Abstract
OBJECTIVE
To estimate the prevalence of and risk factors for diabetic peripheral neuropathy (DPN) in a pilot study among youth participating in the SEARCH for Diabetes in Youth study.
RESEARCH DESIGN AND METHODS
DPN was assessed using the Michigan Neuropathy Screening Instrument (MNSI) (examination for foot abnormalities, distal vibration perception, and ankle reflexes). An MNSI exam (MNSIE) score >2 is diagnostic for DPN.
RESULTS
The MNSIE was completed in 399 subjects, including 329 youth with type 1 diabetes (mean age 15.7 ± 4.3 years, duration 6.2 ± 0.9 years) and 70 with type 2 diabetes (mean age 21.6 ± 4.1 years, duration 7.6 ± 1.8 years). Glycated hemoglobin (A1C) was similar in both groups (8.8 ± 1.8% for type 1 vs. 8.5 ± 2.9% for type 2). The prevalence of DPN was significantly higher in youth with type 2 compared with those with type 1 diabetes (25.7 vs. 8.2%; P < 0.0001). In unadjusted analyses, diabetes type, older age, longer duration of diabetes, increased waist circumference, elevated blood pressure, lower HDL cholesterol, and presence of microalbuminuria (urinary albumin-to-creatinine ratio >30 mg/g) were associated with DPN. The association between diabetes type and DPN remained significant after adjustment for age and sex (odds ratio 2.29 [95% CI 1.05–5.02], P = 0.03).
CONCLUSIONS
DPN prevalence among youth with type 2 diabetes approached rates reported in adult populations with diabetes. Our findings suggest not only that youth with diabetes are at risk for DPN but also that many already show measurable signs of DPN.
The incidence of both type 1 and type 2 diabetes in youth is increasing worldwide (1,2). Recent reports have projected that, if this trend continues, the prevalence of diabetes among the young in the U.S. could triple by the year 2050 (3). This could incur a significant burden on health care costs and on society, especially as these young people enter their peak working and earning capacity at the time when diabetes complications begin to occur. Diabetic peripheral neuropathy (DPN) is among the most distressing of all the chronic complications of diabetes and is a cause of significant disability and poor quality of life (4). Depending on the patient population and diagnostic criteria, the prevalence of DPN among adults with diabetes ranges from 30 to 70% (5–7). However, there are insufficient data on the prevalence and predictors of DPN among the pediatric population. Furthermore, early detection and good glycemic control have been proven to prevent or delay adverse outcomes associated with DPN (5,8,9). Near-normal control of blood glucose beginning as soon as possible after the onset of diabetes may delay the development of clinically significant nerve impairment (8,9). Therefore, children and adolescents with diabetes represent a critical target for primary prevention of this complication.
The American Diabetes Association (ADA) recommends screening for DPN in children and adolescents with type 2 diabetes at diagnosis and 5 years after diagnosis for those with type 1 diabetes, followed by annual evaluations thereafter, using simple clinical tests (10). Since subclinical signs of DPN may precede development of frank neuropathic symptoms, systematic, preemptive screening is required in order to identify DPN in its earliest stages.
There are various measures that can be used for the assessment of DPN. The Michigan Neuropathy Screening Instrument (MNSI) is a simple, sensitive, and specific tool for the screening of DPN (11). It was validated in large independent cohorts (12,13) and has been widely used in clinical trials and longitudinal cohort studies including the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) (13).
The aim of this pilot study was to provide preliminary estimates of the prevalence of and factors associated with DPN among children and adolescents with type 1 and type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study participants and data collection
SEARCH for Diabetes in Youth is a population-based study of diabetes in young people of diverse racial and ethnic backgrounds in the U.S. (14). Study methods for SEARCH have previously been described (14). SEARCH is an observational longitudinal study of youth with diabetes diagnosed before the age of 20 years in the U.S. SEARCH participants are drawn from four geographically defined populations in Ohio, Washington, South Carolina, and Colorado; health plan enrollees in Hawaii and California; and Indian Health Service beneficiaries from four American Indian populations. Prior to protocol implementation, local institutional review board approval was obtained for each center. Participants 18 years and older and parents of youth under age 18 years provided written informed consent for participation; youth provided assent.
