Abstract
OBJECTIVE
To test the hypothesis that acute hypoglycemia induces endothelial dysfunction and inflammation through the generation of an oxidative stress. Moreover, to test if the antioxidant vitamin C can further improve the protective effects of glucagon-like peptide 1 (GLP-1) on endothelial dysfunction and inflammation during hypoglycemia in type 1diabetes.
RESEARCH DESIGN AND METHODS
A total of 20 type 1 diabetic patients underwent four experiments: a period of 2 h of acute hypoglycemia with or without infusion of GLP-1 or vitamin C or both. At baseline, after 1 and 2 h, glycemia, plasma nitrotyrosine, plasma 8-iso prostaglandin F2a (PGF2a), soluble intracellular adhesion molecule-1a (sICAM-1a), interleukin-6 (IL-6), and flow-mediated vasodilation were measured. At 2 h of hypoglycemia, flow-mediated vasodilation significantly decreased, while sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 significantly increased. The simultaneous infusion of GLP-1 or vitamin C significantly attenuated all of these phenomena. Vitamin C was more effective. When GLP-1 and vitamin C were infused simultaneously, the deleterious effect of hypoglycemia was almost completely counterbalanced.
RESULTS
At 2 h of hypoglycemia, flow-mediated vasodilation significantly decreased, while sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 significantly increased. The simultaneous infusion of GLP-1 or vitamin C significantly attenuated all of these phenomena. Vitamin C was more effective. When GLP-1 and vitamin C were infused simultaneously, the deleterious effect of hypoglycemia was almost completely counterbalanced.
CONCLUSIONS
This study shows that vitamin C infusion, during induced acute hypoglycemia, reduces the generation of oxidative stress and inflammation, improving endothelial dysfunction, in type 1 diabetes. Furthermore, the data support a protective effect of GLP-1 during acute hypoglycemia, but also suggest the presence of an endothelial resistance to the action of GLP-1, reasonably mediated by oxidative stress.
Recent evidence suggests that hypoglycemia can be considered a new risk factor in favoring diabetes vascular complications (1). It has been reported that hypoglycemia produces endothelial dysfunction and inflammation (2,3), which are well-recognized pathogenic factors for vascular disease, particularly in diabetes (4). Oxidative stress is considered the key player in the pathogenesis of diabetes complications (5), and it has been suggested that hypoglycemia produces endothelial dysfunction and inflammation through oxidative stress generation both in vitro and in humans (2,3). However, clear evidence in humans is still lacking (6): particularly, what is still missed is the evidence that by using an antioxidant, the elevation of the markers of oxidative stress induced by hypoglycemia can be reversed.
Recently, a possible beneficial effect of glucagon-like peptide 1 (GLP-1) analogs in the management of type 1 diabetes has been suggested (7,8). GLP-1 and its analogs, in addition to their insulin-tropic action in alleviating hyperglycemia, have beneficial effects in protecting progressive impairment of pancreatic β-cell function, preservation of β-cell mass, and suppression of glucagon secretion, gastric emptying, and appetite, all characteristics that could be beneficial for the management of type 1 diabetes (7,8).
Apart from the well-documented incretin effect of GLP-1, its role in the cardiovascular system also arouses interest. GLP-1 effects on the cardiovascular system may include a direct action on the endothelium, where the presence of specific receptors for GLP-1 has been demonstrated (9). Consistently, GLP-1 has been demonstrated to improve endothelial function in diabetes (10,11), possibly increasing the antioxidant defenses of the endothelium (12) and decreasing oxidative stress generation (11). Recently, we have reported that in type 1 diabetes, during induced-hypoglycemia, GLP-1 can partially protect endothelial function and partially decrease the appearance of inflammation, reducing, concomitantly, the level of several markers of oxidative stress (13). However, it is worthy of interest that in type 2 diabetes, hyperglycemia induces an endothelial resistance to the action of GLP-1, oxidative stress being the mediator of such a phenomenon (11).
The aim of this study was to test, in patients with type 1 diabetes, whether 1) the concomitant infusion of an antioxidant, vitamin C, can protect endothelial function and reduce the generation of oxidative stress and inflammation during acute induced-hypoglycemia and 2) the effects of GLP-1 and vitamin C on both endothelial dysfunction and oxidative stress induced by acute hypoglycemia were additive.
