Abstract
OBJECTIVE
Custom-made footwear is the treatment of choice to prevent foot ulcer recurrence in diabetes. This footwear primarily aims to offload plantar regions at high ulcer risk. However, ulcer recurrence rates are high. We assessed the effect of offloading-improved custom-made footwear and the role of footwear adherence on plantar foot ulcer recurrence.
RESEARCH DESIGN AND METHODS
We randomly assigned 171 neuropathic diabetic patients with a recently healed plantar foot ulcer to custom-made footwear with improved and subsequently preserved offloading (∼20% peak pressure relief by modifying the footwear) or to usual care (i.e., nonimproved custom-made footwear). Primary outcome was plantar foot ulcer recurrence in 18 months. Secondary outcome was ulcer recurrence in patients with an objectively measured adherence of ≥80% of steps taken.
RESULTS
On the basis of intention-to-treat, 33 of 85 patients (38.8%) with improved footwear and 38 of 86 patients (44.2%) with usual care had a recurrent ulcer (relative risk −11%, odds ratio 0.80 [95% CI 0.44–1.47], P = 0.48). Ulcer-free survival curves were not significantly different between groups (P = 0.40). In the 79 patients (46% of total group) with high adherence, 9 of 35 (25.7%) with improved footwear and 21 of 44 (47.8%) with usual care had a recurrent ulcer (relative risk −46%, odds ratio 0.38 [0.15–0.99], P = 0.045).
CONCLUSIONS
Offloading-improved custom-made footwear does not significantly reduce the incidence of plantar foot ulcer recurrence in diabetes compared with custom-made footwear that does not undergo such improvement, unless it is worn as recommended.
Every 30 seconds, a limb is lost somewhere in the world as a consequence of diabetes (1). These amputations are nearly always preceded by a foot ulcer, which has a lifetime risk of 15–25% in patients with diabetes (2,3). Foot disorders, including ulcers, are a leading cause of hospitalization and high treatment costs in patients with diabetes (4). Therefore, prevention of ulceration is important to decrease the large patient and economic burden of diabetic foot disease.
Approximately one-half of all diabetic foot ulcers occur on the plantar foot surface and are mainly caused by elevated levels of mechanical pressure acting on the foot during ambulation in the presence of lost protective foot sensation from peripheral neuropathy (5,6). Therefore, to reduce risk of ulceration, relief of mechanical pressure (also called offloading) is indicated. For this purpose, custom-made therapeutic footwear is recommended by international guidelines (7) and is the standard of care in the Netherlands for patients with foot deformity and a history of ulceration.
Despite widespread prescription of custom-made footwear, foot ulcers often recur (8). A limited number of randomized trials have shown inconsistent results in custom-made footwear efficacy to prevent ulcer recurrence in diabetes (7,9–11). These studies varied considerably in prescription methods and shoe designs, and foot pressure was not measured. To explain clinical outcomes in footwear studies, an indication for effective pressure relief seems important, as does an accurate estimate of patient adherence to wearing prescription footwear. Footwear cannot be effective if it is not worn, and adherence is known to be low in diabetic patients. Observational studies and one randomized trial have shown that only 22–29% of patients wear their prescription footwear for >80% of the daytime (10,12–14). High-quality randomized trials are needed to better inform clinical practice about effective footwear designs and the importance of adherence in footwear effectiveness (15).
Within this context, the lack of existing evidence-based prescription guidelines and the proven variation in the offloading effect of custom-made footwear designs suggests that prescription footwear is suboptimal in relieving pressure and should be improved to increase clinical benefit (16–18). We previously showed that the use of in-shoe plantar pressure measurements to evaluate footwear can effectively guide footwear modifications to improve pressure relief in individual patients (19). Significant reductions in peak pressure between 17 and 52% were achieved across patients. We hypothesized that with this approach, ulcer recurrence can be reduced significantly, provided that pressure reduction is maintained over time. Therefore, the objective of the present study was to compare in an intention-to-treat analysis the effect of pressure-improved custom-made footwear with that of usual care (i.e., nonimproved custom-made footwear) on plantar foot ulcer recurrence incidence in 18 months. In addition, we evaluated whether adherence to wearing custom-made footwear influences the outcomes on ulcer recurrence.
RESEARCH DESIGN AND METHODS
We enrolled patients from the multidisciplinary outpatient diabetic foot clinics of two academic and eight large general public hospitals across the Netherlands. Inclusion criteria were age ≥18 years, confirmed type 1 or type 2 diabetes, loss of protective foot sensation as a result of peripheral neuropathy, a healed plantar foot ulcer (i.e., full epithelialization without exudate) in the 18 months preceding randomization, and a new prescription of custom-made footwear. Exclusion criteria were bilateral amputation proximal to the tarsometatarsal joint, the use of walking aids that offload the foot, severe illness that would make 18-month survival unlikely (as judged by the patient’s physician), and inability to follow the study instructions. Each patient provided written informed consent before inclusion.
Study design and randomization
In this investigator-initiated parallel-group study, we randomly assigned patients between November 2007 and October 2010 in a balanced design to 1) custom-made footwear of which the offloading properties were improved and subsequently preserved based on in-shoe plantar pressure measurement and analysis or 2) custom-made footwear that did not undergo improvement based on in-shoe pressure measurement (i.e., usual care). At footwear delivery, the study investigator randomly assigned subjects using an online-accessible computer-generated allocation sequence (TENALEA Clinical Trial Data Management System; National Cancer Institute, Amsterdam, the Netherlands) that used the nondeterministic minimization method. The allocation sequence was prepared and managed by a noninvolved investigator. Participating center and sex were used as factors for stratification.
