Abstract
OBJECTIVE
Knowledge on mortality in autoimmune diabetes with adult onset is limited. We compared mortality in adult-onset autoimmune diabetes and type 2 diabetes, taking into account metabolic risk factors, HbA1c, lifestyle, and socioeconomic factors.
RESEARCH DESIGN AND METHODS
Participants of the population-based HUNT2 Study (second survey of the Norwegian HelseUndersøkelsen i Nord-Trøndelag Study; n = 64,264) were followed up prospectively for mortality in the Cause of Death Registry (1995–2009). Diabetes with onset ≥35 years was classified as autoimmune diabetes in adults if anti-GAD was positive (n = 208) and as type 2 diabetes if anti-GAD was negative (n = 2,425). Hazard ratios (HRs) of mortality from all-causes, cardiovascular disease (CVD), and ischemic heart disease (IHD) were calculated using the Cox proportional hazards model.
RESULTS
Prevalence of the metabolic syndrome was lower in autoimmune diabetes than in type 2 diabetes (55 vs. 77%, P < 0.001). Still, autoimmune diabetes was associated with an increased risks of mortality from all-causes (HR 1.55 [95% CI 1.25–1.92]), CVD (1.87 [1.40–2.48]), and IHD (2.39 [1.57–3.64]), equally high as in type 2 diabetes in analyses where individuals without diabetes were used as the reference group. The increased risk was not explained by overweight, lifestyle, socioeconomic position, or presence of the metabolic syndrome. Excess mortality was primarily observed in individuals with elevated HbA1c.
CONCLUSIONS
Mortality in autoimmune diabetes was as high as in type 2 diabetes, despite a more favorable baseline metabolic risk profile. Excess risk was associated with poor glycemic control. The results from this study, the largest so far on mortality in autoimmune diabetes in adults, underscore the importance of optimal treatment modalities to improve survival in adult-onset autoimmune diabetes.
All forms of diabetes are associated with increased mortality, primarily from cardiovascular disease (CVD) and, in particular, ischemic heart disease (IHD) (1–3). A meta-analysis of 102 prospective studies reported that diabetes confers about a twofold excess risk of CVD, independent of other risk factors (4). Prolonged exposure to hyperglycemia is a well-recognized etiological factor. Hyperglycemia initiates a complex chain of events that damage blood vessels and potentially accelerate the atherosclerotic process (5). However, other factors are also bound to be important and may modify the total risk differently in different forms of diabetes. Hence, excess mortality in type 2 diabetes has also been attributed to traditional risk factors, such as obesity and other components of the metabolic syndrome (6,7), and to socioeconomic factors (8).
The effect of juvenile-onset type 1 diabetes on CVD and mortality is well recognized (1). Less so is the effect of autoimmune diabetes with adult onset. Still, autoimmune diabetes constitutes a significant part of diabetes, especially in the phenotype termed latent autoimmune diabetes in adults (LADA), which is estimated to account for ∼10% of all diabetes (9–11). Autoimmune diabetes is characterized by antibodies against the insulin-producing β-cells (9,12). Compared with “classic” type 1 diabetes, LADA progresses more slowly and is typically not treated with insulin at the time of diagnosis.
On one hand, fewer features of the metabolic syndrome have been reported in individuals with adult-onset autoimmune diabetes than in individuals with type 2 diabetes (13–17), and this may indicate a lower risk of complications. On the other hand, worse glycemic control has been suggested in LADA patients than in patients with type 2 diabetes (15,18–20), something that could put these individuals at increased risk of complications and mortality. Only two previous studies, based on Australian and Finnish populations, have addressed mortality in autoimmune diabetes in adults/LADA (18,21). In addition, a recent U.S. article (22) reported markedly worse prognosis among normal-weight compared with overweight diabetic patients. They speculated about the role of LADA, but did not have information on indicators of autoimmunity to separate patients with LADA from those with type 2 diabetes.
From the large population-based and prospective Norwegian HUNT Study (an acronym for the Norwegian name: HelseUndersøkelsen i Nord-Trøndelag), in conjunction with the Cause of Death Registry, it is possible to identify different forms of diabetes and to analyze mortality and cause of death. The size of the study would provide data for the largest study to date on mortality in autoimmune diabetes in adults.
Here, we aimed to investigate the risk of all-cause, CVD, and IHD mortality in autoimmune diabetes in adults and type 2 diabetes, taking into account the influence of metabolic risk factors, glycemic control, lifestyle, and socioeconomic factors.
RESEARCH DESIGN AND METHODS
Study population
The HUNT Study consists of three surveys in 1984–2008 where the entire population of the Norwegian County of Nord-Trøndelag was invited to participate. In 1995–1997, 66,140 men and women (71.2%), aged 20 years or older, participated in the second of these surveys (HUNT2), consisting of questionnaires, physical examinations, and blood sampling. Individuals with diabetes were invited to a supplementary examination during which information on anti-GAD, fasting glucose, and HbA1c was gathered. The design of the HUNT Study has previously been presented in detail (23). Among the participants of the HUNT2 survey, complete baseline information on diabetes status was available for 64,815 individuals. During 1995–2008, 3,184 individuals with diabetes were identified, including 208 adults with autoimmune diabetes and 2,425 with type 2 diabetes. Our final study population consisted of 64,264 individuals (2,633 with autoimmune and type 2 diabetes together with 61,631 individuals without diabetes).