Once enrolled, the parent/guardian or study participants age 18 years and older were invited to complete a short survey that included questions about race and ethnicity, diabetes treatment, and other information. Participants who completed the initial survey were invited to a baseline study visit; surveys were administered to obtain clinical and demographic information, as well as psychosocial burden. In addition, a physical examination was completed to measure systolic and diastolic blood pressure, height, weight, and waist circumference. A blood sample was collected by venipuncture. Youth whose diabetes was incident in 2002 through 2005 and who completed a baseline study visit were invited to return for follow-up visits at ~12, 24, and 60 months after their baseline visit. Youth who missed a follow-up visit window remained eligible to complete the subsequent follow-up visit(s).
Data for this pilot study were collected from five of the SEARCH sites and included 399 youth diagnosed with diabetes before 20 years of age whose diabetes was incident in 2002–2005. DPN assessment was conducted at the 60-month follow-up visit. In addition, a subset of youth with type 2 diabetes who were part of the 2001 prevalent cohort that participated in a baseline visit were also invited to participate in the DPN pilot study. The pilot study was approved by the institutional review board(s) at each study site. Diabetes type was categorized as type 1 or type 2 based on the health care provider diagnosis. Race/ethnicity was self-reported using the 2000 census questionnaire format, and five categories were created: non-Hispanic white, Hispanic (regardless of race), non-Hispanic black, Asian/Pacific Islander, and Native American. Current cigarette smoking was defined as having smoked cigarettes on ≥1 of the 30 days preceding the survey. Blood samples were obtained under conditions of metabolic stability after at least 8 h of fasting. Specimens were processed locally at the sites and shipped within 24 h to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington), where they were analyzed for measurement of total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, and glycated hemoglobin (A1C) as previously described (14). Urinary albumin levels were assessed on a random spot urine sample, and microalbuminuria was defined as urine albumin-to-creatinine ratio >30 mg albumin/g creatinine.
Assessment of DPN
DPN was assessed using the MNSI, a validated screening tool (11). SEARCH staff from each center were centrally trained and certified to perform the MNSI. The MNSI is a 15-item self-administered questionnaire (MNSIQ) and structured examination of the feet (MNSIE) scored for abnormalities of appearance (deformities, infection, and dry skin/callus), presence of ulcers, vibration perception threshold (VPT) at the distal great toe, and ankle reflexes. Threshold for DPN, established by prior validation studies performed among adults, is a score of >2 on the MNSIE (12,13).
Statistical analyses
Differences in the demographic, anthropometric, clinical, and metabolic parameters between adolescents and young adults with type 1 and type 2 diabetes were compared using Student t test for continuous variables and the χ2 test for categorical variables. Factors associated with DPN were assessed independently using the cutoff score of 2 on the MNSIE to define DPN. Logistic regression analysis was conducted to estimate the odds of DPN associated with diabetes type, controlling for the variables found to be significant in the univariate analysis. The data were analyzed using SAS 9.2 (SAS Institute, Cary, NC). The level of significance was set at α = 0.05.
