Abstract
OBJECTIVE
To investigate the long-term safety and efficacy of empagliflozin, a sodium glucose cotransporter 2 inhibitor; sitagliptin; and metformin in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
In this randomized, open-label, 78-week extension study of two 12-week, blinded, dose-finding studies of empagliflozin (monotherapy and add-on to metformin) with open-label comparators, 272 patients received 10 mg empagliflozin (166 as add-on to metformin), 275 received 25 mg empagliflozin (166 as add-on to metformin), 56 patients received metformin, and 56 patients received sitagliptin as add-on to metformin.
RESULTS
Changes from baseline in HbA1c at week 90 were −0.34 to −0.63% (−3.7 to −6.9 mmol/mol) with empagliflozin, −0.56% (−6.1 mmol/mol) with metformin, and −0.40% (−4.4 mmol/mol) with sitagliptin. Changes from baseline in weight at week 90 were −2.2 to −4.0 kg with empagliflozin, −1.3 kg with metformin, and −0.4 kg with sitagliptin. Adverse events (AEs) were reported in 63.2–74.1% of patients on empagliflozin and 69.6% on metformin or sitagliptin; most AEs were mild or moderate in intensity. Hypoglycemic events were rare in all treatment groups, and none required assistance. AEs consistent with genital infections were reported in 3.0–5.5% of patients on empagliflozin, 1.8% on metformin, and none on sitagliptin. AEs consistent with urinary tract infections were reported in 3.8–12.7% of patients on empagliflozin, 3.6% on metformin, and 12.5% on sitagliptin.
CONCLUSIONS
Long-term empagliflozin treatment provided sustained glycemic and weight control and was well tolerated with a low risk of hypoglycemia in patients with type 2 diabetes.
Type 2 diabetes is characterized by insulin resistance and progressive deterioration of β-cell function (1). Metformin is the recommended first-line antidiabetes agent for patients with type 2 diabetes (2). However, in order to achieve and maintain glycemic control as the disease progresses, patients often require therapies in addition to metformin (2,3).
Despite the availability of a number of antihyperglycemic agents, the side effects associated with existing treatments and their gradual loss of efficacy over time (2,3) mean that many patients with type 2 diabetes do not reach therapeutic goals (3,4). In addition, treatment is often complicated by common comorbidities of type 2 diabetes such as obesity and hypertension, which are not addressed by existing oral antidiabetes agents (5–7).
Inhibition of sodium glucose cotransporter 2 (SGLT2), located in the proximal tubule of the kidney, represents an approach for the treatment of type 2 diabetes that is independent of β-cell function and insulin resistance (8,9). SGLT2 mediates most of renal glucose reabsorption, and inhibition of this transporter leads to reduced reabsorption of filtered glucose and increased urinary glucose excretion (8,10), resulting in reduced plasma glucose levels in patients with type 2 diabetes (8–10). In addition, this mechanism leads to weight loss owing to the loss of calories via urinary glucose excretion (8,11).
Empagliflozin is a potent and selective inhibitor of SGLT2 (12), which in patients with type 2 diabetes causes urinary glucose excretion of up to 90 g/day (13). In two placebo- and active-controlled, dose-finding trials, treatment with empagliflozin for 12 weeks in patients with type 2 diabetes was generally well tolerated and resulted in placebo-corrected reductions in HbA1c of up to 0.72% (7.9 mmol/mol) and placebo-corrected reductions in weight of up to 1.7 kg (14,15). In these studies, reductions in HbA1c were comparable to those of the active comparators metformin and sitagliptin (14,15). The objective of this study was to assess the long-term safety and efficacy of empagliflozin, sitagliptin, and metformin in a 78-week, open-label extension study of two dose-finding trials.
