Abstract
OBJECTIVE
To investigate the effect of biliopancreatic diversion (BPD) surgery on β-cell function in grade I and II obese patients with type 2 diabetes using oral and intravenous glucose loads.
RESEARCH DESIGN AND METHODS
Sixty-eight women were divided into the following three groups: 19 lean-control (23.0 ± 2.2 kg/m2) and 18 obese-control (35.0 ± 4.8 kg/m2) subjects with normal glucose tolerance, and 31 obese patients with type 2 diabetes (36.3 ± 3.7 kg/m2). Of the 31 diabetic women, 64% underwent BPD (n = 20, BMI: 36.5 ± 3.7 kg/m2) and were reassessed 1 month after surgery. Oral glucose tolerance tests and hyperglycemic clamps were performed. Mathematical modeling was used to analyze basal and stimulated β-cell function, insulin sensitivity (IS), hepatic extraction (HE) of insulin, and delay time of β-cell response to a specific plasma glucose concentration.
RESULTS
After BPD, restoration of the basal disposition index (P < 0.001) and improvement of the stimulated disposition indices in oral and intravenous glucose stimulation of the β-cell were observed (P < 0.05). In both dynamic tests, there were no changes in the delay time of β-cell response. IS for oral glucose stimulation (ISoral) and intravenous clamp glucose stimulation (ISclamp) was completely normalized (P < 0.001). ISoral and ISclamp increased approximately 5.0-fold and 3.5-fold, respectively (P < 0.01). The HE of insulin increased in the basal (P < 0.05) and stimulated states (P < 0.01).
CONCLUSIONS
β-Cell function, IS, and HE of insulin improved after BPD, which improved glycemic control.
Type 2 diabetes is a complex metabolic disease that results from two main pathophysiological defects: impaired insulin sensitivity (IS) and β-cell failure (1). In a small proportion of obese individuals with type 2 diabetes, conventional medical therapy is effective to maintain adequate blood glucose control. However, significant, long-term weight reduction, which may help control type 2 diabetes, is often difficult to attain in clinical practice (2).
In 1987, Pories and colleagues (3) published an unexpected finding in which 99% of morbidly obese patients with type 2 diabetes or prediabetes who underwent gastric bypass rapidly restored euglycemia although they were still morbidly obese. Despite the diagnostic bias of type 2 diabetes that leads to an overestimation of remission, this study has significant historical value and is considered a former benchmark. According to several subsequent studies, bariatric surgery has become an alternative therapeutic strategy for morbidly obese patients with poorly controlled type 2 diabetes (4).
According to a recent meta-analysis of bariatric surgery, 78.1% of patients with type 2 diabetes had complete remission of the disease, and 86.6% of patients showed improvement after surgery. Weight loss and type 2 diabetes remission were highest in patients undergoing biliopancreatic diversion (BPD) compared with other techniques (3).
The mechanism of type 2 diabetes remission after BPD is not completely understood. Some studies have demonstrated a dramatic improvement in IS after BPD (5–11). There are only a few, disparate studies on changes in β-cell function with BPD. Unlike IS, there is no gold standard method to assess β-cell function because insulin secretion differs depending on the stimulus (oral vs. intravenous). In addition, peripheral insulin concentrations do not accurately reflect pancreatic insulin secretion because the hepatic extraction (HE) of insulin rate varies significantly under different metabolic conditions. To circumvent these difficulties, the C-peptide measurements may be used because this peptide is cosecreted with insulin in equimolar concentrations. C-peptide measurements and mathematical modeling methods provide a more accurate characterization of β-cell function (12).
The studies that assessed β-cell function in type 2 diabetic patients after BPD included grade III obese patients and applied either the classical plasma insulin measurement method (5,8,10) or the plasma C-peptide measurement modeling method (6,7,9,13). There is only one study that assessed β-cell function after BPD in overweight and obese grade I patients with type 2 diabetes using insulin measurements (11).
To provide additional evidence for the underlying pathophysiological mechanisms associated with type 2 diabetes remission after BPD, we assessed grade I and II obese type 2 diabetes patients 1 month after BPD (before significant weight loss) to determine β-cell function, IS, HE of insulin, and delay time using oral and intravenous glucose.
RESEARCH DESIGN AND METHODS
Subjects
The current study was performed with 68 premenopausal women divided into three groups according to their BMI and glucose tolerance level, as follows: lean, normal glucose tolerant (LeanNGT; n = 19; BMI: 23.0 ± 2.2 kg/m2); obese, normal glucose tolerant (ObeseNGT; n = 18; BMI: 35.0 ± 4.8 kg/m2); and obese with overt type 2 diabetes (ObeseT2DM; n = 31; BMI: 36.3 ± 3.7 kg/m2). Of the 31 ObeseT2DM subjects, 20 underwent the BPD surgery. The surgical group was studied at baseline and 1 month postsurgery. Diabetes was diagnosed according to the American Diabetes Association criteria (14). In the ObeseT2DM group, the mean duration of diabetes was 4.7 ± 4.5 years. Of the patients who underwent the BPD surgery, 12 were treated exclusively with metformin, 7 were treated with metformin and sulfonylureas, and 1 was treated with diet and exercise.
The inclusion criteria were age >20 years, premenopause, and negative islet autoimmunity. The exclusion criteria were use of incretin mimetics, dipeptidyl peptidase-4 inhibitors, or insulin; significant kidney or liver dysfunction; recent neoplasia (<5 years); and use of oral or injectable corticosteroids for >14 consecutive days in the last 3 months.
This study was approved by the Ethics Committee of the State University of Campinas. All participants provided written informed consent before participation.