RESULTS
The characteristics of the study population are depicted in Table 1. A total of 399 youth (329 with type 1 and 70 with type 2 diabetes) participated in the pilot study. Youth with type 1 diabetes were younger (mean age 15.7 ± 4.3 years) and had a shorter duration of diabetes (mean duration 6.2 ± 0.9 years) compared with youth with type 2 diabetes (mean age 21.6 ± 4.1 years and mean duration 7.6 ± 1.8 years). Participants with type 2 diabetes had a higher BMI z score and waist circumference, were more likely to be smokers, and had higher blood pressure and lipid levels than youth with type 1 diabetes (all P < 0.001). A1C, however, did not significantly differ between the two groups (mean A1C 8.8 ± 1.8% [73 ± 2 mmol/mol] for type 1 diabetes and 8.5 ± 2.9% [72 ± 3 mmol/mol] for type 2 diabetes; P = 0.5) but was higher than that recommended by the ADA for this age-group (A1C ≤7.5%) (10). The prevalence of DPN (defined as the MNSIE score >2) was 8.2% among youth with type 1 diabetes and 25.7% among those with type 2 diabetes.
Table 1.
Characteristics of the study population by diabetes status: the SEARCH for Diabetes in Youth DPN pilot study
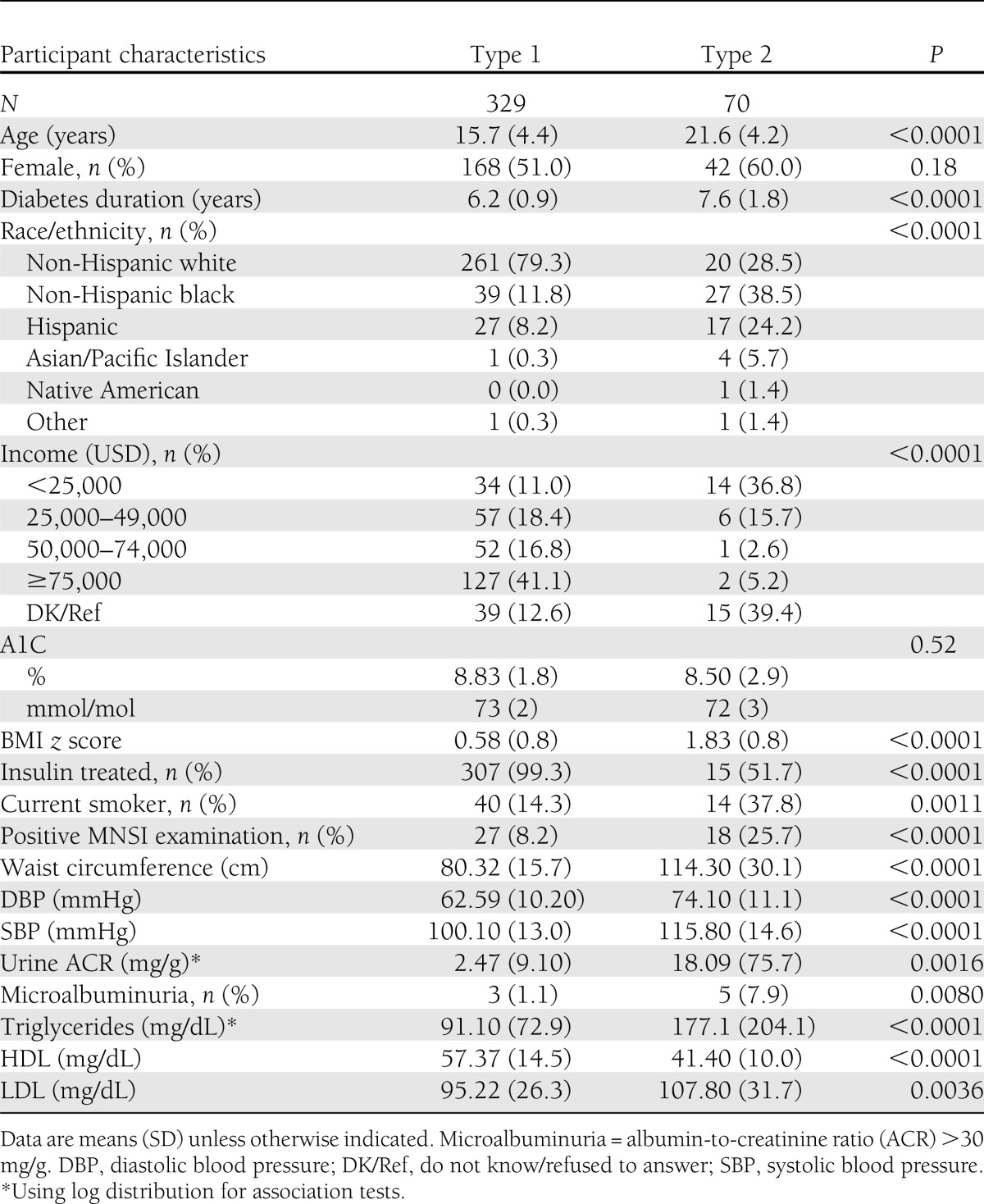
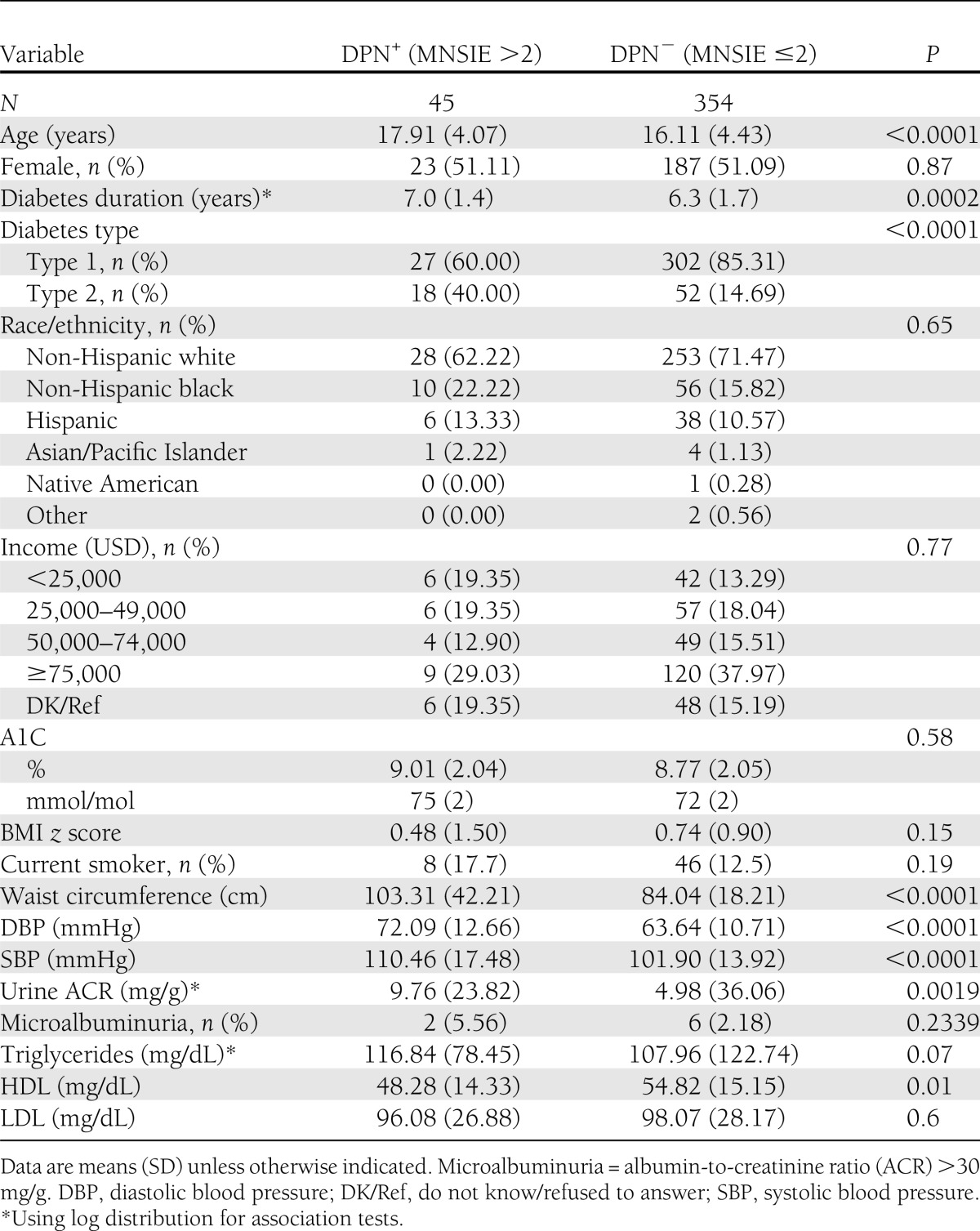
Unadjusted associations between demographic, anthropometric, and metabolic parameters and DPN are presented in Table 2. Youth with DPN were older and had a longer duration of diabetes, greater central obesity (increased waist circumference), higher blood pressure, an atherogenic lipid profile (low HDL cholesterol and marginally high triglycerides), and microalbuminuria. A1C, although above that recommended by ADA for optimal glycemic control among youth, was not significantly different between those with and without DPN (9.0% ± 2.0 or 75.0 ± 2 0.0 mmol/mol vs. 8.8% ± 2.1 or 72.0 ± 2.0 mmol/mol, P = 0.58). Although nearly 37% of youth with type 2 diabetes came from lower-income families with annual income <25,000 USD per annum (as opposed to 11% for type 1 diabetes), socioeconomic status was not significantly associated with DPN (P = 0.77).