Primary outcome assessors were blinded to group assignment. Caregivers and investigators were not blinded to group assignment and were instructed not to communicate treatment allocation with patients. We attempted to blind patients by measuring in-shoe plantar pressures in both study groups at equal intervals and by evaluating and modifying the footwear outside the view of patients. The study was registered in the Dutch Trial Register (study ID NTR1091) and was approved by the medical ethics committees of all 10 participating centers.
Custom-made footwear
All patients received their new prescription custom-made footwear at study entry. This footwear consisted of custom-made insoles worn in custom-made shoes (i.e., fully customized footwear [85.4% of patients]) or custom-made insoles worn in off-the-shelf (extra-depth) shoes (i.e., semicustomized footwear [14.6% of patients]). Any additional pair of custom-made footwear that patients already possessed at study entry (i.e., earlier prescriptions) or were prescribed during follow-up was included in the study. Plantar pressures were measured inside this additional footwear, and if indicated, the footwear was modified in the intervention group. In each center, the local specialist in physical and rehabilitation medicine prescribed the footwear, and the local orthopedic shoe technician manufactured the footwear; both professionals were experienced in diabetic foot care. There was no cross-training between centers in footwear prescription or modification.
Although not enforced by any protocol, footwear design generally resembled design recommendations from a previously published algorithm (20). Shoe lasts for the fully customized shoes were generally created from a plaster cast molding of the foot, and in some cases, they were created from three-dimensional digital scans of a fiberglass cast of the foot. Insoles for these shoes consisted of multilayered materials, generally with a cork base added with microcork and a mid-layer of ethylene vinyl acetate–based multiform. Insoles for the semicustomized shoes were mostly manufactured by attaching a thermoplastic polyurethane-based material (Rhenoflex GmbH, Ludwigshafen am Rhein, Germany) to a positive plaster cast of the foot that was created from static impressions of the foot in a foam box. A multiform or cork base was then added. In a minority of cases, insoles for semicustomized shoes were created from stock multiform insoles, which were individually adapted. For both footwear types, static foot pressure prints on carbon paper sheets were used to identify locations for placement of a metatarsal pad or bar or additional medial arch support in the insole. Areas of interest (e.g., Charcot deformity, previous ulcer location) were regularly targeted by applying a softer, more cushioning material at the corresponding location in the insole or by removing material in the insole to create more support around the location. The insoles were finished with a Plastazote (Zotefoams plc, Croydon, U.K.) (60.8% of insoles), leather (29.3%), or Professional Protective Technology (Langer, Inc., Deer Park, NY) (9.9%) top cover. The stiffened rubber or Poron (Zotefoams) shoe outsole had a roller configuration with, in the majority of cases, a 1- to 2-cm toe spring and a pivot point location just proximal to the offloading target (20). Geometric foot measurements (length, circumference, and instep height) were taken and used to help to appropriately fit the shoes. Patient preferences about style and color of the shoes were taken into account as long as biomechanical function was not jeopardized.
Assessments
All study data were collected, postprocessed, and entered into a database by three trained and centrally appointed researchers. At baseline, data on demographics, diabetes, and foot complication history were collected. Loss of protective sensation was assessed with a 10-g Semmes-Weinstein monofilament and biothesiometer (Biomedical Instruments, Newbury, OH) testing (5). Peripheral arterial status was assessed according to the PEDIS (perfusion, extent/size, depth/tissue loss, infection, sensation) classification (21). Presence of foot deformity was assessed from standardized digital photographs of the foot. Barefoot dynamic plantar pressure distribution was measured at a 100-Hz sampling rate with an Emed-X pressure platform (Novel GmbH, Munich, Germany) (22). Regional mean peak pressures over 5 steps per foot were calculated and used for analysis. Each patient received written and verbal instructions on foot care and on proper use of footwear.
All footwear in both study groups was evaluated at delivery and at 3-month follow-up visits with the Pedar-X in-shoe pressure measurement system (Novel GmbH), which measured peak pressure distribution at a 50-Hz sampling rate at the sock-insole interface during comfortable walking (23). In the improved footwear group, the measured in-shoe plantar pressures guided the modification of footwear that followed a previously described protocol (24). In short, the previous ulcer location with peak pressure >200 kPa and, per foot, the two forefoot or midfoot locations that showed the highest peak pressures >200 kPa were identified and targeted for pressure relief. The shoe technician modified the footwear until peak pressure at these regions of interest was reduced by 25% or below an absolute level of 200 kPa (whichever was reached first) or until a maximum of three rounds of modifications and pressure evaluations were used (19,25). Choice of footwear modification was left to the shoe technician, and multiple modifications were allowed within one round. At each 3-month follow-up visit, this offloading-improvement protocol was applied when the offloading criteria were not yet met at study entry or when peak pressure at the region of interest increased ≥5% over time.
At least 3 months after randomization, footwear use was measured objectively during 7 consecutive days with the use of a temperature-based monitor (@monitor, Department of Medical Technology and Innovation, Academic Medical Center, Amsterdam, the Netherlands) placed inside all custom-made shoes the patient had at the time of testing (14,26). Walking activity was measured simultaneously with a step activity monitor (StepWatch, Orthocare Innovations LLC, Oklahoma City, OK) worn around the ankle. Both monitors produced valid and reliable data (26,27). Average daily step count and footwear adherence were calculated from these measurements. Footwear adherence was defined as the percentage of cumulative steps over 7 days of recording that custom-made footwear was worn.
Patients were followed for 18 months or until plantar foot ulceration, whichever came first. The primary outcome was the percentage of patients with a plantar foot ulcer in 18 months. Ulcers were defined as cutaneous erosions through the dermis without reference to time present (21,28). A panel of three (or in case of disagreement, five) blinded and independently operating foot care specialists who were not directly involved in the study diagnosed the ulcer. Ulcer diagnosis was done from digital photographs of the plantar foot taken at each follow-up visit, or between visits when the patient or treating physician reported the lesion, and from descriptions of the lesion. The same specialists classified ulcers by the University of Texas system (29). Nonulcerative plantar lesions (i.e., hemorrhage, blister, abundant callus, or erythema) were also scored from these photographs by two teams of two blinded researchers who reached consensus on outcome. This scoring of nonulcerative lesions was done after the last study visit to avoid influencing treatment during the study.