We monitored the study population for mortality between 1995 and 2009 by linkage to the Norwegian Cause of Death Registry. In addition to all-cause mortality, we identified individuals with CVD (ICD-10 codes I00–I99 and ICD-9 codes 390–459) and IHD (ICD-10 codes I20–I25 and ICD-9 codes 410–414) as the underlying or contributing cause of death. Migration from the Nord-Trøndelag County was low (<1%) during this time period, as shown in a previous study (24), and was considered negligible.
The HUNT Study and the mortality follow-up were approved by the Norwegian Data Inspectorate and the Regional Medical Research Ethical Committee. The participants of the HUNT Study gave written informed consent.
Classification of diabetes
Individuals with diabetes were identified by questionnaire. Fasting blood samples from all diagnosed diabetic individuals were obtained through the clinical investigation of HUNT and analyzed for anti-GAD, as previously described (25). Antibody levels were expressed as an antibody index relative to a standard serum. An index of ≥0.08 was considered positive. This cutoff was used to achieve the highest possible specificity (1.00) with an acceptable corresponding sensitivity (0.64). Anti-GAD values are reported in World Health Organization (WHO) units (0.08 antibody index = 43 WHO units/mL).
Diabetes with age at onset ≥35 years was classified as autoimmune diabetes in adults (n = 208) if anti-GAD was positive and as type 2 diabetes (n = 2,425) if anti-GAD was negative. Anti-GAD–positive individuals aged <35 years at onset were classified as having “classic” type 1 diabetes (n = 66). To distinguish LADA from all autoimmune diabetes in adults, we included a criterion of insulin independence at diagnosis as an indicator of slow onset, as suggested previously (26). In the HUNT Study, however, information on insulin treatment is available yearly, and not monthly, and we defined LADA case subjects as individuals free of insulin during the year of diagnosis (rather than free of insulin use up to 6 months after diagnosis). We used information on insulin treatment (yes/no) and year of initiation, available for 76% (n = 159) of all individuals with autoimmune diabetes, and classified anti-GAD–positive individuals (with onset ≥ 35 years) as having LADA if they reported not being treated with insulin during the year of diagnosis (n = 141). On the basis of this criterion, 89% of our case subjects with autoimmune diabetes with adult onset (with information on insulin treatment) were classified as having LADA.
Metabolic risk factors
Examinations in HUNT included measurements of height, weight, waist and hip circumference, blood pressure, total cholesterol, HDL-cholesterol, and triglycerides. Hypertension was defined as a blood pressure >140/90 mmHg or as current use of antihypertensive medication. Criteria for the metabolic syndrome were applied as recommended by the International Diabetes Federation (27). Hence, central obesity (waist circumference ≥94 cm for men and ≥80 cm for women) was required, plus at least two of the following four components: raised triglycerides (≥1.7 mmol/L) or specific treatment for this lipid abnormality, reduced HDL-cholesterol (<1.03 mmol/L in men and <1.29 mmol/L in women) or specific treatment for this lipid abnormality, raised blood pressure (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg) or treatment for previously diagnosed hypertension, or raised fasting plasma glucose (≥5.6 mmol/L) or previously diagnosed type 2 diabetes. Information on pharmacological treatment for lipid abnormalities was not available, but such treatment was uncommon in Norway at the time.
Lifestyle, socioeconomic factors, and family history of diabetes
We used questionnaire information on physical activity, smoking, and alcohol consumption (referred to as lifestyle factors) to classify participants as physically active or inactive; never, former, or current smokers; and as abstainers, occasional, low, moderate, or high consumers of alcohol. Participants were also classified as having an educational level corresponding to primary school, upper secondary school, or university, and as having a low-grade (manual workers), middle (nonmanual employees, farmers, fishermen, other self-employment), or high-grade (higher- and lower-grade professionals, administrators, and officials) occupational position. Information on family history of diabetes was collected by separate questions on diabetes in parents, siblings, and children, as well as age at onset of diabetes for each relative.
Statistical analyses
We used left-truncated Cox proportional hazards models to estimate hazard ratios (HRs) and corresponding 95% CI for mortality from all causes, CVD, and IHD in relation to diabetes status at baseline. Person-years of follow-up were accumulated from age at baseline until age at death or until age at the end of follow-up, December 31, 2009. We used age (in years) as the underlying time scale in the Cox model, which provides efficient adjustment for confounding from age (28). Kaplan-Meier curves were estimated to illustrate all-cause mortality by diabetes type.