BPD surgery
All of the procedures in this study were performed by the same surgical team. BPD promotes a permanent and selective maldigestion and malabsorption of energy-rich nutrients, specifically fat and protein, by displacing digestive juices and rerouting the food transit in the small gut. The original technique reduces the total length of intestinal absorption to 250 cm with a 50-cm common channel, increasing malnutrition risk (15), particularly in less obese individuals.
This study uses an adapted BPD technique to avoid nutritional complications. The BPD is performed with an ∼60% distal gastric resection and a long Roux-en-Y reconstruction. The volume of the stomach after surgery is ∼300 mL. The small bowel is transected at 2.8–3.2 m from the ileocecal valve, and its distal end is anastomosed to the remaining stomach. The proximal end of the ileum, comprising the remaining small bowel (involved in carrying biliopancreatic juice but excluded from food transit), is anastomosed to the bowel in an end-to-side fashion 80–120 cm proximal to the ileocecal valve. Consequently, the total length of absorbing bowel is reduced to 280–320 cm, of which the final 80–120 cm, the common channel, is where biliopancreatic juices and ingested food mix.
Anthropometrical and body composition assessment
BMI was calculated. The waist circumference was measured at the umbilical level. The amounts of body fat and fat-free mass were determined using a bioimpedance analyzer (model BIA 310) according to the manufacturer’s protocol.
Dynamic tests for β-cell function and IS
To assess different aspects of the complex β-cell response to different stimuli, we performed oral and intravenous glucose tests. Oral hypoglycemic drugs were discontinued 24–48 h before the dynamic tests.
The oral stimulus was an oral glucose tolerance test (OGTT); at 8 a.m., after a 12-h overnight fast, an intravenous catheter was placed into the antecubital vein. At time 0, the subjects ingested a 75-g glucose load. Blood samples were collected at −30, −15, 0, 10, 20, 30, 60, 90, 120, 180, and 240 min to measure glucose, insulin, and C-peptide concentrations (16).
The intravenous stimulus was a hyperglycemic clamp test; at 8 a.m., after a 12-h overnight fast, a cannula was retrogradely inserted into a peripheral hand vein and kept patent by a constant saline infusion. The hand was kept warm in a hot box maintained between 50 and 60°C for blood arterialization. Glucose was infused into an antecubital vein in the opposite arm and divided into the following two phases: the “first dose,” which included a sufficient amount of glucose to increase the blood glucose levels to the desired plateau (180 mg/dL); and the “maintenance dose,” which was calculated every 5 min during the test and was dependent on blood glucose measurements. Blood samples were obtained every 2.5 min during the first 10 min of glucose infusion and then every 5 min up to 180 min. Glucose levels were measured in all blood samples. Insulin and C-peptide levels were measured at the same time as glucose under basal conditions, and for the first 20 min of infusion and every 20 min thereafter (17).
The homeostasis model assessment (HOMA)-insulin resistance (IR) index, a measure of fasting hepatic IR, was calculated as follows: ([glucose] [times] [insulin])/22.5 (18).
Minimal model indexes
For the OGTT, the oral C-peptide minimal model was used to derive the following β-cell responsivity (Φ) indices: basal Φ is nonstimulated; dynamic Φ (Φd) refers to multiple distal steps of insulin secretion; static Φ (Φs) refers to earlier steps of insulin secretion; and total Φ (Φoral) is the overall response calculated from Φd and Φs (16). The oral glucose minimal model was used to derive IS (ISoral) (19). The basal, dynamic, static, and total disposition indexes (DIb, DId, DIs, and DIoral, respectively), representing β-cell function adjusted to IS, were calculated by multiplying β-cell responsivity indices by IS.
For the hyperglycemic clamp test, the C-peptide minimal model was used to obtain the following β-cell responsivity indices: Φd refers to the first phase of insulin secretion; Φs refers to the second phase of insulin secretion; and total Φ (Φclamp) is the overall response calculated from Φd and Φs (16). The glucose minimal model was used to obtain IS (ISclamp) (20). The disposition indices (DId, DIs, and DIclamp) were then calculated by multiplying β-cell responsivity indices by IS.
The basal HE (HEb) of insulin and the stimulated HEs of insulin during both dynamic tests (i.e., HEoral and HEclamp) were calculated from plasma C-peptide and insulin measurements (12).
Under normal physiological conditions, β-cells respond to acute, intravenous glucose stimulation with biphasic insulin secretion. The readily releasable pool of insulin granules contributes to the first phase of insulin secretion. When this pool is depleted, new granules translocate to the cell membrane to produce the second phase of insulin secretion. The time required to recruit new insulin granules to form the pool of readily releasable granules in response to increased glycemia is called the delay time (12). The C-peptide minimal model was used to calculate the delay time during the OGTT and the hyperglycemic clamp test (12).
Assays
Plasma glucose levels were promptly measured in the fasting state and during the dynamic tests using a glucose analyzer (YSI 2700; YSI Life Sciences, Yellow Springs, OH) with a coefficient of variation of 2%. The glycated hemoglobin was measured with high-performance liquid chromatography. Plasma insulin and C-peptide levels were analyzed using an automated two-site chemiluminescent immunometric assay (Immulite 1000 System; Siemens Health Diagnostics). The intra-assay and interassay coefficients of variation were 5.2–6.4% and 5.9–8.0%, respectively, for insulin, and 1.9–3.3% and 3.8–5.5%, respectively, for C-peptide. Adiponectin levels were measured by an ELISA (Linco Research), and all had coefficients of variation below 10%.
Statistical analysis
Statistical analyses were performed using IBM SPSS-Statistics version 20.0. The data are presented as the mean ± SD for normally distributed data and as median (interquartile range) for nonparametric data, according to the Shapiro-Wilk test. The Kruskal-Wallis test was used to compare three groups (LeanNGT vs. ObeseNGT vs. ObeseT2DM presurgery and LeanNGT vs. ObeseNGT vs. ObeseT2DM postsurgery). A post hoc analysis with a Duncan multiple range test was used to show which groups differ from the other groups. The Wilcoxon signed rank test was used to compare the ObeseT2DM group before surgery versus after surgery. Significance was set at P < 0.05.