RESEARCH DESIGN AND METHODS
Study design
This was a phase IIb, randomized, active-controlled, open-label extension trial. The trial was conducted at 132 trial sites in 21 countries (Austria, Croatia, Czech Republic, Estonia, Finland, France, Germany, Hungary, Italy, Korea, Latvia, Lithuania, Norway, Romania, Russia, Slovakia, Spain, Sweden, Taiwan, Ukraine, and U.S.). The trial was registered with ClinicalTrials.gov (NCT00881530) and was carried out in compliance with the protocol and the principles of the Declaration of Helsinki in accordance with the International Conference on Harmonization Harmonized Tripartite Guideline for Good Clinical Practice. The trial was approved by respective institutional review boards, and independent ethics committees, and competent authorities according to national and international regulations. All patients provided signed and dated informed consent prior to participation in the extension study.
Patients with type 2 diabetes were eligible for inclusion in the extension study if they had successfully completed one of two 12-week, blinded, randomized, placebo-controlled dose-finding trials (study 1 and study 2). In these preceding trials, male and female patients with type 2 diabetes were eligible for inclusion if they were aged ≥18 and ≤79 years with a BMI ≤40 kg/m2 and had insufficient glycemic control (HbA1c ≥7.0 to <10.0% [≥53 to <86 mmol/mol]) at the start of the placebo run-in period. Full details of the inclusion and exclusion criteria for these trials have previously been described (14,15). At inclusion in the preceding blinded trial, patients were either treatment naïve (study 1) or had been on a stable dose of metformin immediate release (IR) of ≥1,500 mg/day or maximum tolerated dose for ≥10 weeks (study 2). In study 1, treatment-naïve patients were randomized to receive 5, 10, or 25 mg empagliflozin once daily (qd), placebo (all double blind), or open-label metformin-IR (up to a maximum of 1,000 mg twice daily or the maximum tolerated dose) for 12 weeks. In study 2, patients on stable metformin background therapy were randomized to receive 1, 5, 10, 25, or 50 mg empagliflozin qd, placebo (all double blind), or open-label sitagliptin (100 mg qd) for 12 weeks.
In the current open-label extension trial, patients from the comparator arms of the preceding trials (study 1, metformin monotherapy, or study 2, sitagliptin as add-on to metformin) continued open-label treatment for an additional 78 weeks and patients on 10 mg or 25 mg empagliflozin in the preceding trials continued on the same dose for a further 78 weeks (Supplementary Fig. 1). Patients on placebo or 1, 5, or 50 mg empagliflozin in the preceding trials were rerandomized to either 10 mg or 25 mg empagliflozin for 78 weeks (Supplementary Fig. 1).
Safety and efficacy end points
The primary objective was to investigate the safety of long-term treatment with empagliflozin. Safety and tolerability were assessed descriptively based on the frequency of adverse events (AEs), the frequency of hypoglycemic events (plasma glucose of ≤70 mg/dL [≤3.9 mmol/L]), changes from baseline in vital signs, and changes from baseline in clinical laboratory parameters. Only AEs and hypoglycemic events that occurred during the extension trial were analyzed. Events consistent with urinary tract infection and genital infection were identified from AEs reported spontaneously by the investigator based on a special search of MedDRA preferred terms. The baseline source for analyses of changes in vital signs and clinical laboratory parameters was before the first intake of active treatment in either the preceding trial (study 1 or study 2) or extension trial. In addition, changes in estimated glomerular filtration rate (eGFR) estimated using the Modification of Diet in Renal Disease (MDRD) equation, were analyzed over 90 weeks in patients who were randomized to 10 or 25 mg empagliflozin, metformin, or sitagliptin in the preceding trials without rerandomization in the extension trial. The baseline for this analysis was defined as the last observed measurement before the first administration of the study drug in the preceding trial.