To take advantage of the repeated measures, the analyses were time-dependent; that is, we updated the baseline (HUNT2) information on diabetes status using information on diabetes onset from the HUNT3 survey. This means that the analyses allow for individuals who developed diabetes during follow-up (i.e., sometime between participating in HUNT2 and HUNT3) to change exposure (i.e., diabetes) status during follow-up. Of the 2,633 individuals with diabetes, 1,583 were identified at baseline (i.e., in the HUNT2 survey, 1995–1997), whereas 1,050 individuals without diabetes at baseline developed diabetes sometime between the HUNT2 and the HUNT3 surveys. These individuals were identified at the time of the third HUNT survey in 2006–2008 (HUNT3), and time exposed was accounted from year of diagnosis.
In Table 2, HRs of mortality by diabetes status are reported after adjustment for age and sex (model 1), age, sex, BMI, waist-to-hip ratio, physical activity, smoking, alcohol consumption, educational level, and family history of diabetes (model 2), and additionally, adjustment for the metabolic syndrome (as a composite variable) and LDL-cholesterol (model 3). The proportional hazard assumption was fulfilled for all these covariates at the Cox regression analyses, with use of the test for all time-dependent covariate variables added. All analyses were conducted using SAS 9.2 software (SAS Institute Inc., Cary, NC).
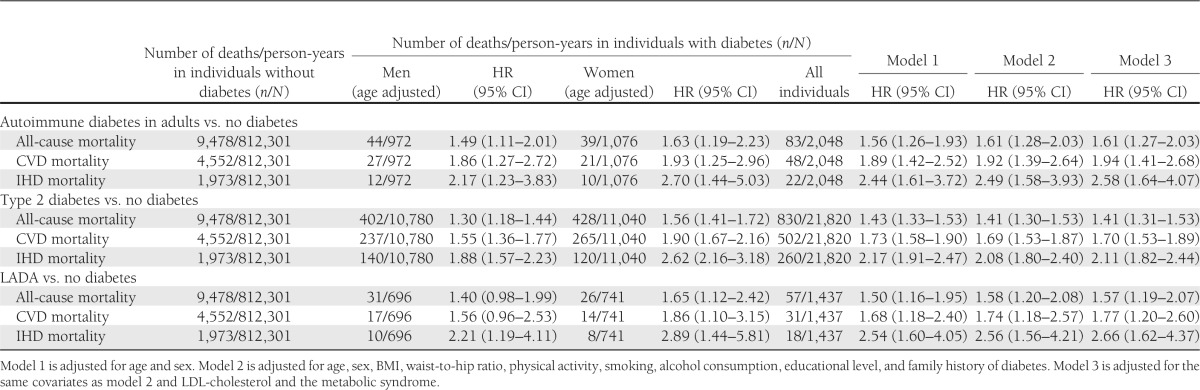
Table 2.
HRs of mortality from all-causes, CVD, and IHD in adult-onset autoimmune diabetes, type 2 diabetes, and LADA compared with individuals without diabetes
RESULTS
Characteristics of the study population
The 64,264 participants contributed to the analyses with 836,586 person-years. During the 14 years of follow-up, there were 10,537 deaths, 5,183 from CVD, and of those, 2,301 from IHD. Mean follow-up time was 13.2 years among individuals without diabetes and 9.1 years among those with diabetes. During follow-up, 15% among those without diabetes died (17% among men and 13% among women) compared with 33% among those with diabetes (33% among men and 34% among women). Overall mortality was 18% lower in the HUNT population than in the general population of Norway compared with age-specific mortality rates for Norway obtained from WHO (29).