RESULTS
Clinical and metabolic characteristics at baseline and postsurgery
Interestingly, even with the same grade of obesity (BMI and body fat percentage), patients within the ObeseT2DM group had larger waist circumferences than those within the ObeseNGT group (P < 0.001), indicating increased central fat distribution. The HOMA-IR index was significantly increased in the ObeseT2DM group compared with the LeanNGT and ObeseNGT groups (P < 0.01). Plasma insulin and adiponectin levels were similar between the ObeseNGT and ObeseT2DM groups, which were different from those of the LeanNGT group (Table 1).
Table 1.
Clinical and metabolic characteristics of the study subjects at baseline and 1 month after BPD surgery
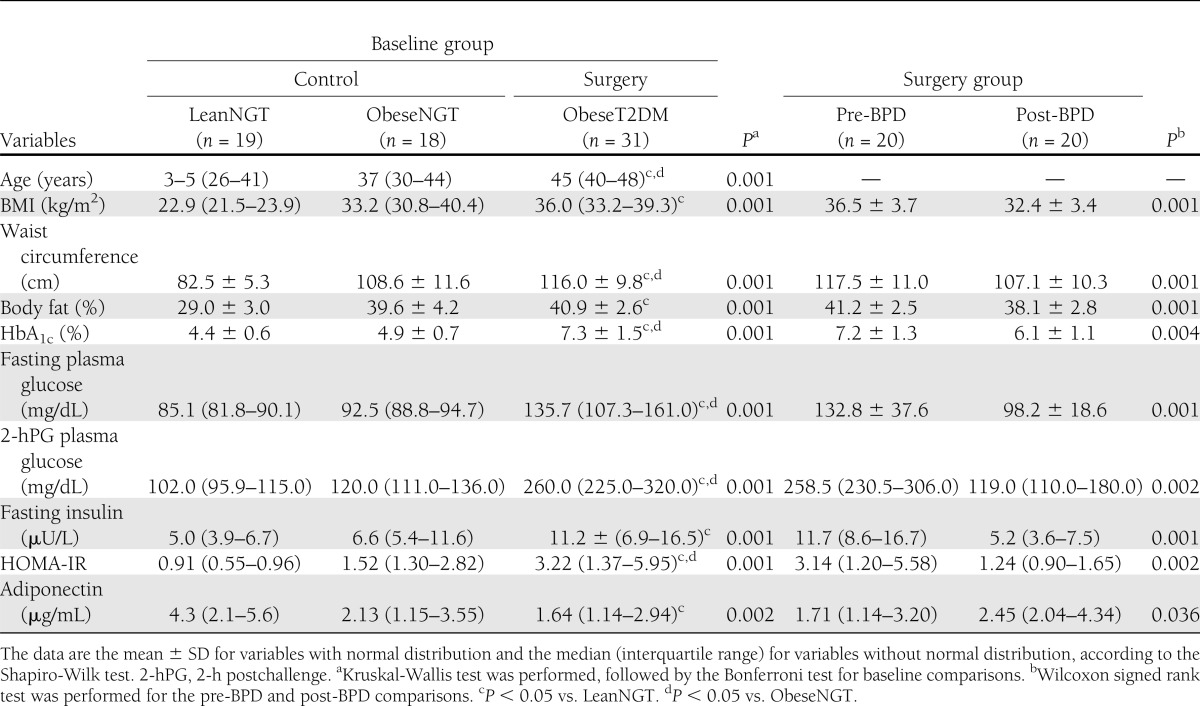
All patients from the surgical group were studied 1 month after the BPD. BMI, waist circumference, and body fat percentage significantly decreased (P < 0.01). HbA1c levels, fasting glycemia levels, glycemia 2-h postchallenge levels, HOMA-IR index, and plasma insulin levels were reduced, and adiponectin levels were increased (P < 0.05) (Table 1).
Antidiabetes treatment continued after surgery in two patients: one received metformin, and the other received a combination of metformin and a sulfonylurea. The fasting glycemia (P < 0.001) and glycemia 2-h postchallenge (P < 0.01) levels were significantly improved after surgery. Twelve patients had normal fasting plasma glucose levels (<100 mg/dL), 3 patients were in the intermediate range (100–126 mg/dL), and 5 patients had elevated levels (>126 mg/dL). According to the OGTT, the plasma glucose levels 2 h postchallenge were adequate in 14 patients (<140 mg/dL), impaired in 5 patients (140–200 mg/dL), and elevated in one patient (>200 mg/dL).
Acute effect of BPD on IS, HE of insulin, β-cell function, and delay time
IS.