RESEARCH DESIGN AND METHODS
Subjects
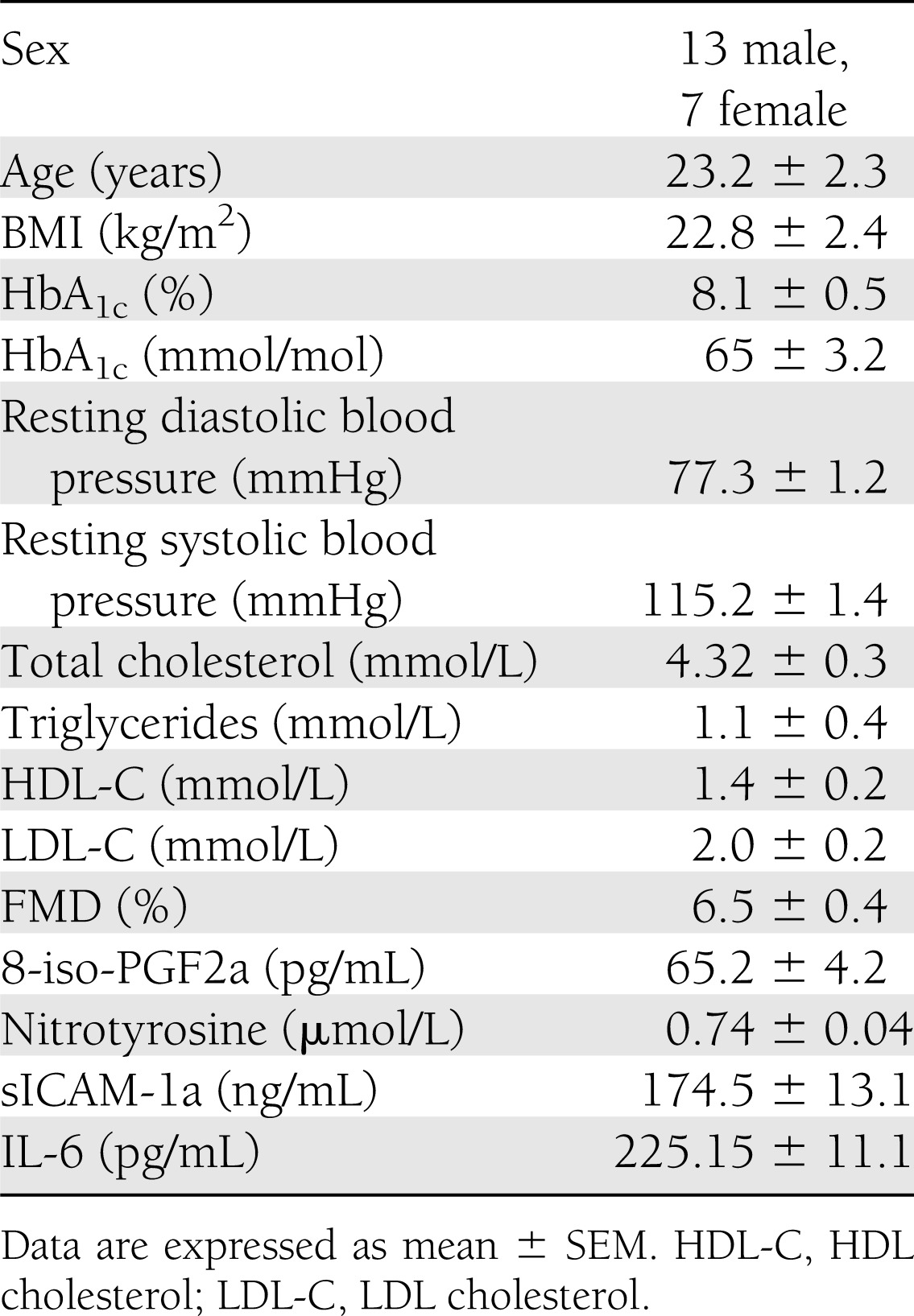
Twenty persons with type 1 diabetes were studied (Table 1). They had normal bedside tests of autonomic function (14) and did not have hypoglycemia unawareness based on the methods of Gold et al. (15) or major macro- or microcomplications of diabetes. They were treated with multiple daily insulin injections. All subjects were nonsmokers and had a normal blood count; had normal plasma lipids, plasma electrolytes, and liver and renal function; were normotensive; and were not taking medications, which can influence neuroendocrine responses to hypoglycemia or anti-inflammatory drugs.