Statistical analysis
Statistical analysis was performed after the last follow-up visit in April 2012 with SPSS version 19.0 software (IBM Corporation, Armonk, NY), unless otherwise stated. All tests assessed group effects, were two-sided, and used P < 0.05 for significance. Baseline patient characteristics, in-shoe peak pressures at footwear delivery, daily step count, and adherence were assessed with independent sample t tests when data were normally distributed or Mann-Whitney U tests when data were not normally distributed. In-shoe peak pressures over time were modeled by multilevel linear regression analysis using MLwiN software version 2.23 (Institute of Education, University of London, London, U.K.) and nested at three levels (time, patient, and center) to account for any dependency on these factors. Fixed factors were group, time, and group–time interaction. To analyze group effects for in-shoe peak pressure over time, pressures were corrected for baseline values at study entry.
In an intention-to-treat analysis, the primary outcome was assessed with Pearson χ2 tests. Ulcer outcome data from patients who died during the study was based on outcome at the moment of death (last observation carried forward). From patients who withdrew participation, information on ulcer outcome at 18 months was obtained from their files with their consent. Survival of ulcer recurrence was assessed by Kaplan-Meier plots and log-rank testing using censored data for death. χ2 tests were conducted to test for the percentage of patients who had ulcer recurrence at the previous ulcer location and the percentage of patients with nonulcerative lesions. Fisher exact test was conducted to test for the percentage of patients with complicated foot ulcers (i.e., infected, ischemic). To assess the influence of footwear adherence on ulcer recurrence, χ2 tests compared ulcer recurrence between study groups in the subgroups of patients with high adherence and low adherence. These subgroups were predefined (before statistical analysis) by a cutoff of 80% adherence, which is indicated from previous studies as being appropriate for creating similar-sized patient groups of high and low adherence (13,14).
