Abstract
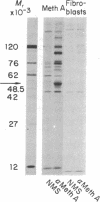
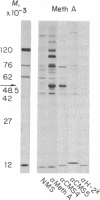
Antisera prepared against BALB/c Meth A sarcoma in syngeneic or compatible F1 mice recognize a protein with an apparent molecular weight of 53,000 in extracts of [35S]methionine-labeled transformed BALB/c cells. This component, designated p53, was not detected in normal adult mouse fibroblasts, lymphoid cells, or hematopoietic cells or in mouse embryo cells or 3T3 cells. An extensive variety of antisera, including alloantisera and heterologous antisera directed against structural antigens of murine leukemia viruses, was tested for reactivity with p53; other than Meth A antisera, only comparably prepared antisera against another BALB/c sarcoma, CMS4, had anti-p53 activity. All transformed mouse cells tested were found to express p53; these tests included chemically induced sarcomas, leukemias, spontaneously transformed fibroblasts, and cells transformed by simian virus 40 and murine sarcoma virus. The presence of p53 in tumors of no known viral etiology indicates coding by resident cellular genes; this does not exclude endogenous viruses as the source of coding sequences or the possibility that transforming viruses code directly for p53.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Brown J. P., Klitzman J. M., Hellström I., Nowinski R. C., Hellström K. E. Antibody response of mice to chemically induced tumors. Proc Natl Acad Sci U S A. 1978 Feb;75(2):955–958. doi: 10.1073/pnas.75.2.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone G., Piazza R., Parmiani G. Effect of different sera on growth and "spontaneous" neoplastic transformation of mouse fibroblasts in vitro. J Natl Cancer Inst. 1974 Feb;52(2):387–393. doi: 10.1093/jnci/52.2.387. [DOI] [PubMed] [Google Scholar]
- DeLeo A. B., Shiku H., Takahashi T., John M., Old L. J. Cell surface antigens of chemically induced sarcomas of the mouse. I. Murine leukemia virus-related antigens and alloantigens on cultured fibroblasts and sarcoma cells: description of a unique antigen on BALB/c Meth A sarcoma. J Exp Med. 1977 Sep 1;146(3):720–734. doi: 10.1084/jem.146.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLEY E. J. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953 Dec;13(12):835–837. [PubMed] [Google Scholar]
- GLYNN J. P., BLANCO A. R., GOLDIN A. STUDIES ON INDUCED RESISTANCE AGAINST ISOTRANSPLANTS OF VIRUS-INDUCED LEUKEMIA. Cancer Res. 1964 Apr;24:502–508. [PubMed] [Google Scholar]
- Gaudray P., Rassoulzadegan M., Cuzin F. Expression of simian virus 40 early genes in transformed rat cells is correlated with maintenance of the transformed phenotype. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4987–4991. doi: 10.1073/pnas.75.10.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. P., Bigner D. D., Fischinger P. J., Bolognesi D. P. Expression of murine leukemia virus structural antigens on the surface of chemically induced murine sarcomas. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5037–5041. doi: 10.1073/pnas.71.12.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Klein R., Toni R., Walker J., Gilden R. Mutants of nonproducer cell lines transformed by murine sarcoma viruses. I. Induction, isolation, particle production, and tumorigenicity. J Exp Med. 1973 Aug 1;138(2):356–363. doi: 10.1084/jem.138.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Translation of polyoma virus T antigens in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5917–5921. doi: 10.1073/pnas.75.12.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Jay F. T., Friedman R. M., Levine A. S. Simian virus 40-specific ribosome-binding proteins induced by a nondefective adenovirus 2-simian virus 40 hybrid. J Virol. 1977 Sep;23(3):692–699. doi: 10.1128/jvi.23.3.692-699.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Shiu R. P., Jay F. T., Levine A. S., Pastan I. Identification of a transformation-specific protein induced by a Rous sarcoma virus. Cell. 1978 Mar;13(3):527–534. doi: 10.1016/0092-8674(78)90326-4. [DOI] [PubMed] [Google Scholar]
- KLEIN G., SJOGREN H. O., KLEIN E., HELLSTROM K. E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561–1572. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Natori T., Law L. W., Appella E. Biological and biochemical properties of Nonidet P40-solubilized and partially purified tumor-specific antigens of the transplantation type from plasma membranes of a methylcholanthrene-induced sarcoma. Cancer Res. 1977 Sep;37(9):3406–3413. [PubMed] [Google Scholar]
- Old L. J., Stockert E. Immunogenetics of cell surface antigens of mouse leukemia. Annu Rev Genet. 1977;11:127–160. doi: 10.1146/annurev.ge.11.120177.001015. [DOI] [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. In vitro translation of Harvey murine sarcoma virus RNA. J Virol. 1977 Jun;22(3):711–719. doi: 10.1128/jvi.22.3.711-719.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Williams D. R., Weeks M. O., Maryak J. M., Vass W. C., Scolnick E. M. Comparison of the genomic organization of Kirsten and Harvey sarcoma viruses. J Virol. 1978 Jul;27(1):45–55. doi: 10.1128/jvi.27.1.45-55.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., DeLeo A. B., O'Donnell P. V., Obata Y., Old L. J. G(AKSL2): a new cell surface antigen of the mouse related to the dualtropic mink cell focus-inducing class of murine leukemia virus detected by naturally occurring antibody. J Exp Med. 1979 Jan 1;149(1):200–215. doi: 10.1084/jem.149.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Aaronson S. A. Properties of clonal lines of murine sarcoma virus transformed Balb-3T3 cells. Virology. 1969 May;38(1):174–179. doi: 10.1016/0042-6822(69)90140-8. [DOI] [PubMed] [Google Scholar]