Exploratory efficacy analyses investigated the change from baseline of the preceding trial to week 78 of the extension trial (i.e., over 90 weeks) in HbA1c, fasting plasma glucose (FPG), body weight, waist circumference, systolic blood pressure (SBP), and diastolic blood pressure (DBP) in patients who took empagliflozin (any dose as monotherapy or as add on to metformin), metformin only, or sitagliptin as add-on to metformin in both the preceding trial (study 1 or study 2) and extension trial. The proportions of patients who reached HbA1c <7% (<53 mmol/mol) were recorded over the extension study. The baseline for these analyses was defined as the last observed measurement before the first administration of the study drug in study 1 or study 2.
Post hoc efficacy analyses investigated changes over 90 weeks from baseline of the preceding trial to week 78 of the extension trial in HbA1c, FPG, body weight, SBP, and DBP in patients who were randomized to 10 or 25 mg empagliflozin, metformin, or sitagliptin in study 1 or study 2 without rerandomization in the extension trial. The baseline for these analyses was defined as the last observed measurement before the first administration of the study drug in study 1 or study 2.
Statistical analysis
Safety and tolerability were assessed descriptively for all patients who received at least one dose of study drug in the extension trial. Reports and analysis of AEs were based on treatment-emergent AEs (those occurring between first drug intake and 7 days after last treatment administration or that started before first drug intake and worsened under treatment). Laboratory values taken after the first dose of study medication to 3 days after the last dose were assigned to the treatment period. Changes in laboratory values were presented descriptively. No imputation was used for missing safety data. Efficacy data after rescue medication were set to missing; missing data were imputed using the last-observation-carried-forward approach.
The efficacy analyses used an analysis of covariance (ANCOVA) model with treatment group and number of previously-used antidiabetes medications as fixed effects, the corresponding baseline as a covariate, and country as a random effect. The analyses were performed for HbA1c, FPG, body weight, waist circumference, SBP, and DBP on the full analysis set (all randomized patients who were treated with at least one dose of study drug and had a baseline HbA1c assessment). The percentage of patients reaching HbA1c <7% (<53 mmol/mol) at week 78 was assessed descriptively using observed cases for all patients who received at least one dose of study drug in the extension trial.