We anticipated an 18-month ulcer recurrence rate of 30% in the usual care group according to estimates from the literature (8–10,30) and 15% in the improved footwear group on the basis of what we considered a relevant risk reduction compared with usual care. With α set to 0.05 (one-sided), power to 0.80 by χ2 analysis, and an anticipated loss to follow-up of 20%, we intended to include 240 patients. Because of a lower recruitment rate in the time available, the actual sample size was 171, which, based on intention-to-treat, yielded a power of 0.76 (one-sided) and 0.65 (two-sided).
RESULTS
Study participants
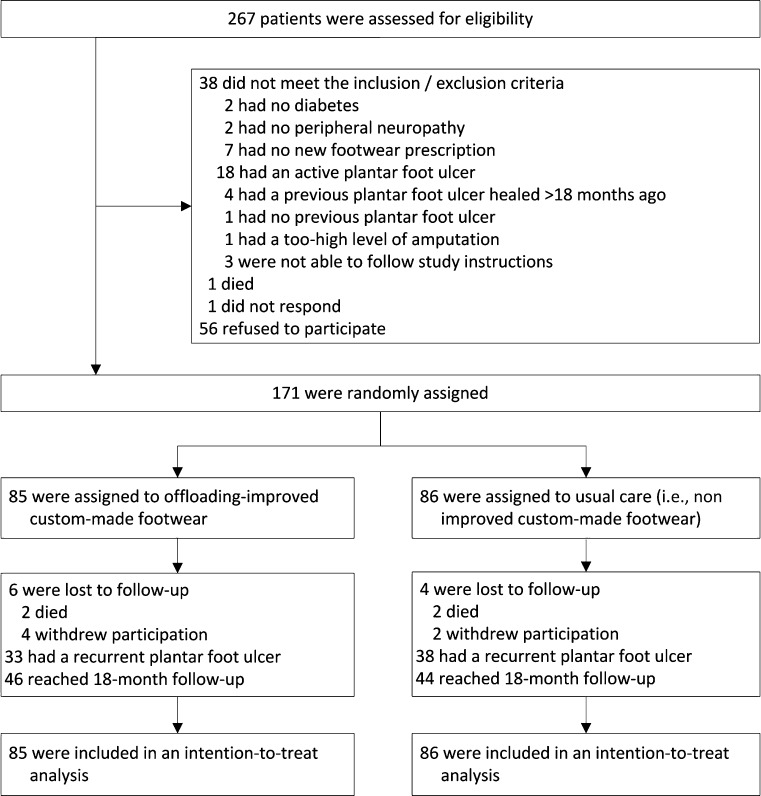
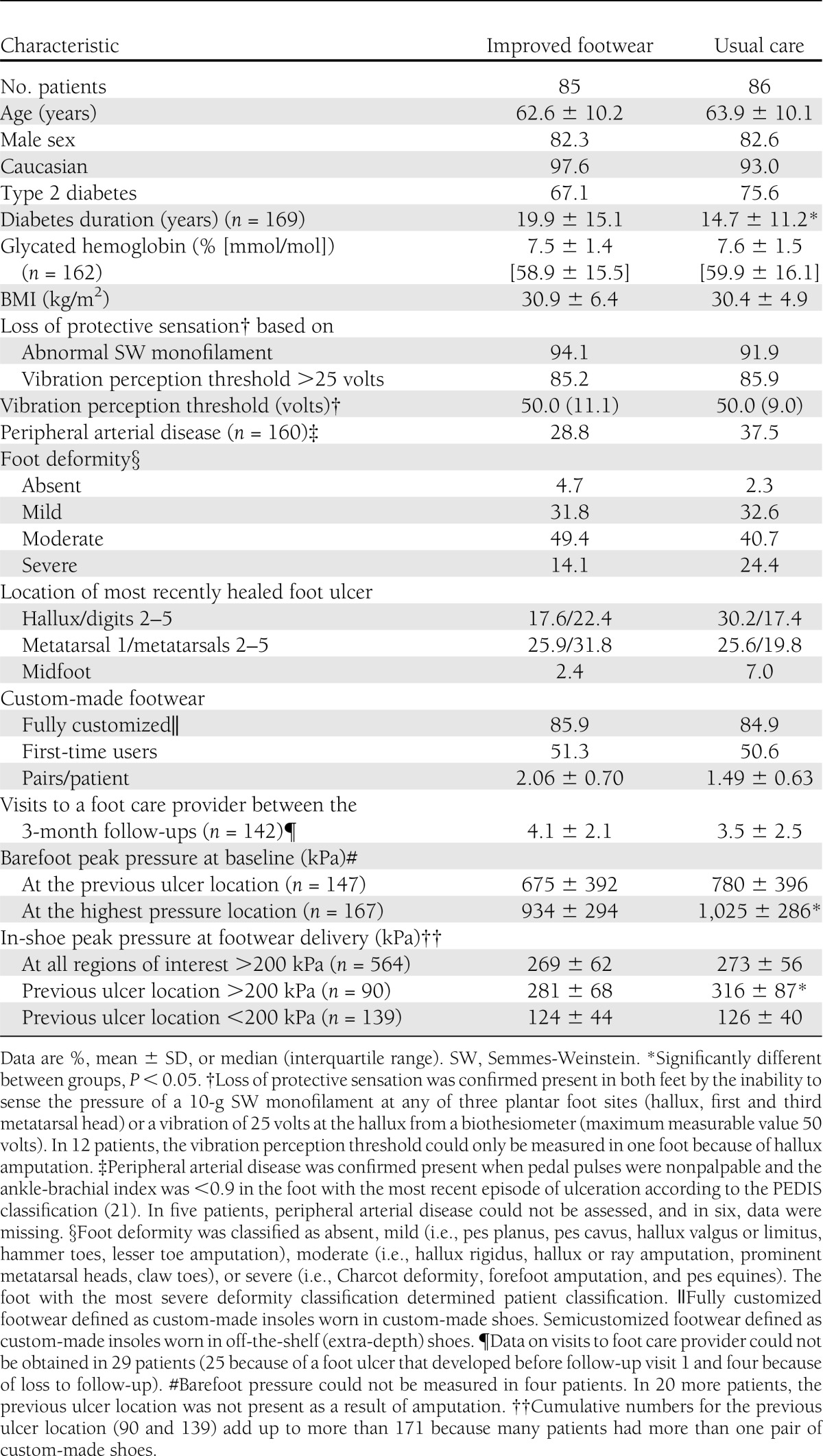
A study flow diagram is shown in Figure 1. The number of included patients varied between six and 32 across participating centers. Baseline patient characteristics are shown in Table 1, and Supplementary Table 1 shows those patients who adhered to footwear use. For those patients lost to follow-up, causes of death and reasons for withdrawal were not related to the study intervention. Of all planned 3-month follow-up visits, 97% took place. In a random sample of 74 patients surveyed at 18 months or at ulcer recurrence for success of patient blinding, 71 did not remember the existence of two study groups or to which study group they were allocated. There was no effect of sex or ethnicity on the primary and secondary outcomes.
Figure 1.
Study flow diagram.
Table 1.
Patient baseline characteristics (n = 171)
In-shoe pressures and footwear modifications
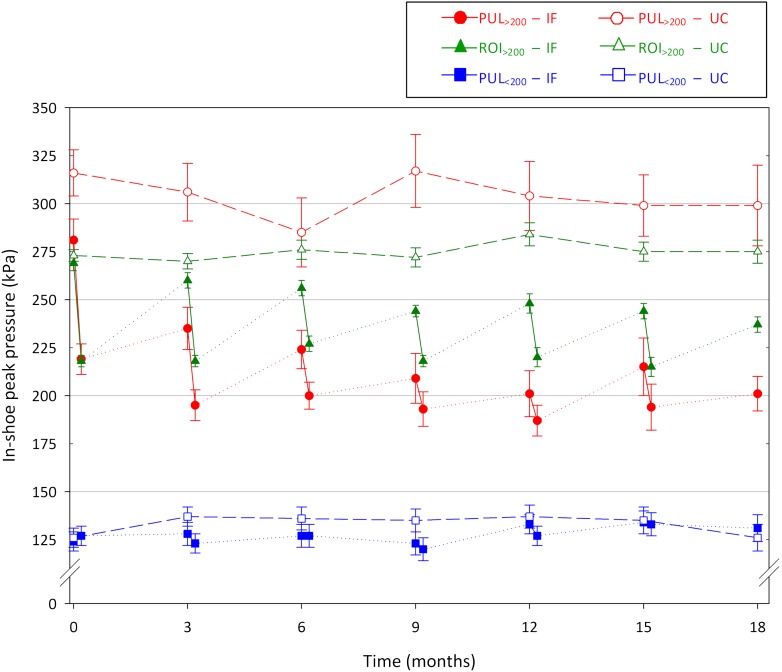
At footwear delivery and over time, in-shoe peak pressures were significantly lower in the improved footwear group after modifying the footwear than in the usual care group in regions with peak pressure >200 kPa (Fig. 2 and Table 2). No time or group–time interaction effects were found. A total of 1,183 footwear modifications in a mean 1.2 rounds of modifications per shoe pair per visit per patient were made in the improved footwear group. Between study visits, the footwear of the improved footwear group was not modified, whereas a total of 33 modifications were made to the footwear of 20 of the 86 usual care group patients following normal clinical practice.