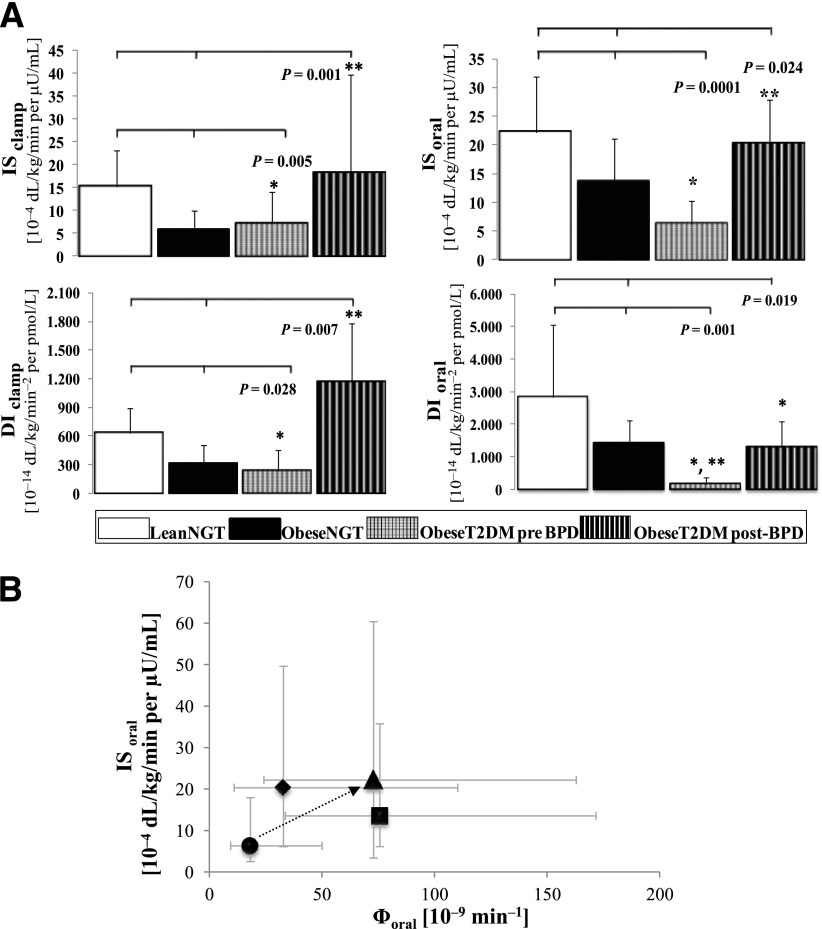
At baseline, the ObeseT2DM group had reduced IS compared with the LeanNGT group and had similar IS compared with the ObeseNGT group (Fig. 1A and Table 2).
Figure 1.
A: Comparison of the IS and the DIs obtained from the oral and the intravenous mathematical minimal models in the pre-BPD and post-BPD groups and the two control groups. B: Relationship between IS and overall β-cell responsivity during the OGTT in the pre-BPD and post-BPD groups and in the two control groups. A: Bars represent the median and semi-interquartile range. The Kruskal-Wallis test and Duncan post hoc test were used. *P < 0.05 vs. LeanNGT; **P < 0.05 vs. ObeseNGT. B: Data are presented as the median and interquartile range. Black circle, ObeseT2DM pre-BPD group; black triangle, ObeseT2DM post-BPD group; black square, ObeseNGT group; black diamond, LeanNGT group.
Table 2.
Comparison of the IS, HE of insulin, β-cell responsivity indexes, and DIs obtained from the oral and the intravenous mathematical minimal models among the three study groups, pre-BPD and post-BPD
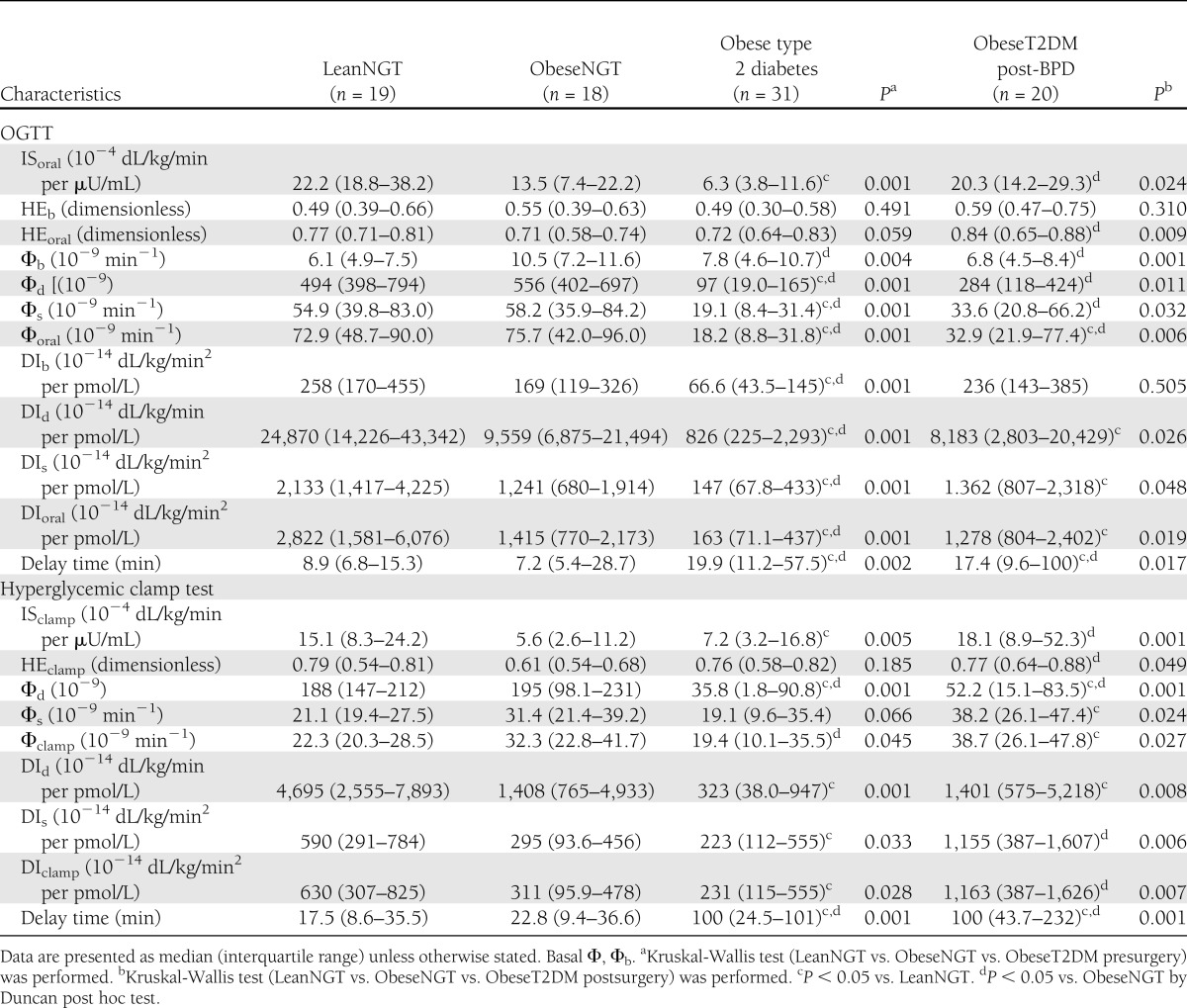
After surgery, ISoral increased approximately 5.0-fold, and ISclamp increased approximately 3.5-fold (P < 0.01) (Fig. 2). After surgery, the IS of the ObeseT2DM group was comparable to that of the LeanNGT group and higher than that of the ObeseNGT group in both the oral and intravenous tests (Fig. 1A and Table 2).