RESULTS
Patients
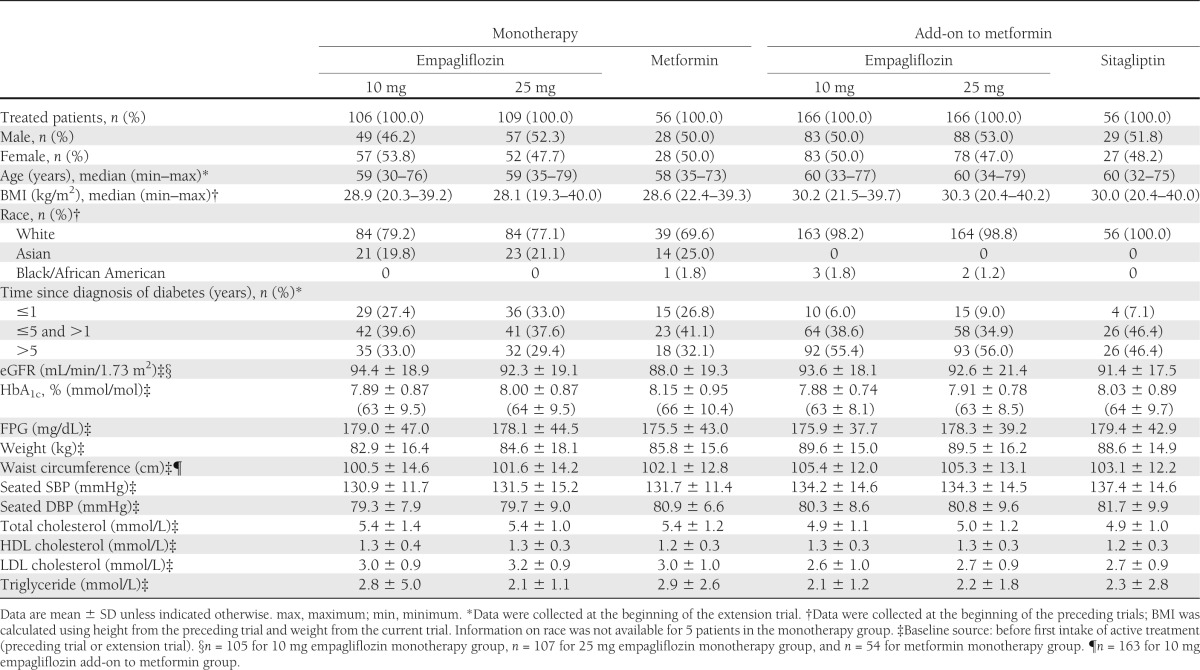
In total, 67.0% of patients from the 12-week monotherapy dose-finding trial (study 1) continued in the extension trial. A total of 106 patients were randomized to receive 10 mg empagliflozin, 109 patients were randomized to 25 mg empagliflozin, and 56 patients continued on metformin. In total, 78.4% of patients from the 12-week add-on to metformin dose-finding trial (study 2) continued in the extension trial. A total of 166 patients were randomized to receive 10 mg empagliflozin, 166 patients were randomized to 25 mg empagliflozin, and 56 patients continued on sitagliptin. Patient demographics and baseline characteristics were balanced between groups (Table 1). Of 660 patients enrolled from both study 1 and study 2, 607 patients (92%) completed the trial. One patient withdrew consent before taking the first dose of study medication during the extension, and 52 patients (7.9%) prematurely discontinued trial medication. The most frequent reason for discontinuation was AEs (23 patients [3.5%]).
Table 1.
Patient demographics and baseline characteristics of all treated patients in the extension trial
Efficacy
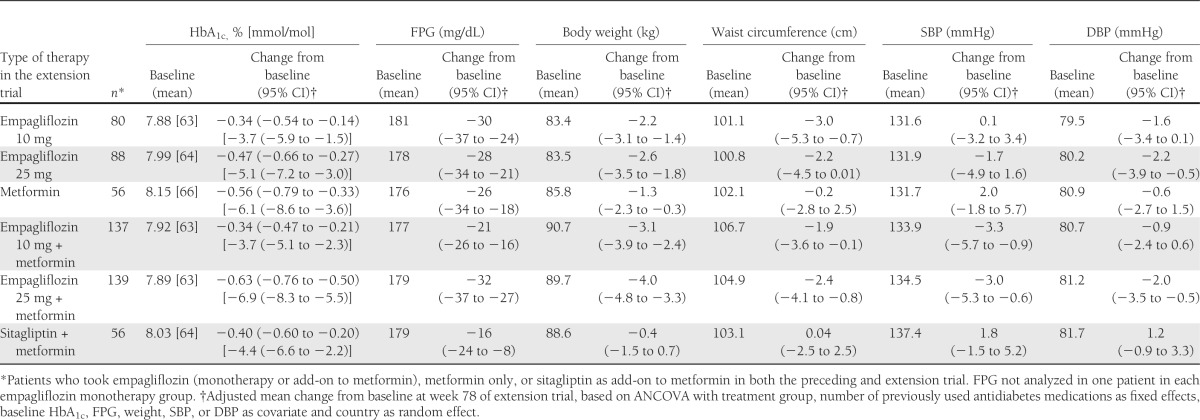
Patients who received empagliflozin over 90 weeks (any dose of empagliflozin during study 1 or 2, 10 or 25 mg during the extension trial) showed clinically meaningful reductions in HbA1c, FPG, body weight, and waist circumference at week 78 of the extension study compared with baseline of the preceding trial (Table 2). In the monotherapy groups, 31.9, 32.1, and 31.0% of patients on 10 mg empagliflozin, 25 mg empagliflozin, and metformin, respectively, reached HbA1c <7% (<53 mmol/mol) at week 78 of the extension study. Of patients on background metformin therapy, 27.0, 44.6, and 36.8% of patients on 10 mg empagliflozin, 25 mg empagliflozin, and sitagliptin, respectively, reached HbA1c <7% (<53 mmol/mol) at week 78 of the extension study. Clinically meaningful reductions from baseline in blood pressure were observed in most empagliflozin groups except for the 10 mg empagliflozin monotherapy group (Table 2). Reductions in blood pressure were not associated with increases in heart rate (data not shown).