Table 1.
Baseline characteristics of the type 1 diabetic patients
Studies were approved by the ethical committees of our institutions, and all participants gave written informed consent.
Patients were asked to avoid any exercise and consume their usual weight-maintaining diet for 3 days before each experiment. All participants were asked to perform intensive home blood glucose monitoring and to avoid hypoglycemia for at least 5 days before a study. On the day prior to a study, intermediate or long-acting insulin was discontinued and replaced by injections of regular insulin before breakfast and lunch. Each subject was admitted to the Research Center the evening before an experiment. At this time, two intravenous cannulas were inserted under 1% lidocaine local anesthesia. One cannula was placed to be used for blood drawing. The other cannula was placed in the contralateral arm for infusions. All subjects received an evening meal, with a continuous low-dose infusion of insulin to normalize plasma glucose. The insulin infusion was adjusted overnight to maintain blood glucose between 4.4 and 7.2 mmol/L.
Acute hypoglycemia experiments
All of the subjects were studied after an overnight, 10-h fast. Four different experiments were planned for each subject in a randomized order: a period of 2 h of hypoglycemia with or without infusion of GLP-1 [Synthetic GLP-1 (7–36)amide; PolyPeptide Laboratories, Wolfenbuttel, Germany] (0.4 pmol · kg−1 · min−1 [16]), vitamin C (30 mg/min [16]), or both. Each subject underwent each experiment with at least 1 week between each.
At time zero, a primed constant (9.0 pmol · kg−1 · min−1) infusion of insulin (Actrapid; NovoNordisk, Copenhagen, Denmark) was started and continued until 120 min. The rate of fall of glucose was controlled (∼0.08 mmol/min), and the glucose nadir (2.9 mmol/L) was achieved using a modification of the glucose clamp technique (16). During the clamp period, plasma glucose was measured every 5 min, and a 20% dextrose infusion was adjusted so that plasma glucose levels were held constant at 2.9 ± 0.1 mmol/L (16). Potassium chloride (20 mmol/L) was infused during the clamp to reduce insulin-induced hypokalemia. The experiment was repeated with the infusion of GLP-1, vitamin C, or both.
At baseline and after 1 and 2 h, blood samples were withdrawn for biochemical assays: glycemia, plasma nitrotyrosine (a stable end product of peroxynitrite oxidation) and plasma 8-iso prostaglandin F2a (PGF2a; a biomarker of lipid peroxidation), both markers of oxidative stress, and intercellular adhesion molecule 1 (ICAM-1) and interleukin-6 (IL-6), both markers of inflammation. Endothelial function was measured by flow-mediated dilation (FMD).
Biochemical and clinical measurements
Cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, and plasma nitrotyrosine were measured according to Ceriello et al. (17). Plasma glucose was measured by the glucose-oxidase method, HbA1c by high-performance liquid chromatography, and insulin by microparticle enzyme immunoassay (Abbott Laboratories, Wiesbaden, Germany). Plasma 8-iso-PGF2a (Cayman Chemical, Ann Arbor, MI), soluble ICAM-1 (sICAM-1; British Bio-technology, Abington, Oxon, U.K.), and IL-6 (R&D Systems, Minneapolis, MN) were determined with commercially available kits.
FMD.
Endothelial function at macrovascular level was evaluated by measuring FMD of the brachial artery (12,16). The examination was carried out in a temperature- and light-controlled room on subjects who were lying comfortably flat on a couch. Brachial arteries in this study were imaged with a standard ultrasound system (VIVID 7 ECHO machine; GE Vingmed System V) connected with a 12-MHz linear transducer probe. The ultrasound system was connected to a personal computer equipped with a frame grabber and artificial neural network wall detection software (vessel image analysis). Brachial artery FMD was determined using protocol similar to published studies (12–16).
At the end of the study each day, 250 µg of sublingual glyceryl trinitrate was administered in order to assess endothelium-independent vasodilatation.
The intraobserver variability for repeated measurements of resting arterial diameter was 0.02 ± 0.02 mm.
Statistical analysis
The sample size was selected according to previous studies (12,16,18).