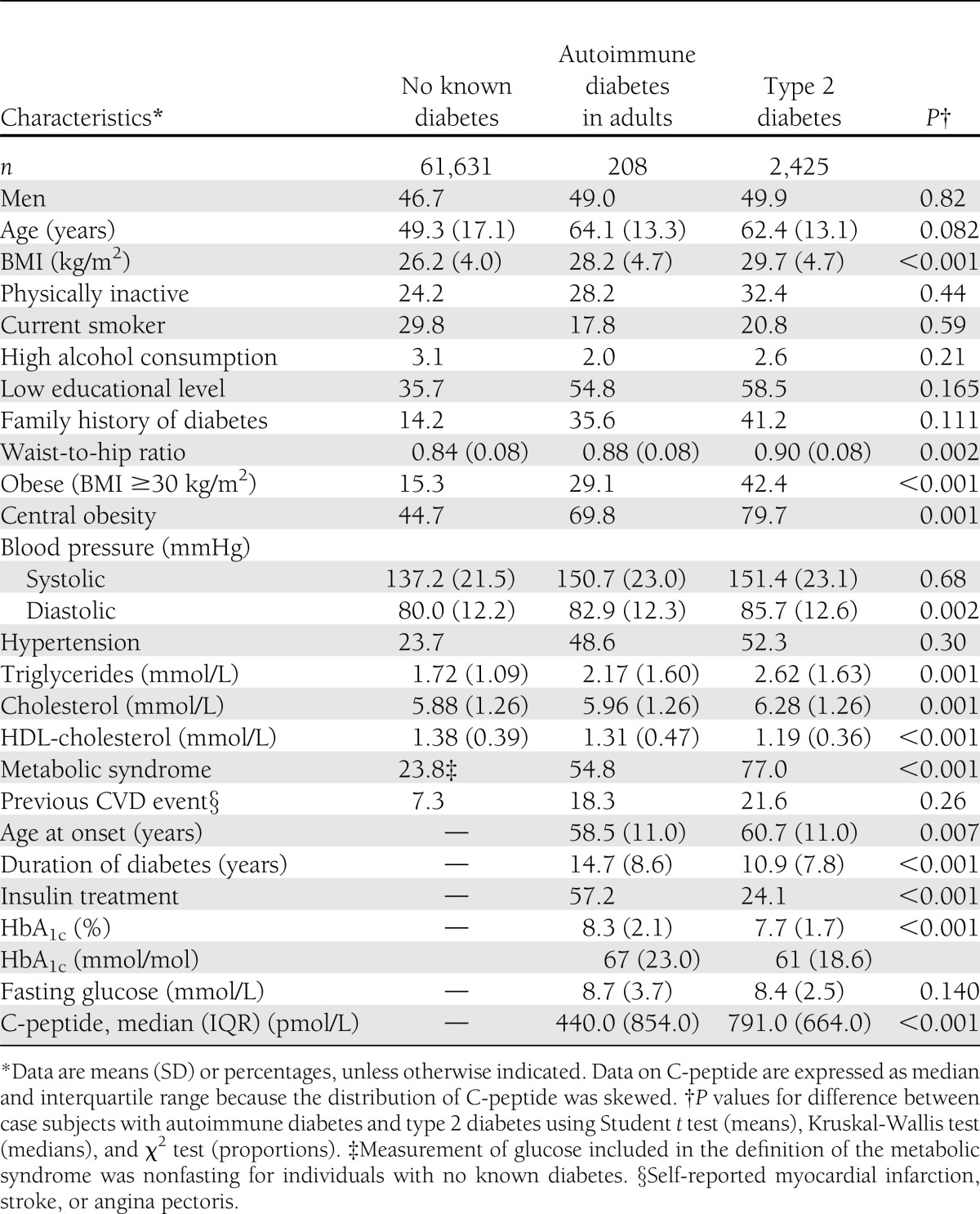
A comparison of individuals with and without diabetes showed those without diabetes were younger, more physically active and leaner, had lower blood pressure and more “healthy” blood lipids, and were less likely to have a low educational background (Table 1). A comparison of adult-onset autoimmune diabetes with type 2 diabetes showed the former was associated with less central obesity, lower levels of triglycerides, and higher HDL-cholesterol levels. Accordingly, prevalence of the metabolic syndrome was lower in autoimmune diabetes, at 55 vs. 77%. Mean duration of diabetes was longer and HbA1c levels were higher in autoimmune diabetes compared with type 2 diabetes.
Table 1.
Baseline characteristics of the study population from the HUNT2 survey in 1995–1997
Increased mortality in adult-onset autoimmune diabetes and type 2 diabetes
Autoimmune diabetes was associated with excess mortality from all-causes, CVD, and IHD compared with individuals without diabetes (Table 2). Increased risks were seen in men and women. These results were similar to those seen for type 2 diabetes. Associations were seen after adjustment for age, sex, BMI, physical activity, smoking, alcohol consumption, educational level, family history of diabetes (model 2), and the metabolic syndrome (model 3). In addition to the analyses where the metabolic syndrome was included as a combined variable (model 3), we also performed analyses that were adjusted for the components of the metabolic syndrome in which each one of these was included separately in the model. This adjustment did not significantly affect the estimates; for example, no effect of blood pressure or cholesterol levels was noted when analyzed separately in relation to mortality.
In stratified analyses, autoimmune diabetes and type 2 diabetes were both associated with excess mortality across levels of BMI; for example, in autoimmune diabetes, the HR of CVD mortality (adjusted for age and sex) was 1.69 (95% CI 0.97–2.95) in individuals with a BMI <25 kg/m2, 2.00 (95% CI 1.30–3.08) in overweight (BMI 25–29.9 kg/m2), and 1.88 (95% CI 1.09–3.26) in obese (BMI ≥30 kg/m2) individuals. The cohort was also stratified according to educational level and occupational position, and increased mortality was seen across levels of education and occupation (results not shown). For these stratified analyses, additional adjustment for lifestyle factors or metabolic factors did not change the results. At baseline, 18% of individuals with autoimmune diabetes and 22% of those with type 2 diabetes reported a previous CVD event (i.e., self-reported myocardial infarction, stroke, or angina). Subgroup analyses in individuals with and without prior CVD events found the age- and sex-adjusted HR of CVD mortality in autoimmune diabetes was 1.55 (95% CI 1.06–2.27) in individuals without and 2.80 (95% CI 1.82–4.30) in individuals with a previous CVD event, and corresponding results for type 2 diabetes were, respectively, HR 1.68 (95% CI 1.48–1.91) and 1.46 (95% CI 1.28–1.67).