Table 2.
Adjusted mean changes from baseline in HbA1c, FPG, body weight, waist circumference, SBP, and DBP at week 78 for patients who took empagliflozin (any dose as monotherapy or add-on to metformin), metformin IR only, or sitagliptin as add-on to metformin in both the preceding trial (study 1 or study 2) and extension trial
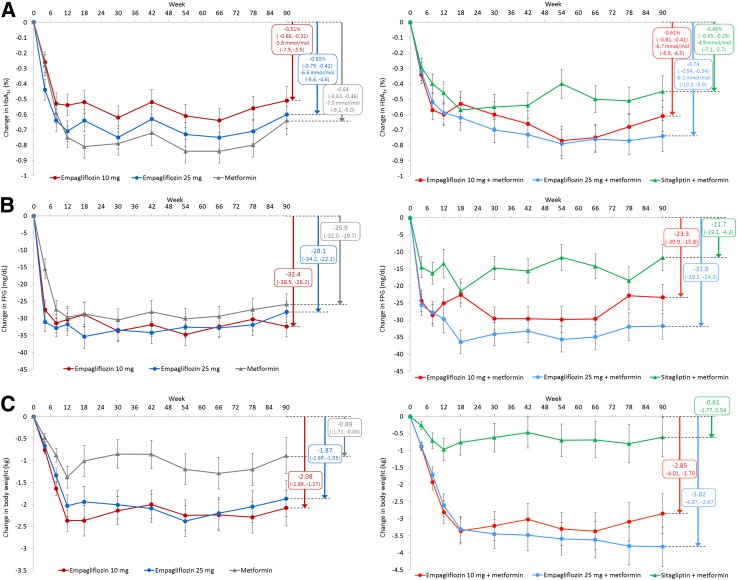
Reductions in HbA1c, FPG, and body weight were sustained with empagliflozin over 90 weeks, as highlighted in patients who received 10 mg empagliflozin, 25 mg empagliflozin, metformin, or sitagliptin without rerandomization after 12 weeks of the preceding trials (Fig. 1A–C).
Figure 1.
Mean ± SE change from baseline in efficacy variables over 90 weeks in patients who received 10 mg empagliflozin, 25 mg empagliflozin, or metformin IR in study 1 or 10 mg empagliflozin, 25 mg empagliflozin, or sitagliptin as add-on to metformin in study 2 without rerandomization at the start of the extension trial. Changes in HbA1c (A), changes in FPG (B), and changes in body weight (C) in patients on monotherapy (left panels) and on add-on to metformin therapy (right panels). Full analysis set, last-observation-carried-forward. Based on ANCOVA with treatment group, number of previously used antidiabetes medications as fixed effects, the corresponding baseline value of the end point under consideration as a covariate, and country as random effect. Boxes show mean change from baseline (95% CI) at week 78 of the extension trial (i.e., after 90 weeks’ treatment).