Figure 2.
Mean in-shoe peak pressures over 18 months of follow-up for all previous ulcer locations with peak pressure at footwear delivery >200 kPa (red), all previous ulcer locations with peak pressure <200 kPa (blue), and all regions of interest with peak pressure >200 kPa (green) for both the improved footwear (closed symbols) and the usual care (open symbols) groups. Changes in peak pressure at each follow-up in the improved footwear group are pressure changes after footwear modification. Error bars represent SEMs. IF, improved footwear; PUL, previous ulcer location; ROI, region of interest; UC, usual care.
Table 2.
Clinical and biomechanical outcomes
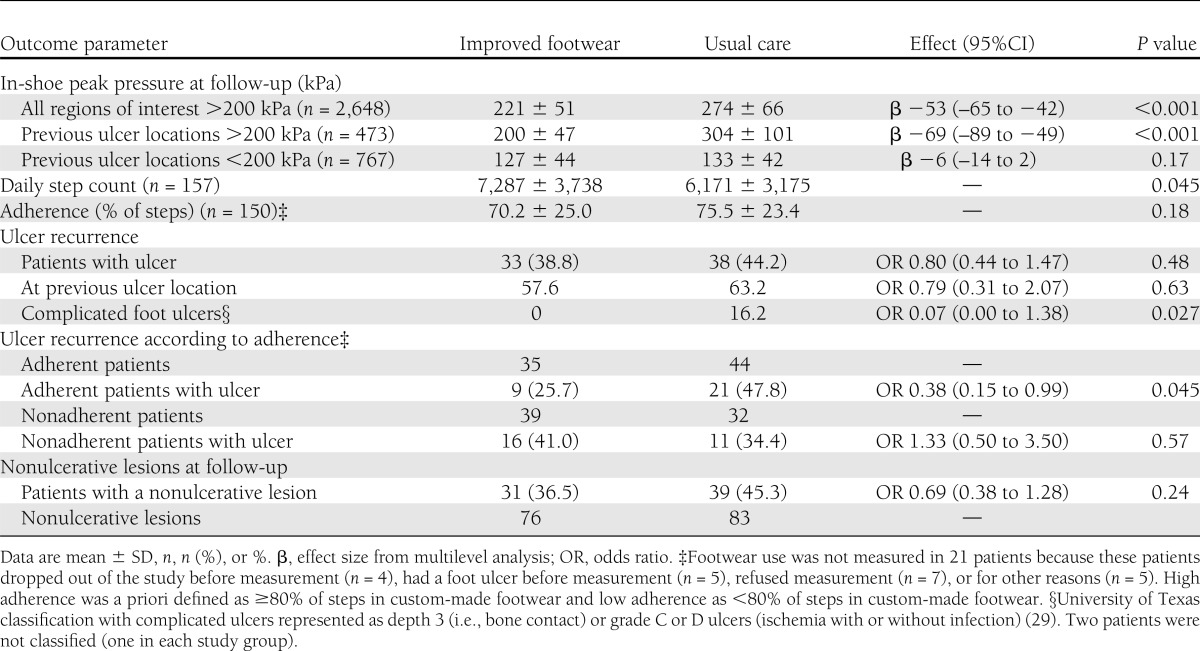
Ulcer recurrence
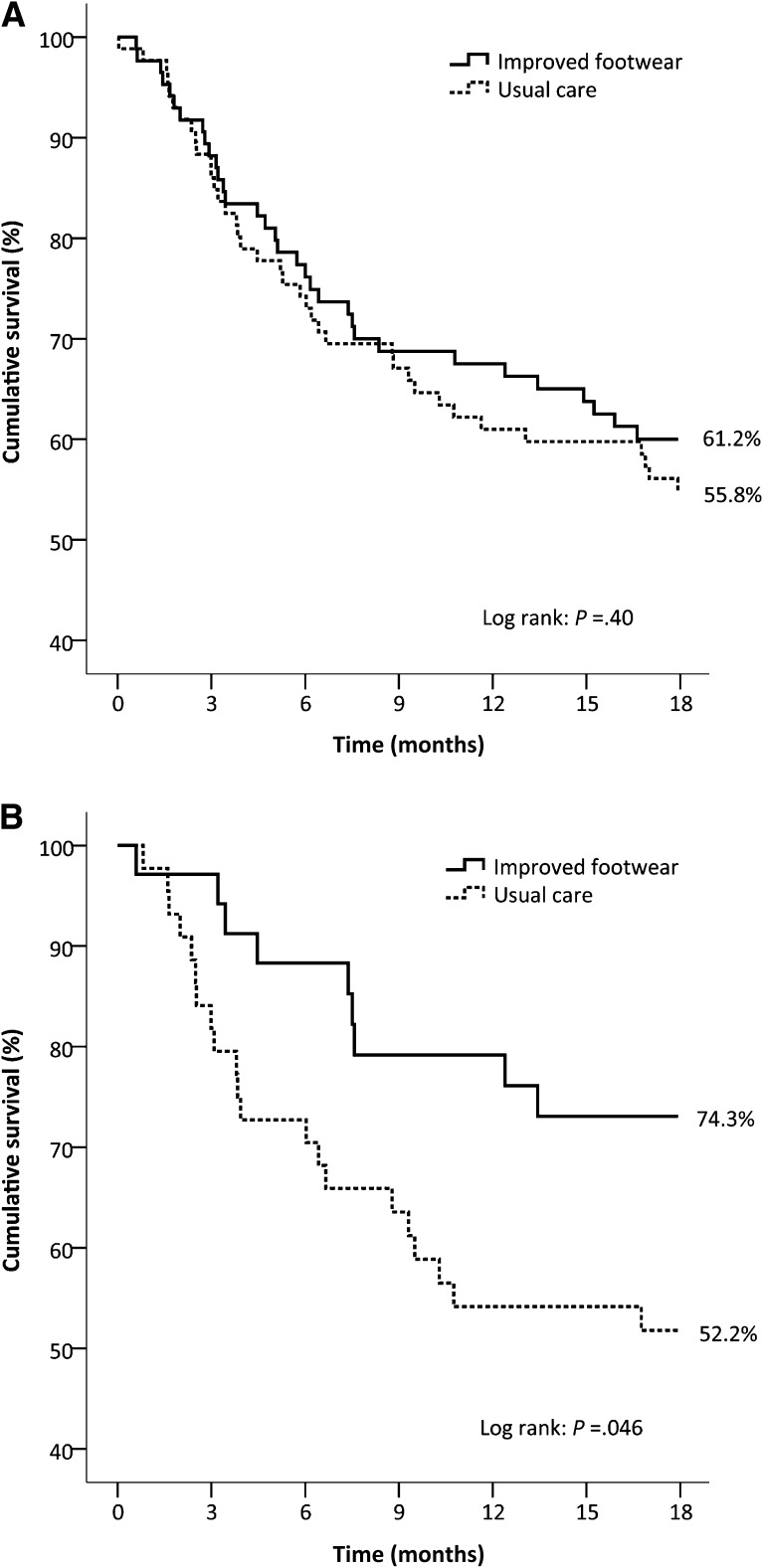
Seventy-one patients (42% of the total group) had a recurrent plantar foot ulcer in 18 months (Table 2). In the improved footwear group, 38.8% of patients had a recurrent ulcer, which was not significantly different from the 44.2% recurrence in the usual care group (relative risk reduction 11%, odds ratio 0.80 [95% CI 0.44–1.47], P = 0.48). Ulcer survival curves were also not significantly different between study groups (P = 0.40) (Fig. 3). The improved footwear group showed significantly fewer complicated foot ulcers (i.e., Texas depth 3 or grade C and D ulcers) than the usual care group.
Figure 3.
Kaplan-Meier plots on cumulative survival of plantar foot ulcer recurrence over 18 months of follow-up with censored data for patients who died. A: Intention-to-treat (n = 171). B: Patients who adhered to wearing custom-made footwear (i.e., ≥80% of steps taken in custom-made footwear) (n = 79).
Seventy-nine patients, 46% of the total group, adhered to wearing their custom-made footwear. In this subgroup, 25.7% of patients with improved footwear had a recurrent ulcer (Table 2), which was significantly lower than the 47.8% of patients in the usual care group (relative risk reduction 46%, odds ratio 0.38 [95% CI 0.15–0.99], P = 0.045). Ulcer survival curves were also significantly different between study groups in favor of the improved footwear group (P = 0.046) (Fig. 3).
Adverse events and nonulcerative lesions
Thirty serious adverse events occurred during follow-up (four deaths, 26 hospital admissions) and were equally divided between groups. None of these events could be related to the intervention. No significant group differences were present for nonulcerative lesions occurring during the study (Table 2). Of the 71 patients who had a recurrent foot ulcer, 29 (41%) had a nonulcerative plantar lesion at study entry compared with 17 of the 100 patients (17%) who did not reulcerate (odds ratio 3.4 [95% CI 1.7–6.8], P < 0.001).