Kaplan-Meier curves of all-cause mortality rates were similar in autoimmune diabetes and type 2 diabetes (Supplementary Fig. 1). HRs of all-cause, CVD, and IHD mortality in autoimmune diabetes compared with type 2 diabetes were estimated at HRs of 1.02 (95% CI 0.81–1.28), 0.99 (95% CI 0.74–1.33), and 0.88 (95% CI 0.57–1.36), respectively, after adjustment for age, sex, and duration of diabetes. The associations were somewhat attenuated after adjustment for duration of diabetes; HRs without adjustment for duration of diabetes were 1.11 (95% CI 0.88–1.39) for all-cause mortality, 1.06 (95% CI 0.79–1.43) for CVD mortality, and 0.95 (95% CI 0.62–1.47) for IHD mortality. The analyses of autoimmune diabetes versus type 2 diabetes were, however, based on small numbers, and we cannot exclude a possible difference between types of diabetes.
Mortality in LADA
Increased all-cause and CVD mortality was seen also when autoimmune diabetes was restricted to LADA (Table 2). The age and sex adjusted HRs were 1.50 (95% CI 1.16–1.95) for all-cause mortality, 1.68 (95% CI 1.18–2.40) for CVD mortality, and 2.54 (95% CI 1.60–4.05) for IHD mortality. This excess risk was not explained by metabolic risk factors, lifestyle, or socioeconomic factors.
Glycemic control
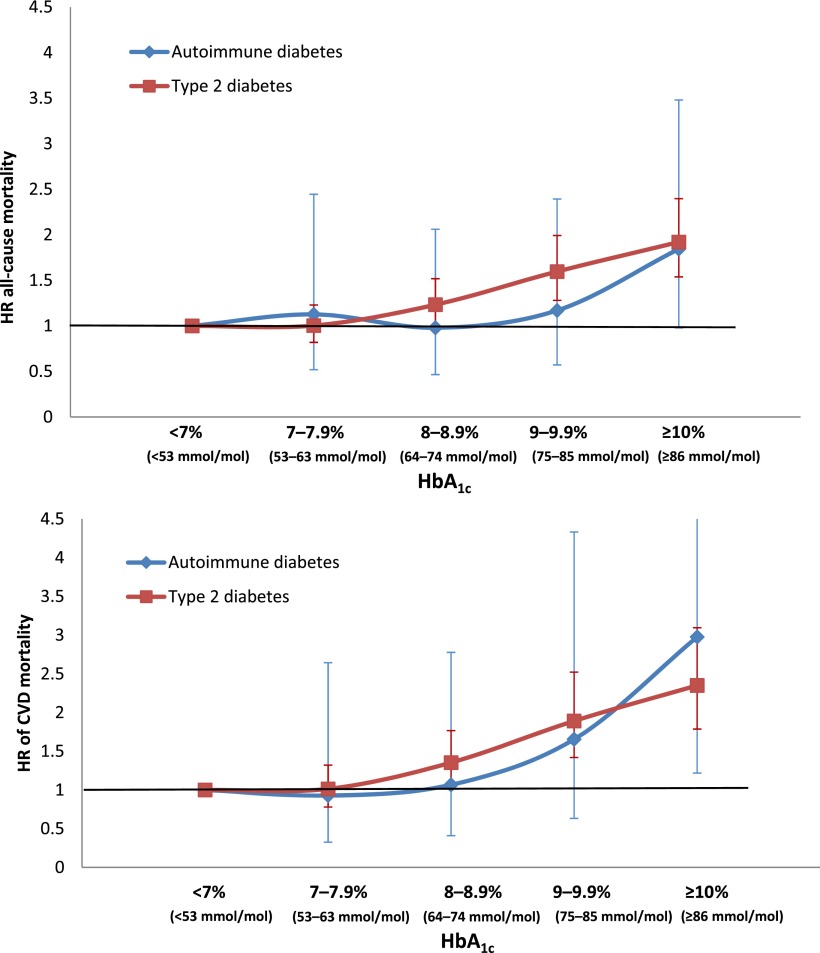
Excess mortality in autoimmune diabetes and type 2 diabetes was mainly seen in individuals with HbA1c ≥7% (≥53 mmol/mol) (Table 3). Mortality risks were also higher in individuals with higher fasting glucose (individuals being dichotomized according to the median of fasting glucose levels). Figure 1 displays HRs of all-cause and CVD mortality by HbA1c levels and shows that HbA1c was a strong indicator of mortality risk for both autoimmune diabetes and type 2 diabetes. For every unit of HbA1c, the HR of CVD mortality increased, with 1.22 (95% CI 1.06–1.40) in autoimmune diabetes and 1.15 (95% CI 1.10–1.20) in type 2 diabetes. The analyses in autoimmune diabetes were, however, limited by small numbers.
