking rate and high level of physical activity, although they had adverse dietary behaviors such as increased intake of energy, saturated fatty acid, cholesterol, and sodium. Furthermore, it is difficult, although not impossible under strong assumptions for mediation analysis, to separate the effects specific to dietary fiber and the generic effect mediated by the quantity of energy consumed in this observational study. Finally, our results may not be generally applicable to populations with different lifestyle or genetic factors. For example, BMI and body weight are markedly different between patients in Japan and Western countries (30). Our systematic review found that earlier studies were conducted in U.S., Europe, and Japan and that the findings were moderately heterogeneous. Furthermore, a cohort study suggests that dietary fiber intake may modify the association between TCF7L2 rs7903146 and the incidence of type 2 diabetes, leading to preventive effects of dietary fiber from type 2 diabetes only among non–risk allele carriers (31). The contribution of such ethnic and genetic differences remains uncertain and is worthy of further research. These limitations notwithstanding, we conclude that high intakes of dietary fiber, particularly soluble fiber, and vegetables and fruits reduce incident stroke but not CHD in patients with type 2 diabetes.
Acknowledgments
This study was financially supported by the Ministry of Health, Labour and Welfare of Japan.
No potential conflicts of interest relevant to this article were reported.
The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Sh.T. performed statistical analysis and drafted the manuscript. Y.Y. performed the dietary survey. C.K. contributed to the writing of the manuscript. Sa.T. was responsible for statistical analysis and data management. C.H. and R.O. contributed to the writing of the manuscript. H.I. contributed to the design and conduct of the JDCS. Y.O. was responsible for statistical analysis and data management. Y.A. contributed to the design and conduct of the JDCS. N.Y. and H.S. are the principal investigators of the JDCS. H.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank all of the patients and diabetologists at the 59 participating institutes for long-standing collaboration in the JDCS. Thanks are extended to Mami Haga and Natsuko Tada at the Niigata University Faculty of Medicine for excellent secretarial assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0654/-/DC1.
References
- 1.Bantle JP, Wylie-Rosett J, Albright AL, et al. American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 2.Mann JI, De Leeuw I, Hermansen K, et al. Diabetes and Nutrition Study Group (DNSG) of the European Association Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis 2004;14:373–394 [DOI] [PubMed] [Google Scholar]
- 3.Canadian Diabetes Association Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2008;32:1–201 [Google Scholar]
- 4.Japan Diabetes Society Evidence-Based Practice Guideline for the Treatment of Diabetes in Japan. Tokyo, Japan, Nankodo Co., Ltd., 2010. [in Japanese] [Google Scholar]
- 5.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30–42 [DOI] [PubMed] [Google Scholar]
- 6.Potter JG, Coffman KP, Reid RL, Krall JM, Albrink MJ. Effect of test meals of varying dietary fiber content on plasma insulin and glucose response. Am J Clin Nutr 1981;34:328–334 [DOI] [PubMed] [Google Scholar]
- 7.Streppel MT, Arends LR, van ’t Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005;165:150–156 [DOI] [PubMed] [Google Scholar]
- 8.Marckmann P, Sandström B, Jespersen J. Effects of total fat content and fatty acid composition in diet on factor VII coagulant activity and blood lipids. Atherosclerosis 1990;80:227–233 [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Whincup PH, Thomas MC, Sattar N. Associations between dietary fiber and inflammation, hepatic function, and risk of type 2 diabetes in older men: potential mechanisms for the benefits of fiber on diabetes risk. Diabetes Care 2009;32:1823–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JW, Tietyen-Clark J. Dietary fiber: hyperlipidemia, hypertension, and coronary heart disease. Am J Gastroenterol 1986;81:907–919 [PubMed] [Google Scholar]