Table 2.
Differences in the demographic, anthropometric, and metabolic parameters between youth with and youth without DPN: the SEARCH for Diabetes in Youth DPN pilot study
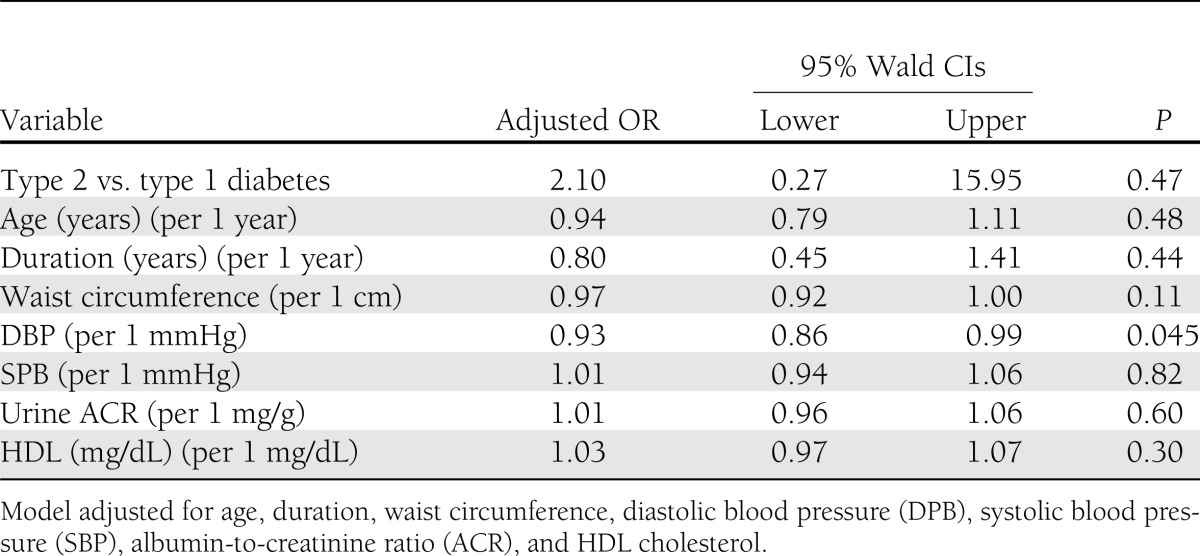
In the unadjusted logistic regression model, the odds of having DPN was nearly four times higher among those with type 2 diabetes compared with youth with type 1 diabetes (odds ratio [OR] 3.8 [95% CI 1.9–7.5, P < 0.0001). This association was attenuated, but remained significant, after adjustment for age and sex (OR 2.3 [95% CI 1.1–5.0], P = 0.03). However, this association was no longer significant (OR 2.1 [95% CI 0.3–15.9], P = 0.47) when additional covariates, significant in the univariate analyses (age, diabetes duration, waist circumference, blood pressure, HDL cholesterol, and microalbuminuria), were added to the model (Table 3).