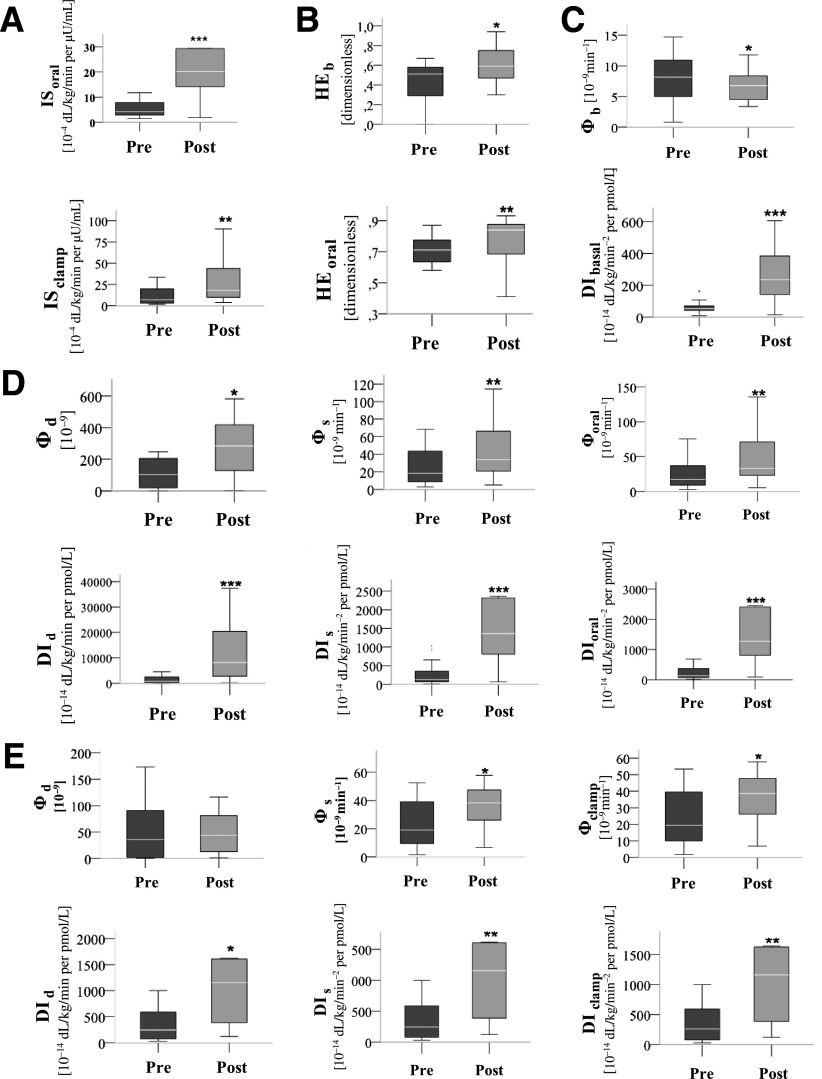
Figure 2.
Effect of BPD on IS (A); basal and orally stimulated HE of insulin (B); basal β-cell function (C); the dynamic, static, and total β-cell functional parameters from the OGTT (D); and the hyperglycemic clamp test (E). *P < 0.05; **P < 0.01; ***P < 0.001. Wilcoxon signed rank test for related samples, considering only the patients who underwent the BPD procedure (n = 20).
HE of insulin.
The preoperative basal and stimulated HE of insulin did not differ among the three study groups in both dynamic tests (Table 2).
The postoperative HEb, HEoral (Fig. 2), and HEclamp (data not shown) improved in the ObeseT2DM group (P < 0.05).
The postsurgical HEb remained similar among all groups, whereas the postsurgical HEoral and HEclamp were significantly higher in the ObeseT2DM group than in the ObeseNGT group. There was no observed difference between the postsurgical ObeseT2DM group and the LeanNGT group (Table 2).
β-Cell function.
IS measurements must be used to accurately determine β-cell function. The DI adjusts β-cell secretion measurements to IS to calculate β-cell function with the oral and intravenous minimal models. The following β-cell function results are reported using the DI.
Basal insulin secretion.
At baseline, the DIb was significantly diminished in the ObeseT2DM group compared with the control groups (Table 2). After surgery, the DIb increased significantly (Fig. 2), reaching a level similar to that of both control groups (Table 2).
Dynamic and first phase of insulin secretion.