Table 3.
Risk of mortality in adult-onset autoimmune diabetes and type 2 diabetes compared with individuals without known diabetes, stratified on levels of HbA1c, fasting glucose, and insulin treatment
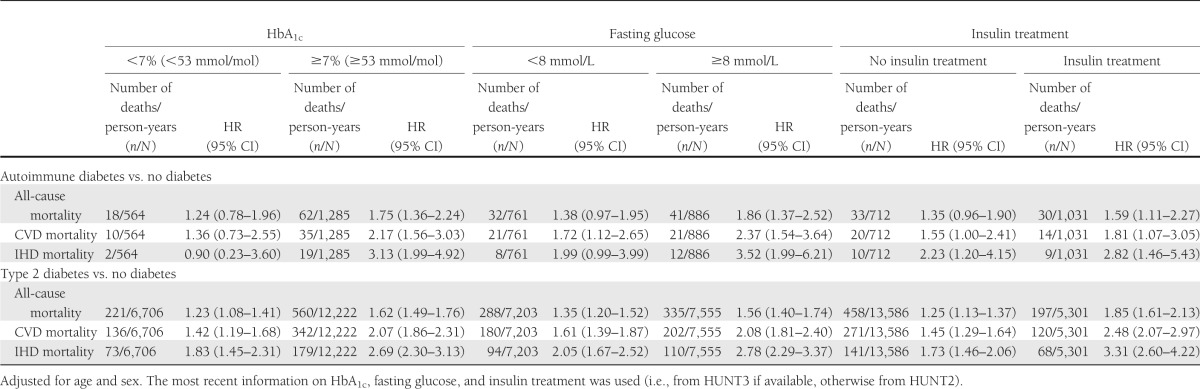
Figure 1.
HR of all-cause and CVD mortality in adult-onset autoimmune diabetes and type 2 diabetes by levels of HbA1c (reference HbA1c <7% [<53 mmol/mol]).
Duration of diabetes
Mortality increased with diabetes duration. For every year with diabetes, the HR for all-cause mortality was 1.02 (95% CI 0.99–1.05) in autoimmune diabetes and 1.03 (95% CI 1.02–1.04) in type 2 diabetes. The effect of duration on mortality in autoimmune diabetes was seen mainly in individuals with high HbA1c, with a HR of 1.02 (95% CI 0.99–1.05) vs. 0.97 (95% CI 0.88–1.06). A longer duration of diabetes was seen in insulin-treated versus non–insulin-treated individuals, and the HRs of all-cause mortality for insulin-treated versus non–insulin-treated individuals were 0.83 (95% CI 0.49–1.40) in autoimmune diabetes and 1.18 (95% CI 0.98–1.43) in type 2 diabetes, adjusted for age, sex, duration of diabetes, and HbA1c.
CONCLUSIONS
Our findings indicate that mortality in autoimmune diabetes in adults is at least as high as in type 2 diabetes, including a more than twofold increased risk of death from IHD. The excess risk was seen in men and women and was not explained by components of the metabolic syndrome or by socioeconomic factors but appeared for the major part to be associated with poor glycemic control.
We confirm previous findings (15,18–20) of worse glycemic control, as measured by HbA1c, in autoimmune diabetes compared with type 2 diabetes. Further, we find that excess mortality mainly afflicted patients with poor glycemic control. Also, fasting glucose levels (fasting glucose ≥8 mmol/L), another parameter of metabolic control, was associated with higher mortality risk. Mortality rates were similar for adult-onset autoimmune diabetes and type 2 diabetes, despite a clearly more favorable metabolic risk profile in individuals with autoimmune diabetes. Taken together, our results indicate that the effect of worse metabolic control is the major factor that increases mortality in adult-onset autoimmune diabetes and also in type 2 diabetes. In line with these results are findings from two recent Swedish studies in type 1 diabetes with young (≤30 years) onset (30,31) where a strong independent effect of poor glycemic control on risk of heart failure and CVD mortality was reported.
Our conclusions appear valid also when applied only to LADA and largely extend the findings on LADA of a previous, smaller, study (18). All-cause mortality was increased by 50% for LADA patients compared with individuals without diabetes, corresponding to the results seen for the whole group of adult-onset autoimmune diabetes. In addition, we show that the excess mortality risk pertains to men and women and, in particular, to death from IHD.
The strengths of this study include the use of data from a large population-based study and a long and virtually complete follow-up. Also, Norway is among the countries with the best quality of CVD death certification according to the ICD coding (32). Furthermore, we were able to take into account a large number of factors with potential influence on mortality, including metabolic risk factors, lifestyle, and socioeconomic factors. Lastly, to the best of our knowledge, ours is the largest study so far on mortality in autoimmune diabetes in adults.