Table 3.
Adjusted OR and 95% CI for factors associated with DPN from multiple logistic regression analysis: the SEARCH for Diabetes in Youth DPN pilot study
CONCLUSIONS
In a pilot study of a multiethnic cohort of youth with diabetes, the prevalence of DPN was 8.2% among youth with type 1 diabetes and 25.7% among those with type 2 diabetes. Youth with DPN were more likely to have type 2 diabetes and an adverse cardiovascular risk profile (central obesity, higher blood pressure, and lower HDL) and microalbuminuria. The prevalence of DPN among type 1 diabetes youth in our pilot study is lower than that reported by Eppens et al. (15) among 1,433 Australian adolescents with type 1 diabetes assessed by thermal threshold testing and VPT (prevalence of DPN 27%; median age and duration 15.7 and 6.8 years, respectively). A much higher prevalence was also reported among Danish (62.5%) and Brazilian (46%) cohorts of type 1 diabetes youth (16,17) despite a younger age (mean age among Danish children 13.7 years and Brazilian cohort 12.9 years). The prevalence of DPN among youth with type 2 diabetes (26%) found in our study is comparable to that reported among the Australian cohort (21%) (15). The wide ranges in the prevalence estimates of DPN among the young cannot solely be attributed to the inherent racial/ethnic differences in this population but could potentially be due to the differing criteria and diagnostic tests used to define and characterize DPN. The Australian cohort used thermal and VPT to assess DPN, while the Danish cohort used VPT only.
Metabolic syndrome components such as central obesity, elevated blood pressure, and dyslipidemia have been implicated in the pathogenesis of DPN. In the EURODIAB study which prospectively followed 1,172 subjects with type 1 diabetes for 7.3 years (mean age 30 ± 8.8 years, A1C 8.0 ± 1.8%, and duration 12 ± 8 years) the authors reported that, apart from glycemic control, the incidence of DPN was significantly associated with potentially modifiable cardiovascular risk factors, including raised triglyceride levels, BMI, smoking, and hypertension (18). In the population-based Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA)/Cooperative Health Research in the Region of Augsburg (KORA) study, DPN assessed by MNSI was associated with increasing age and abdominal obesity (19). The Pittsburgh Epidemiology of Diabetes Complications Study (EDC) has established that low HDL cholesterol is associated with prevalent polyneuropathy (20) and that hypertension and smoking are predictors of incident polyneuropathy (21). These data thus support the relationship between DPN and cardiovascular risk factors (central obesity, lower HDL, and elevated blood pressure) found in our study, although in a much younger cohort.
In our study, the duration of diabetes was significantly longer among those with DPN, but A1C values did not differ significantly between the two groups, suggesting that a longer duration with its sustained impact on peripheral nerves is an important determinant of DPN. However, the A1C was above that recommended by ADA for optimal glycemic control among both youth with and youth without DPN. Cho et al. (22) reported an increase in the prevalence of DPN from 14 to 28% over 17 years among 819 Australian adolescents with type 1 diabetes aged 11–17 years at baseline, despite improvements in care and minor improvements in A1C (8.2–8.7%). The prospective Danish Study Group of Diabetes in Childhood also found no association between DPN (assessed by VPT) and glycemic control (23).