At baseline, DId (from OGTT) was diminished in the ObeseDMT2 group compared with both control groups; the ObeseT2DM group DId (from hyperglycemic clamp) was reduced compared with that of the LeanNGT group and was similar to that of the ObeseNGT group (Table 2). The postoperative DId increased in both tests (Fig. 2). The post-BPD DId (from OGTT) of the ObeseT2DM group increased to levels similar to that of the ObeseNGT group but remained smaller than that of the LeanNGT group. No improvement was observed for the DId (from hyperglycemic clamp) after surgery (Table 2).
Static and second phase of insulin secretion.
At baseline, the DIs (from OGTT) was significantly reduced in the ObeseT2DM group compared with that of the control subjects; the DIs (from hyperglycemic clamp) in the ObeseT2DM group was reduced compared with that of the LeanNGT group and similar to that of the ObeseNGT group (Table 2). The postoperative DIs improved in the oral and intravenous tests (Fig. 2). The post-BPD ObeseT2DM DIs (from OGTT) was similar to that of the ObeseNGT group and remained lower than that of LeanNGT group; however, the DIs (from hyperglycemic clamp) was similar to that of the LeanNGT group and higher than that of the ObeseNGT group (Table 2).
Overall responsivity.
At baseline, the DIoral of the ObeseT2DM group was diminished compared with those of the control groups, and the DIclamp was similar to that of the ObeseNGT group but less than that of the LeanNGT group (Fig. 1A and Table 2). The postoperative DIs improved substantially (Fig. 2). Post-BPD, the DIoral of the ObeseT2DM group was similar to that of the ObeseNGT group and remained lower than that of the LeanNGT group; however, the DIclamp of the ObeseT2DM group was similar to that of the LeanNGT group and higher than that of the ObeseNGT group (Fig. 1A and Table 2).
When IS was assessed with the Φoral (Fig. 1B), BPD was associated with concomitant improvements in both parameters, reflecting the acute effect of BPD on glucose tolerance.
Delay time.
At baseline, the delay time was markedly increased in the ObeseT2DM group compared with the control subjects (Table 2).
The postoperative delay time did not change after BPD in the OGTT (pre-BPD 20 min [11–58 min] vs. post-BPD 17 min [9–100 min]; P = 0.795) or the hyperglycemic clamp (pre-BPD 100 min [25–101 min] vs. post-BPD 100 min [44–232 min]; P = 0.124).
Consequently, no improvement in the delay time was observed postsurgery (Table 2).
CONCLUSIONS
This is the first study that investigates the effect of BPD in grade I and II obese subjects with type 2 diabetes using accurate methods to characterize the major aspects of the β-cell function in detail. The data show the following: 1) normalization of IS; 2) increases in basal and stimulated HE of insulin; 3) improvement in basal and stimulated β-cell function with both oral and intravenous glucose loads; and 4) lack of improvement in the delay time of β-cell response during oral and intravenous glucose loads.
The acute improvement of IS 1 month after surgery is in accordance with previous reports that analyzed BPD in obese patients with type 2 diabetes using the fasting index of IS (HOMA-IR) (8,10,11), the stimulated indexes of whole-body IS from the OGTT (7), and the hyperinsulinemic-euglycemic clamp (6,7,9). The acute normalization of IS after BPD may be partially independent of weight loss. Few studies that used the hyperinsulinemic-euglycemic clamp to directly measure IS have shown significant results (6,21,22). Guidone et al. (6) demonstrated that most of the long-term improvement was achieved within 7–10 days after BPD, before significant weight loss, which is partially explained by caloric restriction (23). However, after a very low-calorie diet, a selective improvement in hepatic IS without changes in whole-body IS was demonstrated (21,22). Furthermore, our group has shown that there were no improvements in the whole-body IS of premenopausal women 1 month after Roux-en-Y gastric bypass (22), which supports the hypothesis of differential mechanisms associated with different surgical techniques that was also demonstrated by our group in collaboration with a research group from Italy (24). The mechanisms of rapid improvement in IS have not been fully elucidated. Changes in nutrient absorption and sensing, especially in lipid malabsorption (6); intramyocellular lipid depletion (25); bile acid metabolism alterations (26); the synergy among gut microbiota modifications, intestinal permeability, and intestinal gluconeogenesis (27,28); and increased serum adiponectin (29) may be important in enhancing IS after BPD.
The liver plays a key role in modifying the circulating levels of insulin because a significant portion (∼50%) of secreted insulin undergoes HE. In the current study, the HE of insulin increased after BPD and exceeded the HE rates of the ObeseNGT group. Similar increases in HE were also demonstrated after weight loss with caloric restriction in obese patients with type 2 diabetes (30). Free fatty acids, which are elevated in obesity and diabetes, can impair insulin binding and degradation in isolated rat hepatocytes (31). Studies in animals (32) and humans demonstrated that the HE of insulin is reduced in the presence of obesity (33) and mild glucose intolerance (34). Glucose homeostasis is maintained with increased insulin secretion and/or decreased first-pass HE of insulin, which may provide a physiological mechanism to preserve pancreatic β-cell function.
β-Cell dysfunction is a sine qua non pathophysiological feature for the development of type 2 diabetes. In the current study, significant improvements of basal, stimulated, and overall β-cell function were demonstrated after BPD. Basal insulin secretion (DIbasal) was normalized 1 month after BPD compared with our control groups, which reflects an adaptation to the increased IS. The OGTT dynamic β-cell responsivity (DId) significantly improved and was restored to the ObeseNGT group levels; whereas the static responsivity (DIs), related to the second phase of insulin secretion, and overall β-cell responsivity (DIclamp) had larger improvements with the clamp and were restored to the LeanNGT group levels. Studies using the C-peptide modeling approach with an oral and intravenous glucose load in grade III obese patients with type 2 diabetes after BPD surgery have shown significant improvement in β-cell glucose sensitivity after 7 days (6), fully normalized first phase of insulin secretion after 1 month (9), and a tendency for improvement in β-cell glucose sensitivity 7 days after BPD (7). Additional studies (5,8,10) have demonstrated improved acute insulin response 1 month after BPD.