Among possible limitations, a single measurement of HbA1c, as available here, is clearly suboptimal to repeated measures over time. Self-reporting of diabetes by questionnaire is another potential concern. A previous HUNT Study showed self-reporting was a valid method to identify known cases of diabetes (33), but individuals with undiagnosed diabetes will be missed. If the risk of not being diagnosed is related to diabetes type, this may affect the comparison between mortality in autoimmune diabetes versus type 2 diabetes. Specificity of the anti-GAD assay was calculated to be 100%; hence, individuals with type 2 diabetes would not have been falsely classified as autoimmune diabetes. However, a sensitivity of 64% means that some autoimmune diabetes may be classified as type 2 diabetes. Given that the proportion of autoimmune diabetes is much lower than that of type 2 diabetes, this misclassification is unlikely to distort the association between diabetes type and mortality, which could have been the consequence of choosing a cutoff with higher sensitivity but less specificity, in which case, individuals with type 2 diabetes could have been wrongly classified as having autoimmune diabetes.
Noteworthy is that levels of anti-GAD in juvenile-onset autoimmune diabetes often diminish or disappear with duration of diabetes. However, there are indications that GAD levels are more persistent in LADA (34,35) than in type 1 diabetes in children (36,37). A previous study (34) showed that most individuals who developed LADA between HUNT2 and HUNT3 were GAD-positive already at HUNT2 (i.e., during prediabetes), with no significant change in GAD levels between HUNT2 and HUNT3.
Mortality was lower in HUNT participants compared with the general Norwegian population. Part of the explanation for this difference might be that nonparticipation in HUNT was higher among those with poor health (38). One might speculate that individuals with better controlled diabetes are more prone to participate in a health study such as HUNT, and consequently, the diabetes-related mortality in our population may be an underestimation.
We conclude in this study, the largest so far on mortality in autoimmune diabetes with adult onset, that increased mortality risk in autoimmune diabetes in adults is at least of similar magnitude as seen for type 2 diabetes and that mortality is linked primarily to CVD. The increased risk appears to be associated primarily with poor glycemic control. These results highlight the need to improve treatment of hyperglycemia in autoimmune diabetes with adult onset.
Acknowledgments
The HUNT Study is a collaboration between the HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology, NTNU), the Nord-Trøndelag County Council, Norway, the Central Norway Health Authority, and the Norwegian Institute of Public Health. GlaxoSmithKline Norway supported the diabetes study at HUNT2 and HUNT3 financially through the Norwegian University of Science and Technology. This work was supported by a grant from the Swedish Council for Working Life and Social Research. No other potential conflicts of interest relevant to this article were reported.
All authors contributed to interpretation of results, reviewed and edited the manuscript, and approved the final version of the manuscript. L.O. contributed to developing the objective of the study, analyzed data, and was responsible for the writing of the manuscript. V.G. contributed to developing the objective of the study. K.M. researched data. T.A. analyzed data. S.C. contributed to developing the objective of the study. L.O. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
These data were presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012, and at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0564/-/DC1.
References
- 1.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992-1999. Diabetologia 2006;49:660–666 [DOI] [PubMed] [Google Scholar]
- 2.Laing SP, Swerdlow AJ, Slater SD, et al. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 2003;46:760–765 [DOI] [PubMed] [Google Scholar]
- 3.Dale AC, Midthjell K, Nilsen TI, Wiseth R, Vatten LJ. Glycaemic control in newly diagnosed diabetes patients and mortality from ischaemic heart disease: 20-year follow-up of the HUNT Study in Norway. Eur Heart J 2009;30:1372–1377 [DOI] [PubMed] [Google Scholar]
- 4.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol 2008;45:1–16 [DOI] [PubMed] [Google Scholar]
- 6.Bonora E, Targher G, Formentini G, et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med 2004;21:52–58 [DOI] [PubMed] [Google Scholar]
- 7.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009;52:65–73 [DOI] [PubMed] [Google Scholar]
- 8.Roper NA, Bilous RW, Kelly WF, Unwin NC, Connolly VM. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 2001;322:1389–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuomi T, Carlsson A, Li H, et al. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes 1999;48:150–157 [DOI] [PubMed] [Google Scholar]
- 10.Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab 2009;94:4635–4644 [DOI] [PubMed] [Google Scholar]
- 11.Hawa MI, Kolb H, Schloot N, et al. Action LADA consortium Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care 2013;36:908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groop L, Tuomi T, Rowley M, Zimmet P, Mackay IR. Latent autoimmune diabetes in adults (LADA)—more than a name. Diabetologia 2006;49:1996–1998 [DOI] [PubMed] [Google Scholar]