Nerve conduction studies are still regarded as the gold standard measure to assess DPN, but the discomfort associated with the procedure and significant investment in time, personnel training, and costs preclude their use in routine clinical practice. Assessment using the MNSI, however, is noninvasive and easy to use in large, multicenter cohorts and in adults was shown to be specific (95%) and sensitive (80%) for identifying the presence of DPN, with a positive predictive value of 97% and a negative predictive value of 74% (11,13).
The ADA recommends that health care providers perform an annual comprehensive foot examination for all patients with diabetes to identify neuropathy risk factors predictive of ulcers and amputations (24). This examination should include inspection, assessment of foot pulses, and testing for loss of protective sensation (10-g monofilament plus testing any one of the following: vibration using 128-Hz tuning fork, pinprick sensation, ankle reflexes, or VPT). The MNSI incorporates most of these tests recommended by the ADA and is thus an ideal bedside screening tool for DPN.
Our study has limitations that must be taken into consideration. The results of this study should be interpreted cautiously, as the MNSI has not been validated in children and adolescents, although several of its components (vibration perception using 128-Hz tuning fork, pinprick sensation, and ankle reflexes) have been used among the pediatric population for DPN screening. The cross-sectional design of the study and the lack of information on prior A1C data could be a reason for the lack of association between DPN and A1C, as we could not include a weighted A1C over time into the model. The loss of the association between diabetes type and DPN with addition of covariates in the fully adjusted model could be due to power loss, given the small number of youth with DPN in the sample, or indicative of stronger associations between these covariates and DPN such that conditioning on them eliminates the observed association between DPN and diabetes type. More data are needed to further characterize the relationship between DPN and diabetes type in youth. While the data presented come from a pilot study conducted in 2009–2010, the SEARCH cohort study is currently collecting data on DPN in an estimated sample of 3,000 youth with type 1 and type 2 diabetes, which will have more power and allow a more comprehensive evaluation of prevalence of and risk factors for the development and progression of DPN.
In conclusion, our pilot study found evidence that the prevalence of DPN in adolescents with type 2 diabetes approaches rates reported in adults with diabetes. Several CVD risk factors such as central obesity, elevated blood pressure, dyslipidemia, and microalbuminuria, previously identified as predictors of DPN among adults with diabetes, emerged as independent predictors of DPN in this young cohort and likely accounted for the increased prevalence of DPN in youth with type 2 diabetes. Awareness and periodic assessment of this complication in its early subclinical stage might prevent the poor quality of life associated with DPN in the future by allowing early application of suitable interventions. Further long-term study of DPN in youth is needed.
Acknowledgments
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA no. 00097, DP-05-069 and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site contract numbers are as follows: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Kuakini Medical Center (U58CCU919256 and U01 DP000245), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708-01), University of Washington School of Medicine (U58/CCU019235-4, U01 DP000244, and U18DP002710-01), and Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171). The authors acknowledge the involvement of General Clinical Research Centers at the South Carolina Clinical & Translational Research Institute, at the Medical University of South Carolina (National Institutes of Health [NIH]/National Center for Research Resources [NCRR] grant UL1RR029882), Children’s Hospital and Regional Medical Center (grant M01RR00037), Colorado Pediatric General Clinical Research Center (grant M01 RR00069), the Barbara Davis Center at the University of Colorado at Denver (DERC NIH P30 DK57516), the Institutional Clinical and Translational Science Award, NIH/NCRR at the University of Cincinnati (grant 1UL1RR026314-01), and the Children with Medical Handicaps program managed by the Ohio Department of Health.
No potential conflicts of interest relevant to this article were reported.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Institute of Diabetes and Digestive and Kidney Diseases.