BPD bypasses a wide area of the small intestine and allows nutrients to enter directly into the last part of the ileum. The improvement of β-cell function after BPD may be, in part, related to the modulation of the incretin effect caused by the rapid delivery of food to the distal parts of the gastrointestinal tract. A decreased glucagon-like peptide 1 (GLP-1) meal response has been associated with obesity, type 2 diabetes, or both, whereas an increase of GLP-1 has been reported in many studies early after BPD (6,35,36). In addition to the GLP-1 insulinotropic effect, higher GLP-1 concentrations should suppress glucagon release, possibly through direct activation of GLP-1 receptors on pancreatic α-cells. Although the precise mechanism is not entirely clear, the inhibitory effect of GLP-1 on glucagon secretion in vivo is only observed at glucose levels at or above fasting levels. Thereby, the glucagonostatic effect of GLP-1 may contribute to limit postprandial glucose excursions and improve glucose metabolism (37). In addition, the reduction in lipotoxicity, glucotoxicity (38), and the newly described aminotoxicity, which is due to a reduction in the levels of circulating branched-chain amino acids and aromatic amino acids, after bariatric surgery (39) may also contribute to the improvement of β-cell function and IS.
The unchanged delay time of β-cell insulin secretion response after a glucose load may be caused by a genetic defect that cannot be restored with surgery. The extent of β-cell function restoration appears to be dependent on the age of the patient, the severity and duration of diabetes, metabolic control, and IR. After bariatric surgery, the absence of diabetes remission and the reappearance of glucose intolerance could be related to a genetic susceptibility to dysglycemia, such as unknown β-cell defects (40). Long-term studies will determine the progress of delay time after BPD.
The impact of BPD on type 2 diabetes is huge, with excellent effects on the glucose homeostasis and with the highest and most durable degree of excess weight loss (4). On the other hand, the original BPD technique is used in a minority of obese patients with type 2 diabetes, because its nutritional risks are higher compared with the mainly restrictive procedures. In the current study, an adapted BPD technique was used in selected patients to reduce the long-term risks of nutritional complications.
The current study was limited because all of our subjects were premenopausal women; it did not include other currently approved techniques (Roux-en-Y gastric bypass and sleeve gastrectomy) in the comparison with the BPD group; and it did not assess renal insulin clearance, as much as the kidney is the other important site of insulin clearance, removing almost 50% of peripheral insulin. Furthermore, the hyperinsulinemic state obtained during the hyperglycemic clamp did not completely suppress hepatic glucose output, continuing with hepatic production that contributed ∼5% of the total glucose amount during the test. So, the IS value does underestimate peripheral IS in insulin-resistant states. To accurately measure hepatic glucose production, it is necessary to use glucose tracer methods, which are costly and time-consuming. Unfortunately, the glucose tracer method was not available in the current study.
In summary, the current study shows several acute positive physiological adaptations after BPD surgery in patients with grade I and II obesity and type 2 diabetes, such as restoration of IS, improvement in the HE of insulin, and amelioration in static and dynamic β-cell functions. These adaptations contribute to the observed acute improvement in glycemic control. The lack of improvement in the delay time of insulin secretion highlights a characteristic of type 2 diabetes that may be irreversible and recidivistic. A comprehensive approach using BPD may assist clinicians in defining the role of the gut in the pathophysiology of type 2 diabetes, and in developing new clinical and surgical therapeutic agents to treat this disease.
Acknowledgments
This research was supported by grants 2008/09451-7 and 2008/07312-0 from the São Paulo Research Foundation. Complementary financial support was provided by Ethicon Endo-Surgery Group. No other potential conflicts of interest relevant to this article were reported.
A.C.J.V. participated in the study coordination, participant enrollment, nutrition oversight of participants, data collection, statistical analysis, data interpretation, and the composition of the article. J.C.P. participated in the conception and design of the study, study coordination, medical oversight of participants, and surgical operations. M.d.S.d.O. and F.S.N. participated in the participant enrollment, medical oversight of participants, and data collection. M.M.d.O.L. participated in the participant enrollment and medical oversight of participants. E.A.C. participated in the medical oversight of participants. F.P. and C.D.M. participated in the modeling analysis and data interpretation. C.C. participated in the coordination of modeling analysis and data interpretation. B.G. participated in the conception and design of the study, study coordination, and data interpretation. A.C.J.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Daniella de Oliveira Magro (Department of Internal Medicine, State University of Campinas) and Dr. Walkyria de Paula Pimenta (Department of Internal Medicine, School of Medicine, Universidade Estadual Paulista Júlio Mesquita Filho, Botucatu, São Paulo, Brazil) for their scientific and technical assistance at the beginning of the study.
Footnotes
Clinical trial reg. no. RBR-9kdzdv, www.ensaiosclinicos.gov.br (universal trial no. U1111-1137-0489).