- 13.Isomaa B, Almgren P, Henricsson M, et al. Chronic complications in patients with slowly progressing autoimmune type 1 diabetes (LADA). Diabetes Care 1999;22:1347–1353 [DOI] [PubMed] [Google Scholar]
- 14.Römkens TE, Kusters GC, Netea MG, Netten PM. Prevalence and clinical characteristics of insulin-treated, anti-GAD-positive, type 2 diabetic subjects in an outpatient clinical department of a Dutch teaching hospital. Neth J Med 2006;64:114–118 [PubMed] [Google Scholar]
- 15.Buzzetti R, Di Pietro S, Giaccari A, et al. Non Insulin Requiring Autoimmune Diabetes Study Group High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care 2007;30:932–938 [DOI] [PubMed] [Google Scholar]
- 16.Hawa MI, Thivolet C, Mauricio D, et al. Action LADA Group Metabolic syndrome and autoimmune diabetes: action LADA 3. Diabetes Care 2009;32:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z, Xiang Y, Ji L, et al. LADA China Study Group Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes 2013;62:543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myhill P, Davis WA, Bruce DG, Mackay IR, Zimmet P, Davis TM. Chronic complications and mortality in community-based patients with latent autoimmune diabetes in adults: the Fremantle Diabetes Study. Diabet Med 2008;25:1245–1250 [DOI] [PubMed] [Google Scholar]
- 19.Roh MO, Jung CH, Kim BY, Mok JO, Kim CH. The prevalence and characteristics of latent autoimmune diabetes in adults (LADA) and its relation with chronic complications in a clinical department of a university hospital in Korea. Acta Diabetol 2013;50:129–134. [DOI] [PubMed] [Google Scholar]
- 20.Andersen CD, Bennet L, Nyström L, et al. Worse glycaemic control in LADA patients than in those with type 2 diabetes, despite a longer time on insulin therapy. Diabetologia 2013;56:252–258 [DOI] [PubMed] [Google Scholar]
- 21.Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care 2008;31:714–719 [DOI] [PubMed] [Google Scholar]
- 22.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012;308:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krokstad S, Langhammer A, Hveem K, et al. Cohort Profile: The HUNT Study, Norway. Int J Epidemiol 9 August 2012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Nauman J, Janszky I, Vatten LJ, Wisløff U. Temporal changes in resting heart rate and deaths from ischemic heart disease. JAMA 2011;306:2579–2587 [DOI] [PubMed] [Google Scholar]
- 25.Olsson L, Ahlbom A, Grill V, Midthjell K, Carlsson S. High levels of education are associated with an increased risk of latent autoimmune diabetes in adults: results from the Nord-Trøndelag health study. Diabetes Care 2011;34:102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fourlanos S, Dotta F, Greenbaum CJ, et al. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia 2005;48:2206–2212 [DOI] [PubMed] [Google Scholar]
- 27.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23:469–480 [DOI] [PubMed] [Google Scholar]
- 28.Cologne J, Hsu WL, Abbott RD, et al. Proportional hazards regression in epidemiologic follow-up studies: an intuitive consideration of primary time scale. Epidemiology 2012;23:565–573 [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization Mortality Database. Available from http://www.who.int/healthinfo/mortality_data/en/index.html Accessed 15 April 2012
- 30.Lind M, Bounias I, Olsson M, Gudbjörnsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet 2011;378:140–146 [DOI] [PubMed] [Google Scholar]
- 31.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care 2010;33:1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozano R, Murray CJL, Lopez AD, Satoh T. Miscoding and misclassification of ischemic heart disease mortality. In Global Programme on Evidence for Health Policy working paper No. 12. Geneva, Switzerland, World Health Organization, 2001.
- 33.Midthjell K, Holmen J, Bjørndal A, Lund-Larsen G. Is questionnaire information valid in the study of a chronic disease such as diabetes? The Nord-Trøndelag diabetes study. J Epidemiol Community Health 1992;46:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sørgjerd EP, Skorpen F, Kvaløy K, Midthjell K, Grill V. Time dynamics of autoantibodies are coupled to phenotypes and add to the heterogeneity of autoimmune diabetes in adults: the HUNT study, Norway. Diabetologia 2012;55:1310–1318 [DOI] [PubMed] [Google Scholar]
- 35.Borg H, Gottsäter A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes 2002;51:1754–1762 [DOI] [PubMed] [Google Scholar]
- 36.Decochez K, Keymeulen B, Somers G, et al. Belgian Diabetes Registry Use of an islet cell antibody assay to identify type 1 diabetic patients with rapid decrease in C-peptide levels after clinical onset. Belgian Diabetes Registry. Diabetes Care 2000;23:1072–1078 [DOI] [PubMed] [Google Scholar]
- 37.Decochez K, Tits J, Coolens JL, et al. High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age. The Belgian Diabetes Registry. Diabetes Care 2000;23:838–844 [DOI] [PubMed] [Google Scholar]
- 38.Langhammer A, Krokstad S, Romundstad P, Heggland J, Holmen J. The HUNT study: participation is associated with survival and depends on socioeconomic status, diseases and symptoms. BMC Med Res Methodol 2012;12:143. [DOI] [PMC free article] [PubMed] [Google Scholar]