M.J. wrote the manuscript. A.L. analyzed data. C.L.M. contributed to the analysis plan and discussion and reviewed and edited the manuscript. R.A.B. and J.D. contributed to the discussion and reviewed and edited the manuscript. D.D. contributed to the analysis plan and discussion and reviewed and edited the manuscript. D.J.P., S.S., C.P., D.A.S., and B.L.R. contributed to the discussion and reviewed and edited the manuscript. R.P.-B. and E.L.F. contributed to the analysis plan and discussion and reviewed and edited the manuscript. E.L.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The SEARCH for Diabetes in Youth Study is indebted to the many youth, and their families and their health care providers, whose participation made this study possible. The authors thank Stacey A. Sakowski Jacoby, Deputy Managing Director, A. Alfred Taubman Medical Research Institute, University of Michigan, and Catherine Stables, postdoctoral fellow, Department of Neurology, University of Michigan, for critically reviewing the manuscript.
References
- 1.Patterson CC, Gyürüs E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 [DOI] [PubMed] [Google Scholar]
- 2.Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M, Consensus Workshop Group Type 2 diabetes in the young: the evolving epidemic: the international diabetes federation consensus workshop. Diabetes Care 2004;27:1798–1811 [DOI] [PubMed] [Google Scholar]
- 3.Imperatore G, Boyle JP, Thompson TJ, et al. SEARCH for Diabetes in Youth Study Group Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012;35:2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulton AJ, Vinik AI, Arezzo JC, et al. American Diabetes Association Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28:956–962 [DOI] [PubMed] [Google Scholar]
- 5.Tesfaye S, Stevens LK, Stephenson JM, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 1996;39:1377–1384 [DOI] [PubMed] [Google Scholar]
- 6.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–154 [DOI] [PubMed] [Google Scholar]
- 7.Katulanda P, Ranasinghe P, Jayawardena R, Constantine GR, Sheriff MH, Matthews DR. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol Metab Syndr 2012;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albers JW, Herman WH, Pop-Busui R, et al. Diabetes Control and Complications Trial /Epidemiology of Diabetes Interventions and Complications Research Group Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010;33:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, et al.; DCCT/EDIC Research Group. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed]
- 10.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 12.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg 2006;108:477–481 [DOI] [PubMed] [Google Scholar]
- 13.Herman WH, Pop-Busui R, Braffett BH, et al. DCCT/EDIC Research Group Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SEARCH Study Group SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 15.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 16.Olsen BS, Sjølie A, Hougaard P, et al. Danish Study Group of Diabetes in Childhood A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. J Diabetes Complications 2000;14:295–300 [DOI] [PubMed] [Google Scholar]
- 17.Nery Ferreira BE, Silva IN, de Oliveira JT. High prevalence of diabetic polyneuropathy in a group of Brazilian children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2005;18:1087–1094 [DOI] [PubMed] [Google Scholar]
- 18.Tesfaye S, Chaturvedi N, Eaton SE, et al.; EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005 27;352:341–350 [DOI] [PubMed]
- 19.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care 2008;31:464–469 [DOI] [PubMed] [Google Scholar]
- 20.Maser RE, Steenkiste AR, Dorman JS, et al. Report from Pittsburgh Epidemiology of Diabetes Complications Study Epidemiological correlates of diabetic neuropathy. Diabetes 1989;38:1456–1461 [DOI] [PubMed] [Google Scholar]
- 21.Forrest KY, Maser RE, Pambianco G, Becker DJ, Orchard TJ. Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes 1997;46:665–670 [DOI] [PubMed] [Google Scholar]
- 22.Cho YH, Craig ME, Hing S, et al. Microvascular complications assessment in adolescents with 2- to 5-yr duration of type 1 diabetes from 1990 to 2006. Pediatr Diabetes 2011;12:682–689 [DOI] [PubMed] [Google Scholar]
- 23.Olsen BS, Johannesen J, Sjølie AK, et al. Danish Study Group of Diabetes in Childhood Metabolic control and prevalence of microvascular complications in young Danish patients with Type 1 diabetes mellitus. Diabet Med 1999;16:79–85 [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Executive summary: Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]