Data are expressed as means ± SE. The Kolmogorov-Smirnov algorithm was used to determine whether each variable had a normal distribution. Comparisons of baseline data among the groups were performed using unpaired Student t test or Mann-Whitney U test, where indicated. The changes in variables during the tests were assessed by two-way ANOVA with repeated-measures or Kolmogorov-Smirnov test, where indicated. If differences reached statistical significance, post hoc analyses with two-tailed paired t test or Wilcoxon signed-rank test for paired comparisons were used to assess differences at individual time periods in the study. Statistical significance was defined as P < 0.05.
RESULTS
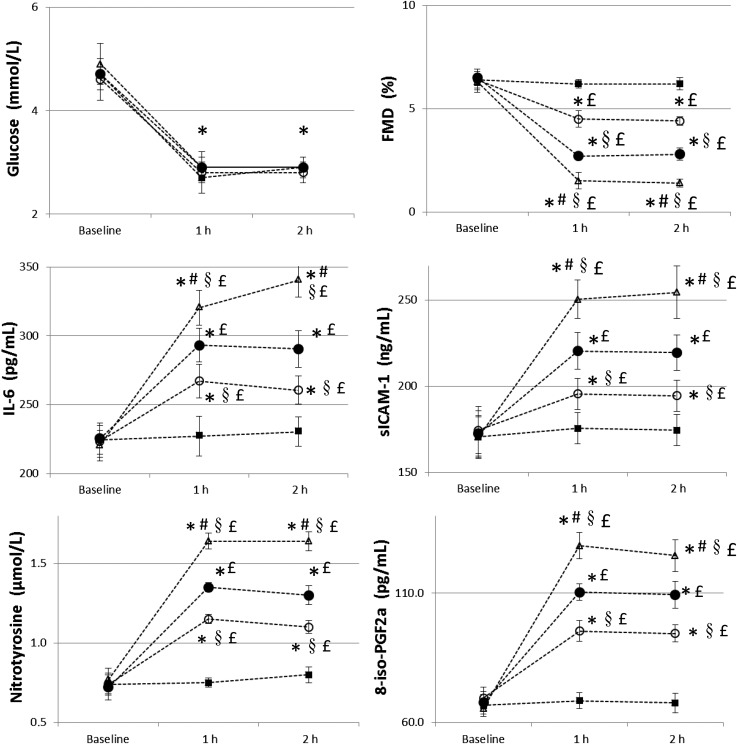
After 2 h of acute hypoglycemia, FMD significantly decreased, while sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 significantly increased, compared with basal values, (Fig. 1). When hypoglycemia was accompanied by the simultaneous infusion of GLP-1, all of these phenomena were significantly attenuated: FMD decreased less, while sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 were less increased (Fig. 1). Vitamin C infusion had an even better effect than GLP-1 (Fig. 1). However, when GLP-1 and vitamin C were simultaneously infused, the deleterious effect of hypoglycemia on FMD, sICAM-1, 8-iso-PGF2a, nitrotyrosine, and IL-6 was almost completely counterbalanced (Fig. 1). Endothelial-independent vasodilatation was not affected in any of the experiments. The amount of insulin infused during the experiments was similar (area under the curve 75,200 ± 350 vs. 75,320 ± 370 vs. 75,150 ± 330 vs. 75,300 ± 320 pmol).
Figure 1.
Glycemia, FMD, IL-6, sICAM-1, nitrotyrosine, and 8-iso-PGF2a changes during hypoglycemia (white triangle), hypoglycemia plus GLP-1 (black circle), hypoglycemia plus vitamin C (white circle), and hypoglycemia plus GLP-1 and vitamin C (black square). Data are mean ± SEM. *P < 0.01 vs. basal, £P < 0.05 vs. hypoglycemia plus GLP-1 and vitamin C, §P < 0.05 vs. hypoglycemia plus GLP-1, #P < 0.05 vs. hypoglycemia plus vitamin C.
CONCLUSIONS
This study confirms that acute hypoglycemia induces endothelial dysfunction and inflammation in people with type 1 diabetes (13). However, in our opinion, it adds two important findings to our previous report showing that GLP-1 can reduce these phenomena (13).