Safety
The proportion of patients with at least one AE was similar between empagliflozin groups (63.2–74.1%) and comparator groups (69.6% with either metformin or sitagliptin as add-on to metformin) (Table 3). More than 90% of AEs were mild or moderate in intensity, as assessed by the investigator. Severe AEs were reported in 2.4–6.6% of patients in empagliflozin groups, 7.1% of patients on metformin, and 8.9% of patients on sitagliptin as add-on to metformin (Table 3). The frequency of investigator-defined drug-related AEs was higher in empagliflozin groups (11.9–14.5%) than in the comparator groups (7.1% with metformin and 8.9% with sitagliptin as add-on to metformin) (Table 3). Serious AEs were reported more frequently with sitagliptin as add-on to metformin (16.1%) compared with empagliflozin (6.0–9.4%) or metformin (5.4%); only one serious AE was considered drug related by the investigator (hyperglycemia in one patient on sitagliptin as add-on to metformin). AEs led to discontinuation in 0.9–5.4% of patients in empagliflozin groups, 1.8% of patients on metformin, and 3.6% of patients on sitagliptin as add-on to metformin (Table 3). The most frequently reported AEs (by preferred term) were hyperglycemia (11.3–21.7% of patients on empagliflozin, 14.3% on metformin only, and 19.6% on sitagliptin as add-on to metformin), nasopharyngitis (7.2–9.4% of patients on empagliflozin, 16.1% on metformin only, and 8.9% on sitagliptin as add-on to metformin), and urinary tract infection (2.8–10.8% of patients on empagliflozin, 1.8% on metformin only, and 8.9% on sitagliptin as add-on to metformin).
Table 3.
Patients with AEs in the extension trial
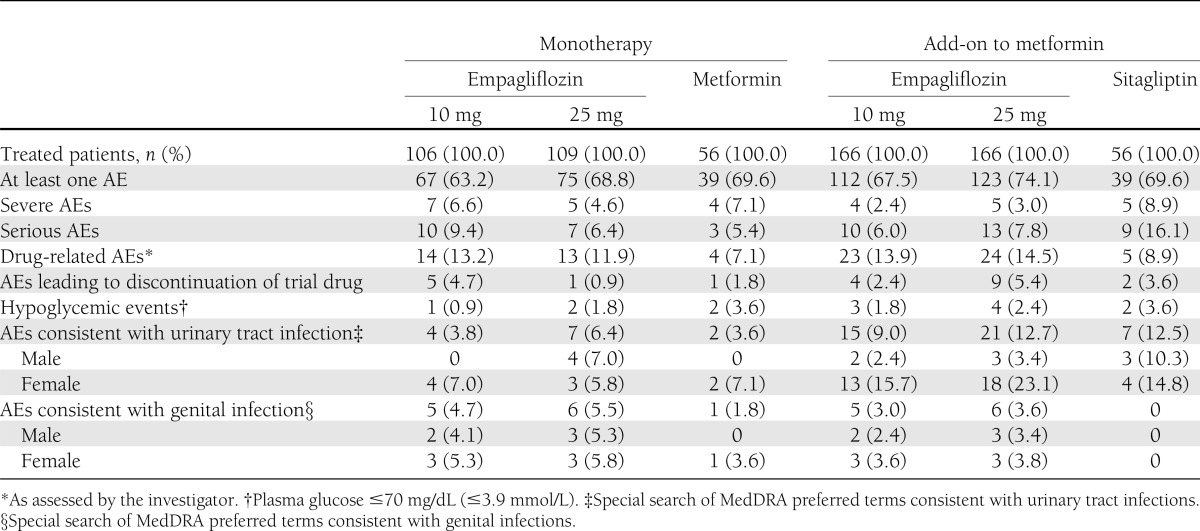
Hypoglycemic events were reported in 0.9–2.4% of patients on empagliflozin, 3.6% on metformin monotherapy, and 3.6% on sitagliptin as add-on to metformin. All hypoglycemic events occurred >28 days after intake of study medication, and none required assistance. No patients discontinued a study drug owing to hypoglycemic events. Events consistent with urinary tract infection (based on a special search of MedDRA preferred terms) were reported in 3.8–6.4% of patients on empagliflozin monotherapy, 3.6% on metformin monotherapy, 9.0–12.7% on empagliflozin as add-on to metformin, and 12.5% on sitagliptin as add-on to metformin. Most events consistent with urinary tract infection were mild in intensity, none were severe or serious, and no patients discontinued a study drug due to these events. More patients on empagliflozin or sitagliptin as add-on to metformin reported events consistent with urinary tract infection compared with empagliflozin or metformin monotherapy (Table 3). AEs consistent with genital infection (based on a special search of MedDRA preferred terms) were observed more frequently with empagliflozin (3.0–5.5%) than metformin monotherapy (1.8%) or sitagliptin as add-on to metformin (no events) and led to discontinuation of empagliflozin treatment in 4 of the 22 affected patients. Most events consistent with genital infection were mild; none were severe or serious.