References
- 1.Ferrannini E. Insulin resistance versus insulin deficiency in non-insulin-dependent diabetes mellitus: problems and prospects. Endocr Rev 1998;19:477–490 [DOI] [PubMed] [Google Scholar]
- 2.Malik VS, Hu FB. Popular weight-loss diets: from evidence to practice. Nat Clin Pract Cardiovasc Med 2007;4:34–41 [DOI] [PubMed] [Google Scholar]
- 3.Pories WJ, Caro JF, Flickinger EG, Meelheim HD, Swanson MS. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg 1978;206:316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256 [DOI] [PubMed] [Google Scholar]
- 5.Polyzogopoulou EV, Kalfarentzos F, Vagenakis AG, Alexandrides TK. Restoration of euglycemia and normal acute insulin response to glucose in obese subjects with type 2 diabetes following bariatric surgery. Diabetes 2003;52:1098–1103 [DOI] [PubMed] [Google Scholar]
- 6.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 2006;55:2025–2031 [DOI] [PubMed] [Google Scholar]
- 7.Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia 2006;49:2136–2143 [DOI] [PubMed] [Google Scholar]
- 8.Briatore L, Salani B, Andraghetti G, et al. Restoration of acute insulin response in T2DM subjects 1 month after biliopancreatic diversion. Obesity (Silver Spring) 2008;16:77–81 [DOI] [PubMed] [Google Scholar]
- 9.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 2009;32:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briatore L, Salani B, Andraghetti G, et al. Beta-cell function improvement after biliopancreatic diversion in subjects with type 2 diabetes and morbid obesity. Obesity (Silver Spring) 2010;18:932–936 [DOI] [PubMed] [Google Scholar]
- 11.Scopinaro N, Adami GF, Papadia FS, et al. The effects of biliopancreatic diversion on type 2 diabetes mellitus in patients with mild obesity (BMI 30-35 kg/m2) and simple overweight (BMI 25-30 kg/m2): a prospective controlled study. Obes Surg 2011;21:880–888 [DOI] [PubMed] [Google Scholar]
- 12.Cobelli C, Toffolo GM, Dalla Man C, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293:E1–E15 [DOI] [PubMed] [Google Scholar]
- 13.Camastra S, Manco M, Mari A, et al. Beta-cell function in severely obese type 2 diabetic patients: long-term effects of bariatric surgery. Diabetes Care 2007;30:1002–1004 [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association Executive summary: standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg 1979;66:618–620 [DOI] [PubMed] [Google Scholar]
- 16.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–158 [DOI] [PubMed] [Google Scholar]
- 17.Mitrakou A, Vuorinen-Markkola H, Raptis G, et al. Simultaneous assessment of insulin secretion and insulin sensitivity using a hyperglycemia clamp. J Clin Endocrinol Metab 1992;75:379–382 [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 19.Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 2002;49:419–429 [DOI] [PubMed] [Google Scholar]
- 20.Cobelli C, Caumo A, Omenetto M. Minimal model SG overestimation and SI underestimation: improved accuracy by a Bayesian two-compartment model. Am J Physiol 1999;277:E481–E488 [DOI] [PubMed] [Google Scholar]
- 21.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima MM, Pareja JC, Alegre SM, et al. Acute effect of roux-en-y gastric bypass on whole-body insulin sensitivity: a study with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010;95:3871–3875 [DOI] [PubMed] [Google Scholar]
- 23.Isbell JM, Tamboli RA, Hansen EN, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010;33:1438–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muscelli E, Mingrone G, Camastra S, et al. Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med 2005;118:51–57 [DOI] [PubMed] [Google Scholar]
- 25.Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 2002;51:144–151 [DOI] [PubMed] [Google Scholar]
- 26.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009;58:1400–1407 [DOI] [PubMed] [Google Scholar]
- 27.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med 2013;34:39–58 [DOI] [PubMed] [Google Scholar]
- 28.Mithieux G. A synergy between incretin effect and intestinal gluconeogenesis accounting for the rapid metabolic benefits of gastric bypass surgery. Curr Diab Rep 2012;12:167–171 [DOI] [PubMed] [Google Scholar]
- 29.Salani B, Briatore L, Andraghetti G, Adami GF, Maggi D, Cordera R. High-molecular weight adiponectin isoforms increase after biliopancreatic diversion in obese subjects. Obesity (Silver Spring) 2006;14:1511–1514 [DOI] [PubMed] [Google Scholar]
- 30.Henry RR, Brechtel G, Griver K. Secretion and hepatic extraction of insulin after weight loss in obese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1988;66:979–986 [DOI] [PubMed] [Google Scholar]
- 31.Svedberg J, Björntorp P, Smith U, Lönnroth P. Effect of free fatty acids on insulin receptor binding and tyrosine kinase activity in hepatocytes isolated from lean and obese rats. Diabetes 1992;41:294–298 [DOI] [PubMed] [Google Scholar]
- 32.Wiesenthal SR, Sandhu H, McCall RH, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 1999;48:766–774 [DOI] [PubMed] [Google Scholar]
- 33.Rossell R, Gomis R, Casamitjana R, Segura R, Vilardell E, Rivera F. Reduced hepatic insulin extraction in obesity: relationship with plasma insulin levels. J Clin Endocrinol Metab 1983;56:608–611 [DOI] [PubMed] [Google Scholar]
- 34.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 1983;32:438–446 [DOI] [PubMed] [Google Scholar]
- 35.Valverde I, Puente J, Martín-Duce A, et al. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg 2005;15:387–397 [DOI] [PubMed] [Google Scholar]
- 36.Astiarraga B, Gastaldelli A, Muscelli E, et al. Biliopancreatic diversion in nonobese patients with type 2 diabetes: impact and mechanisms. J Clin Endocrinol Metab 2013;98:2765–2773 [DOI] [PubMed] [Google Scholar]
- 37.Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 2002;87:1239–1246 [DOI] [PubMed] [Google Scholar]
- 38.Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl. 4):82–90 [DOI] [PubMed] [Google Scholar]
- 39.Laferrère B, Reilly D, Arias S, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 2011;3:80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch FF, Pareja JC, Geloneze SR, Chaim E, Cazzo E, Geloneze B. Comparison of metabolic effects of surgical-induced massive weight loss in patients with long-term remission versus non-remission of type 2 diabetes. Obes Surg 2012;22:910–917 [DOI] [PubMed] [Google Scholar]