It has been suggested that hypoglycemia can produce an oxidative stress (3,13,19), which in turn can favor the appearance of an endothelial dysfunction and inflammation (3–13,19). However, this hypothesis has been, until now, just linked to the evidence that several markers of oxidative stress increase during hypoglycemia (3–13,19), and, to date, it has not been confirmed showing that an antioxidant can counterbalance this phenomenon or, better, that the antioxidant given during hypoglycemia, reducing oxidative stress, can simultaneously reduce endothelial dysfunction and inflammation. This is what, for the first time, our study addresses. The infusion of vitamin C, during acute hypoglycemia, produced an expected improvement in oxidative stress, which was accompanied by the simultaneous improvement of both endothelial function and inflammation. In our opinion, this means a causal role of oxidative stress in favoring the appearance of endothelial dysfunction and inflammation during hypoglycemia.
However, another possibility is that acute hypoglycemia causes activation of the sympathetic system (hence the increase in 8-iso-PGF2a), which causes vasoconstriction (hence the decrease in FMD). The endothelial cells try to respond to this by increasing their nitric oxide production (hence the increase in nitrotyrosine) but cannot overcome the autonomic overactivity. Sympathetic activity is known to influence inflammatory signals (20), hence the increase in IL-6 levels. Therefore, the observed acute hypoglycemia-induced alterations could simply be due to a physiological reflex response, not necessarily a pathological oxidative stress-induced endothelial dysfunction.
The second finding is related to the additive effects of vitamin C and GLP-1.
In this study, a protective effect of GLP-1 on hypoglycemia-induced endothelial dysfunction, inflammation, and oxidative stress is confirmed. Studies are accumulating showing that GLP-1 and its analogs used in clinical practice have an antioxidant activity (12,13). Therefore, it is reasonable that GLP-1 should, by reducing oxidative-stress generation, improve endothelial dysfunction and inflammation generated by hypoglycemia. However, it is worthy of interest that vitamin C was more efficacious than GLP-1 in counterbalancing the effects of hypoglycemia and that only when both vitamin C and GLP-1 were infused simultaneously were the effects of hypoglycemia abolished completely. These data, altogether, support the hypothesis that the endothelium, as in hyperglycemia (11,21,22), became less sensitive to GLP-1 in hypoglycemia, more than GLP-1 itself loses its activity, and that oxidative stress might be the mediator of such phenomenon.
A possible direct influence of insulin on our results cannot be excluded, particularly because it has recently been reported that GLP-1 enhances the vasodilator effect of insulin (23). However, in our experiments, insulin infusion was accompanied by a decrease of the endothelial function, and the amount of insulin infused was the same during all of the experiments, suggesting that the role of insulin in our results could be not of particular relevance. In the same article by Tesauro et al. (23), GLP-1 did not enhance the effect of vitamin C on endothelial dysfunction. However, our experimental conditions are very different. We studied 20 patients with type 1 diabetes, while the study of Tesauro et al. (23) was focused on only 5 patients with metabolic syndrome (23). Moreover, as underlined by the same authors, no control study with vitamin C alone was performed.
Two mechanisms have been suggested to explain this resistance to the GLP-1 action in diabetes: the activation of protein kinase Cβ (PKCβ), induced by hyperglycemia, able to reduce the expression of the GLP-1 receptors (24), and the generation by hyperglycemia of an oxidative stress (11). Nevertheless, the two proposed mechanisms, PKCβ activation with reduction of the expression of GLP-1 receptors and oxidative-stress generation, could be convincingly correlated, because it is well-recognized that PKCβ is activated by the free radicals (25). Even at this stage, it is just a hypothesis; as both hyperglycemia and hypoglycemia work through the same pathways and mainly generating an oxidative stress (1,26), it seems reasonable that the same mechanisms involved in the appearance of GLP-1 resistance in hyperglycemia might also been involved in hypoglycemia.
The risk of a cardiovascular disease in type 1 diabetes, even still partly neglected, is very high (27).
The role of hyperglycemia in favoring cardiovascular disease in type 1 diabetes seems to be relevant; even many other classical and less classical risk factors seem to be also involved (26). However, particularly the role of the oxidative stress seems to be very relevant in the pathogenesis of these complications in type 1 diabetes (26), and our finding suggests that hypoglycemia, a frequent event in the life of type 1 diabetes, which is emerging as a cardiovascular risk factor (1), can also produce an oxidative stress.