Changes in eGFR over 90 weeks were small and comparable across treatment groups, as analyzed in patients who were randomized to 10 or 25 mg empagliflozin, metformin, or sitagliptin in the preceding trials without rerandomization in the extension trial (Supplementary Fig. 2). Treatment with empagliflozin did not increase markers of glomerular or tubular damage such as urinary albumin or α 1 microglobulin concentration (Supplementary Table 1).
There were no changes in electrolyte (sodium, potassium, calcium, phosphate, or magnesium) levels in any treatment group at the end of the 78-week extension (Supplementary Table 1). In empagliflozin groups, mean hematocrit values increased by 2.1–3.4% from baseline compared with decreases of 2.1% with metformin and 1.3% with sitagliptin as add-on to metformin (Supplementary Table 1). In empagliflozin groups, mean uric acid values decreased by 44–51 μmol/L from baseline compared with increases of 25 μmol/L with metformin and 7 μmol/L with sitagliptin as add-on to metformin (Supplementary Table 1). Treatment with empagliflozin resulted in small changes in lipid profiles. Mean changes from baseline in LDL cholesterol were −0.01 to 0.16 mmol/L in empagliflozin groups, −0.14 mmol/L with metformin, and 0.06 mmol/L with sitagliptin as add-on to metformin (Supplementary Table 1). HDL cholesterol levels increased from baseline by 0.15 to 0.18 mmol/L in empagliflozin groups, 0.14 mmol/L with metformin, and 0.09 with sitagliptin as add-on to metformin (Supplementary Table 1).
CONCLUSIONS
This open-label extension trial investigated long-term safety and efficacy of empagliflozin, metformin, and sitagliptin. Significant reductions in HbA1c, FPG, and body weight with empagliflozin compared with placebo have already been reported in the preceding 12-week dose-finding trials (14,15). This extension trial demonstrated that long-term empagliflozin treatment provided sustained glycemic control and body weight loss in patients with type 2 diabetes for up to 90 weeks.
Furthermore, long-term treatment with empagliflozin was well tolerated. In this extension trial, the proportion of patients with at least one AE in empagliflozin groups was similar to that with metformin and sitagliptin as add-on to metformin. Most AEs were mild or moderate in intensity. Serious AEs were reported more frequently with sitagliptin compared with empagliflozin or metformin. The range in the number of patients with AEs leading to discontinuation in empagliflozin groups was similar to the number with metformin and sitagliptin as add-on to metformin. The number of patients with hypoglycemic events was low in all groups. The range in the number of patients with events consistent with urinary tract infection was similar between empagliflozin and comparators on the same background therapy, but more patients on background metformin therapy reported events compared with those on monotherapy. This may be due to differences in patient demographics or investigator or patient reporting; the effect of background metformin therapy is unlikely to cause these differences. In phase III trials of empagliflozin (data not published) and other SGLT2 inhibitors as monotherapy or add-on to metformin (16–19), the overall frequency of events consistent with urinary tract infections did not differ by background therapy. Events consistent with genital infection were reported more frequently in empagliflozin groups compared with metformin or sitagliptin as add-on to metformin, but events were mostly mild and few led to treatment discontinuation. Changes in eGFR were small and comparable among treatment groups.