CONCLUSIONS
Among patients with diabetes, peripheral neuropathy, and a recently healed plantar foot ulcer, offloading-improved custom-made footwear showed no statistically significant protective effect against plantar foot ulcer recurrence over custom-made footwear that did not undergo such improvement (usual care). This unexpected outcome shows that better offloading in protective footwear is by itself not clinically beneficial. The intention-to-treat analysis was slightly underpowered, but we do not expect that inclusion of the originally anticipated number of patients would have given different outcomes. To understand the lack of clinical success, we assessed the influence of footwear adherence, which was accurately measured by objective methods. In the subgroup of patients who were adherent, offloading-improved custom-made footwear significantly reduced plantar foot ulcer recurrence risk with 46% compared with nonimproved custom-made footwear, suggesting that improved offloading makes a clinically important difference when continuous pressure relief is guaranteed by wearing the custom-made footwear. Although such a positive effect should be confirmed in future trials, this outcome implies a reduced risk for infection and amputation, reduced treatment costs, and preserved patient quality of life (4).
The incidence of plantar foot ulcer recurrence (42%) was higher than found in other footwear trials, which suggests that we included patients who are more prone to develop recurrent foot ulcers. Reiber et al. (10) showed 15% recurrence in 2 years in patients wearing therapeutic footwear; however, many of these patients had foot sensation. These authors used a more conservative classification for ulceration and excluded moderate to severe foot deformity. These factors may explain the difference in recurrence rates with the present study. Rizzo et al. (11) reported a 12% ulcer occurrence in 12 months, including in patients with severe deformity. However, only 20% of the studied patients had a prior foot ulcer. All patients in the present study had a recently healed foot ulcer, which could leave the tissue more vulnerable for subsequent breakdown. This is indicated by the high prevalence of nonulcerative lesions at study entry in patients who had an ulcer recurrence and the quick drop in ulcer-free survival (Fig. 3). Uccioli et al. (9) found comparable recurrence rates to the present study, but we assessed only plantar foot ulcers, whereas others, including Uccioli et al., assessed all foot ulcers, regardless of location.