In conclusion, our study shows that hypoglycemia produces endothelial dysfunction and inflammation through oxidative stress and that an antioxidant, such as vitamin C, can partly prevent this effect. While the chronic use of vitamin C cannot be the solution (28), recent development in the antioxidant treatment of diabetes-related complications might provide a future perspective also in preventing the deleterious effects of hypoglycemia on the cardiovascular system (29).
A possible limitation of this study is that type 1 diabetic subjects enrolled in the current study were free of micro- or macrovascular complications and may not represent the entire spectrum of type 1 diabetes.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
A.C. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. A.N., E.O., and L.B. researched data, contributed to discussion, and reviewed and edited the manuscript. S.C., M.R., and S.G. contributed to discussion and reviewed and edited the manuscript. L.L.S. and G.P. researched data and contributed to discussion. A.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008;24:353–363 [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Alexanian A, Ying R, et al. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 2012;32:712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects [published correction appears in Metabolism 2009;58:1046]. Metabolism 2009;58:443–448 [DOI] [PubMed] [Google Scholar]
- 4.Nandish S, Wyatt J, Bailon O, Smith M, Oliveros R, Chilton R. Implementing cardiovascular risk reduction in patients with cardiovascular disease and diabetes mellitus. Am J Cardiol 2011;108(Suppl.):42B–51B [DOI] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snell-Bergeon JK, Wadwa RP. Hypoglycemia, diabetes, and cardiovascular disease. Diabetes Technol Ther 2012;14(Suppl. 1):S51–S58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Issa CM, Azar ST. Possible role of GLP-1 and its agonists in the treatment of type 1 diabetes mellitus. Curr Diab Rep 2012;12:560–567 [DOI] [PubMed] [Google Scholar]
- 8.George P, McCrimmon RJ. Potential role of non-insulin adjunct therapy in Type 1 diabetes. Diabet Med 2013;30:179–188 [DOI] [PubMed] [Google Scholar]
- 9.Mudaliar S, Henry RR. Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med 2010;123(Suppl.):S19–S27 [DOI] [PubMed] [Google Scholar]
- 10.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Esposito K, Testa R, Bonfigli AR, Marra M, Giugliano D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an “endothelial resistance” to glucagon-like peptide 1 in diabetes. Diabetes Care 2011;34:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 2010;30:1407–1414 [DOI] [PubMed] [Google Scholar]
- 13.Ceriello A, Novials A, Ortega E, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care 2013;36:2346–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985;8:491–498 [DOI] [PubMed] [Google Scholar]
- 15.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 16.Ceriello A, Novials A, Ortega E, et al. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes 2012;61:2993–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia 2001;44:834–838 [DOI] [PubMed] [Google Scholar]
- 18.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010;33:1529–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousefzade G, Nakhaee A. Insulin-induced hypoglycemia and stress oxidative state in healthy people. Acta Diabetol 2012;49(Suppl. 1):S81–S85 [DOI] [PubMed] [Google Scholar]
- 20.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation 2005;12:255–269 [DOI] [PubMed] [Google Scholar]
- 21.Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes 2010;59:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green CJ, Henriksen TI, Pedersen BK, Solomon TP. Glucagon like peptide-1-induced glucose metabolism in differentiated human muscle satellite cells is attenuated by hyperglycemia. PLoS ONE 2012;7:e44284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tesauro M, Schinzari F, Adamo A, et al. Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care 2013;36:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mima A, Hiraoka-Yamomoto J, Li Q, et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 2012;61:2967–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003;52:2795–2804 [DOI] [PubMed] [Google Scholar]
- 26.Ceriello A. Hyperglycaemia and the vessel wall: the pathophysiological aspects on the atherosclerotic burden in patients with diabetes. Eur J Cardiovasc Prev Rehabil 2010;17(Suppl. 1):S15–S19 [DOI] [PubMed] [Google Scholar]
- 27.Orchard TJ, Costacou T. When are type 1 diabetic patients at risk for cardiovascular disease? Curr Diab Rep 2010;10:48–54 [DOI] [PubMed] [Google Scholar]
- 28.Frei B, Birlouez-Aragon I, Lykkesfeldt J. Authors’ perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr 2012;52:815–829 [DOI] [PubMed] [Google Scholar]
- 29.Miyata T, Suzuki N, van Ypersele de Strihou C. Diabetic nephropathy: are there new and potentially promising therapies targeting oxygen biology? Kidney Int. 13 March 2013 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]