Long-term safety and efficacy data are important for agents that may be used for chronic treatment. In this trial, empagliflozin showed a favorable benefit-to-risk ratio with clinically meaningful and sustained efficacy with good tolerability for up to 90 weeks. However, its open-label design, and the fact that only 73.3% of patients from the preceding trials continued in the extension study, may impact on the overall benefit-to-risk assessment of empagliflozin. Phase III trials will provide further information on the efficacy and safety of empagliflozin in patients with type 2 diabetes.
In conclusion, this extension trial demonstrated that treatment with empagliflozin in patients with type 2 diabetes provided sustained glycemic control and weight loss over 90 weeks and was generally well tolerated. These data provide support for the evaluation of empagliflozin as a treatment for type 2 diabetes in phase III trials.
Acknowledgments
This study was funded by Boehringer Ingelheim. S.H., S.P., T.H., H.J.W., and U.C.B. are employees of Boehringer Ingelheim. A.B. is an external trial clinical monitor working on behalf of Boehringer Ingelheim. E.F. has received consulting honoraria and/or research grant support from Merck & Co., GlaxoSmithKline, Daiichi Sankyo, Janssen/Johnson & Johnson, Boehringer Ingelheim, Lilly & Co., Sanofi, Halozyme, Bristol-Myers Squibb/AstraZeneca, and Astellas. Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Elizabeth Ng of Fleishman-Hillard Group Ltd during the preparation of this article. No other potential conflicts of interest relevant to this article were reported.
E.F. contributed to the acquisition and interpretation of data and drafted the manuscript. A.B., S.H., S.P., and U.C.B. contributed to the study design and interpretation of data and reviewed and edited the manuscript. T.H. and H.J.W. contributed to the interpretation of data and reviewed and edited the manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version. S.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
Clinical trial reg. no. NCT00881530, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0663/-/DC1, in which a complete list of investigators can be found.
References
- 1.Campbell RK. Fate of the beta-cell in the pathophysiology of type 2 diabetes. J Am Pharm Assoc (2003) 2009;49(Suppl. 1):S10–S15 [DOI] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 3.Turner RC, Cull CA, Frighi V, Holman RR, UK Prospective Diabetes Study (UKPDS) Group Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 4.Alvarez Guisasola F, Mavros P, Nocea G, Alemao E, Alexander CM, Yin D. Glycaemic control among patients with type 2 diabetes mellitus in seven European countries: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) study. Diabetes Obes Metab 2008;10(Suppl. 1):8–15 [DOI] [PubMed] [Google Scholar]
- 5.Aguilar RB. Evaluating treatment algorithms for the management of patients with type 2 diabetes mellitus: a perspective on the definition of treatment success. Clin Ther 2011;33:408–424 [DOI] [PubMed] [Google Scholar]
- 6.Stolar MW, Hoogwerf BJ, Gorshow SM, Boyle PJ, Wales DO. Managing type 2 diabetes: going beyond glycemic control. J Manag Care Pharm 2008;14(5 Suppl. B):s2–s19 [PubMed] [Google Scholar]
- 7.Stolar MW. Defining and achieving treatment success in patients with type 2 diabetes mellitus. Mayo Clin Proc 2010;85(Suppl. 12):S50–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney Int Suppl 2011;120:S20–S27 [DOI] [PubMed] [Google Scholar]
- 9.Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci 2011;32:63–71 [DOI] [PubMed] [Google Scholar]
- 10.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012;8:495–502 [DOI] [PubMed] [Google Scholar]
- 11.Bolinder J, Ljunggren O, Kullberg J, et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–1031 [DOI] [PubMed] [Google Scholar]
- 12.Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab 2012;14:83–90 [DOI] [PubMed] [Google Scholar]
- 13.Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with T2DM. Diabetes Ther. 10 July 2013 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:721–728 [DOI] [PubMed] [Google Scholar]
- 15.Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 1 August 2013 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:2223–2233 [DOI] [PubMed] [Google Scholar]
- 17.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenstock J, Aggarwal N, Polidori D, et al. Canagliflozin DIA 2001 Study Group Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]