The primary goal of custom-made footwear is to protect the foot by reducing pressure at high-risk foot locations. Previous footwear trials failed to identify what role pressure relief plays in ulcer prevention because they did not measure pressure. The nonsignificant relative risk reduction of 11% found in the present study suggests that solely improving offloading to an ∼20% peak pressure difference compared with nonimproved footwear is insufficient to significantly reduce ulcer recurrence risk. As comparison, successful healing of plantar foot ulcers often occurs in devices that reduce peak pressure between 50 and 80% compared with a control condition (31), but such offloading effects seem unrealistic in custom-made footwear. More appropriate would be to target footwear adherence because the data suggest that moderate differences in offloading of ∼20% make a clinically and statistically important difference when footwear adherence is assured. These findings suggest that footwear effectiveness is a function of both offloading and adherence.
Preventive foot care should therefore focus on the combined improvement of footwear offloading and adherence. Footwear offloading can be improved under guidance of in-shoe pressure measurements or by using footwear design methods that are proven to be effective in relieving pressure (16,17,19,24), even though more systematic and evidence-based approaches to footwear design are still needed. To improve adherence, the provision of offloading footwear specifically for indoor use may be effective because adherence in high-risk diabetic patients is much lower when patients are at home than away from home (14). Reported factors for low adherence include low perceived esthetics, comfort, and therapeutic benefit of the shoes; higher BMI; and less severe foot deformity (12–14). To a certain extent, these factors can be managed. Suggestions made to improve adherence include 1) creating an acceptable style and color of footwear, 2) educating and motivating patients to wear their prescription footwear, and 3) introducing technology to alert patients when shoes are not worn (12–14). The effect of these interventions on adherence has yet to be investigated. The current data can help to convince patients of the therapeutic value of their prescription footwear. The relatively high prevalence of nonulcerative lesions found at study entry in patients who had a recurring ulcer also suggests that early recognition and treatment of these lesions is important in preventive foot care.
In conclusion, the findings show that offloading-improved custom-made footwear does not significantly reduce the incidence of plantar foot ulcer recurrence in diabetic patients with high foot ulcer risk compared with custom-made footwear that does not undergo such improvement, unless it is worn as recommended. Although future trials should confirm the positive effect of continuously worn and adequately offloaded footwear, we recommend combined improvement of footwear offloading and adherence to reduce the risk of plantar foot ulcer recurrence in high-risk diabetic patients.
Acknowledgments
The DIAbetic Foot Orthopedic Shoe (DIAFOS) trial was supported by project grants from the Dutch Diabetes Research Foundation (project 2007.00.067), the Dutch Foundation for the Development of Orthopedic Footwear, and the Dutch Organization for Health Research and Development (project 14350054).
No potential conflicts of interest relevant to this article were reported.
None of the sponsors had any involvement in the design and conduct of the study, analysis and interpretation of the data, and preparation, review, or approval of the manuscript.
S.A.B. designed the study and analysis plan, conducted the primary statistical analysis and data interpretation, wrote the manuscript, and gave final approval of the manuscript. R.W. and M.A. collected the data, conducted the primary data analysis, contributed to the statistical analysis and data interpretation, critically reviewed and edited the manuscript, and gave final approval of the manuscript. M.d.H. contributed to the study design and data analysis, critically reviewed and edited the manuscript, and gave final approval of the manuscript. T.B.-W. and J.v.B. contributed to the data collection, reviewed and edited the manuscript, and gave final approval of the manuscript. F.N. contributed to the study design and data interpretation, critically reviewed and edited the manuscript, and gave final approval of the manuscript. S.A.B. is the guarantor of the study and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The findings of the DIAFOS trial were previously presented at conference meetings of the International Foot and Ankle Biomechanics Society, Sydney, Australia, 11–13 April 2012; the Expert Scientific Community on Pressure Measurement, Aalborg, Denmark, 2–4 August 2012; and the Diabetic Foot Study Group of the European Association for the Study of Diabetes, Potsdam, Germany, 28–30 September 2012.
In the DIAFOS trial, the Academic Medical Center in Amsterdam collaborated with nine other hospitals and nine orthopedic footwear companies in the Netherlands. The authors acknowledge the contribution of R. Keukenkamp (Academic Medical Center, Amsterdam) in collecting data for the study and the following persons in recruiting patients and modifying footwear: P.J.A. Mooren (Academic Medical Center, Amsterdam); J.W.E. Verlouw, I. Ruijs, and H. van Wessel (Maxima Medical Centre, Veldhoven); J.P.J. Bakker and C. van den Eijnde (Medical Center Alkmaar); D. Wever and H. Wessendorf (Medisch Spectrum Twente, Enschede); R. Dahmen and B. Koomen (Slotervaart Hospital, Amsterdam); R. Haspels (Hospital group Twente, Almelo); J. Harlaar, V. de Groot, and J. Pulles (VU Medical Center, Amsterdam); W.P. Polomski, R. Lever, and G. du Mont (Spaarne Hospital, Hoofddorp); H.G.A. Hacking and J. de Bruin (St. Antonius Hospital, Nieuwegein); and H. Berendsen, W. Custers, and I. Paardekoper (Reinier de Graaf Gasthuis, Delft). Furthermore, the authors acknowledge the contribution of R.P. Michels, H.A. Manning, C.E.V.B. Hazenberg, E.J. Peters, and N.C. Schaper in assessing the primary outcome in the study and the members of the trial steering committee (N.C. Schaper, F. Elferink, and A.L. de Lange) for valuable advice.
Footnotes
Clinical trial reg. no. NTR1091, www.trialregister.nl.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0996/-/DC1.