- 11.Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med 2000;342:1392–1398 [DOI] [PubMed] [Google Scholar]
- 12.Pereira MA, O’Reilly E, Augustsson K, et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med 2004;164:370–376 [DOI] [PubMed] [Google Scholar]
- 13.Chen GC, Lv DB, Pang Z, et al. Dietary fiber intake and stroke risk: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 2013;67:96–100 [DOI] [PubMed]
- 14.Ascherio A, Rimm EB, Hernán MA, et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation 1998;98:1198–1204 [DOI] [PubMed] [Google Scholar]
- 15.Oh K, Hu FB, Cho E, et al. Carbohydrate intake, glycemic index, glycemic load, and dietary fiber in relation to risk of stroke in women. Am J Epidemiol 2005;161:161–169 [DOI] [PubMed] [Google Scholar]
- 16.Larsson SC, Männistö S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Dietary fiber and fiber-rich food intake in relation to risk of stroke in male smokers. Eur J Clin Nutr 2009;63:1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshak ES, Iso H, Date C, et al. JACC Study Group Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr 2010;140:1445–1453 [DOI] [PubMed] [Google Scholar]
- 18.Kokubo Y, Iso H, Saito I, et al. JPHC Study Group Dietary fiber intake and risk of cardiovascular disease in the Japanese population: the Japan Public Health Center-based study cohort. Eur J Clin Nutr 2011;65:1233–1241 [DOI] [PubMed] [Google Scholar]
- 19.Wallström P, Sonestedt E, Hlebowicz J, et al. Dietary fiber and saturated fat intake associations with cardiovascular disease differ by sex in the Malmö Diet and Cancer Cohort: a prospective study. PLoS ONE 2012;7:e31637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M, van Dam RM, Rimm E, Hu FB, Qi L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010;121:2162–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger KN, Beulens JW, van der Schouw YT, et al. Dietary fiber, carbohydrate quality and quantity, and mortality risk of individuals with diabetes mellitus. PLoS ONE 2012;7:e43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenaker DA, Toeller M, Chaturvedi N, Fuller JH, Soedamah-Muthu SS, EURODIAB Prospective Complications Study Group Dietary saturated fat and fibre and risk of cardiovascular disease and all-cause mortality among type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 2012;55:2132–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129–2140 [DOI] [PubMed] [Google Scholar]
- 24.Sone H, Tanaka S, Tanaka S, et al. Japan Diabetes Complications Study Group Serum level of triglycerides is a potent risk factor comparable to LDL cholesterol for coronary heart disease in Japanese patients with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study (JDCS). J Clin Endocrinol Metab 2011;96:3448–3456 [DOI] [PubMed] [Google Scholar]
- 25.Lee ET, Keen H, Bennett PH, Fuller JH, Lu M. Follow-up of the WHO Multinational Study of Vascular Disease in Diabetes: general description and morbidity. Diabetologia 2001;44(Suppl. 2):S3–S13 [DOI] [PubMed] [Google Scholar]
- 26.Sone H, Tanaka S, Iimuro S, et al. Japan Diabetes Complications Study Group Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia 2010;53:419–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka S, Tanaka S, Iimuro S, et al. Cohort profile: The Japan Diabetes Complications Study: a long-term follow-up of a randomised lifestyle intervention study of type 2 diabetes. Int J Epidemiol. 18 May 2013 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 28.Takahashi K, Yoshimura Y, Kaimoto T, et al. Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake. Jpn J Nutr 2001;59:221–232 [in Japanese] [Google Scholar]
- 29.Ministry of Health, Labour and Welfare. National Health and Nutrition Survey in 2010 [Internet]. Available from http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h22-houkoku-07.pdf Accessed 1 October 2013 [in Japanese]
- 30.Sone H, Yoshimura Y, Ito H, Ohashi Y, Yamada N, Japan Diabetes Complications Study Group Energy intake and obesity in Japanese patients with type 2 diabetes. Lancet 2004;363:248–249 [DOI] [PubMed] [Google Scholar]
- 31.Hindy G, Sonestedt E, Ericson U, et al. Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia 2012;55:2646–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]