References
- 1.International Diabetes Federation Time to Act: Diabetes and Foot Care. Brussels, International Diabetes Federation, 2005 [Google Scholar]
- 2.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990;13:513–521 [DOI] [PubMed] [Google Scholar]
- 3.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217–228 [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 5.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000;23:606–611 [DOI] [PubMed] [Google Scholar]
- 6.Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007;50:18–25 [DOI] [PubMed] [Google Scholar]
- 7.Bus SA, Valk GD, van Deursen RW, et al. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev 2008;24(Suppl. 1):S162–S180 [DOI] [PubMed] [Google Scholar]
- 8.Pound N, Chipchase S, Treece K, Game F, Jeffcoate W. Ulcer-free survival following management of foot ulcers in diabetes. Diabet Med 2005;22:1306–1309 [DOI] [PubMed] [Google Scholar]
- 9.Uccioli L, Faglia E, Monticone G, et al. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care 1995;18:1376–1378 [DOI] [PubMed] [Google Scholar]
- 10.Reiber GE, Smith DG, Wallace C, et al. Effect of therapeutic footwear on foot reulceration in patients with diabetes: a randomized controlled trial. JAMA 2002;287:2552–2558 [DOI] [PubMed] [Google Scholar]
- 11.Rizzo L, Tedeschi A, Fallani E, et al. Custom-made orthesis and shoes in a structured follow-up program reduces the incidence of neuropathic ulcers in high-risk diabetic foot patients. Int J Low Extrem Wounds 2012;11:59–64 [DOI] [PubMed] [Google Scholar]
- 12.Knowles EA, Boulton AJ. Do people with diabetes wear their prescribed footwear? Diabet Med 1996;13:1064–1068 [DOI] [PubMed] [Google Scholar]
- 13.Macfarlane DJ, Jensen JL. Factors in diabetic footwear compliance. J Am Podiatr Med Assoc 2003;93:485–491 [DOI] [PubMed] [Google Scholar]
- 14.Waaijman R, Keukenkamp R, de Haart M, Polomski WP, Nollet F, Bus SA. Adherence to wearing prescription custom-made footwear in patients with diabetes at high risk for plantar foot ulceration. Diabetes Care 2013;36:1613–1618 [DOI] [PMC free article] [PubMed]
- 15.Crawford F. How can we best prevent new foot ulcers in people with diabetes? BMJ 2008;337:a1234. [DOI] [PubMed] [Google Scholar]
- 16.Bus SA, Ulbrecht JS, Cavanagh PR. Pressure relief and load redistribution by custom-made insoles in diabetic patients with neuropathy and foot deformity. Clin Biomech (Bristol, Avon) 2004;19:629–638 [DOI] [PubMed] [Google Scholar]
- 17.Guldemond NA, Leffers P, Schaper NC, et al. The effects of insole configurations on forefoot plantar pressure and walking convenience in diabetic patients with neuropathic feet. Clin Biomech (Bristol, Avon) 2007;22:81–87 [DOI] [PubMed] [Google Scholar]
- 18.Arts ML, Waaijman R, de Haart M, Keukenkamp R, Nollet F, Bus SA. Offloading effect of therapeutic footwear in patients with diabetic neuropathy at high risk for plantar foot ulceration. Diabet Med 2012;29:1534–1541 [DOI] [PubMed] [Google Scholar]
- 19.Bus SA, Haspels R, Busch-Westbroek TE. Evaluation and optimization of therapeutic footwear for neuropathic diabetic foot patients using in-shoe plantar pressure analysis. Diabetes Care 2011;34:1595–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahmen R, van der Wilden GJ, Lankhorst GJ, Boers M. Delphi process yielded consensus on terminology and research agenda for therapeutic footwear for neuropathic foot. J Clin Epidemiol 2008;61:819–826 [DOI] [PubMed] [Google Scholar]
- 21.Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20(Suppl. 1):S90–S95 [DOI] [PubMed] [Google Scholar]
- 22.Bus SA, de Lange A. A comparison of the 1-step, 2-step, and 3-step protocols for obtaining barefoot plantar pressure data in the diabetic neuropathic foot. Clin Biomech (Bristol, Avon) 2005;20:892–899 [DOI] [PubMed] [Google Scholar]
- 23.Arts ML, Bus SA. Twelve steps per foot are recommended for valid and reliable in-shoe plantar pressure data in neuropathic diabetic patients wearing custom made footwear. Clin Biomech (Bristol, Avon) 2011;26:880–884 [DOI] [PubMed] [Google Scholar]
- 24.Waaijman R, Arts ML, Haspels R, Busch-Westbroek TE, Nollet F, Bus SA. Pressure-reduction and preservation in custom-made footwear of patients with diabetes and a history of plantar ulceration. Diabet Med 2012;29:1542–1549 [DOI] [PubMed] [Google Scholar]
- 25.Owings TM, Apelqvist J, Stenström A, et al. Plantar pressures in diabetic patients with foot ulcers which have remained healed. Diabet Med 2009;26:1141–1146 [DOI] [PubMed] [Google Scholar]
- 26.Bus SA, Waaijman R, Nollet F. New monitoring technology to objectively assess adherence to prescribed footwear and assistive devices during ambulatory activity. Arch Phys Med Rehabil 2012;93:2075–2079 [DOI] [PubMed] [Google Scholar]
- 27.Coleman KL, Smith DG, Boone DA, Joseph AW, del Aguila MA. Step activity monitor: long-term, continuous recording of ambulatory function. J Rehabil Res Dev 1999;36:8–18 [PubMed] [Google Scholar]
- 28.Bakker K, Apelqvist J, Schaper NC, International Working Group on Diabetic Foot Editorial Board Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev 2012;28(Suppl. 1):225–231 [DOI] [PubMed] [Google Scholar]
- 29.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998;21:855–859 [DOI] [PubMed] [Google Scholar]
- 30.Chantelau E, Haage P. An audit of cushioned diabetic footwear: relation to patient compliance. Diabet Med 1994;11:114–116 [DOI] [PubMed] [Google Scholar]
- 31.Cavanagh PR, Bus SA. Off-loading the diabetic foot for ulcer prevention and healing. J Vasc Surg 2010;52(Suppl.):37S–43S [DOI] [PubMed] [Google Scholar]