Abstract
OBJECTIVE
To evaluate if presence of cardiovascular (CV) risk factors and their clustering as metabolic syndrome (MetS) is associated with increased arterial stiffness and accelerated progression over time among youth with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Longitudinal study of 298 youth with type 1 diabetes (age 14.5 years; 46.3% female; duration 4.8 years), with two research visits conducted 5 years apart. CV factors included: waist circumference, blood pressure (BP), fasting lipids (HDL cholesterol, LDL cholesterol [LDL-c], triglycerides), albumin/creatinine ratio, and HbA1c. MetS was based on Adult Treatment Panel III criteria modified for youth. Pulse wave velocity (PWV) in the carotid–femoral segment was measured by tonometry. Mixed models were used to assess the rate of progression in PWV and the association between CV factors and PWV over time.
RESULTS
PWV increased significantly over time (0.145 m/s/year; P < 0.0001). MetS (P = 0.0035), large waist (P < 0.0001), and elevated BP (P = 0.0003) at baseline were each associated with worse PWV over time. These baseline factors, however, did not significantly influence the rate of progression. Increases in waist circumference (P < 0.0001), LDL-c levels (P = 0.0156), and declining glucose control (HbA1c; P = 0.0419) were independently associated with higher PWV over time.
CONCLUSIONS
Presence, clustering, and worsening of CV risk factors are associated with increased arterial stiffness over time in youth with type 1 diabetes. Whether improvement in CV risk factors early in life will slow the progression of arterial stiffness and reduce the burden of CV disease in this population requires further study.
Adults with childhood-onset type 1 diabetes are at increased risk of premature cardiovascular disease (CVD) morbidity and mortality (1,2). Increased arterial stiffness independently predicts all-cause and CVD mortality (3), and higher pulse pressure predicts CVD mortality, incidence, and end-stage renal disease development among adults with type 1 diabetes (1,4,5). Several reports have shown that youth and adults with type 1 diabetes have elevated arterial stiffness, though the mechanisms are largely unknown (6). The etiology of advanced atherosclerosis in type 1 diabetes is likely multifactorial, involving metabolic, behavioral, and diabetes-specific cardiovascular (CV) risk factors. Aging, high blood pressure (BP), obesity, the metabolic syndrome (MetS), and type 2 diabetes are the main contributors of sustained increased arterial stiffness in adults (7,8). However, the natural history, the age-related progression, and the possible determinants of increased arterial stiffness in youth with type 1 diabetes have not been studied systematically.
Childhood obesity has been shown to be related to CV risk factors in young adulthood, and adult obesity is also a risk factor for CVD (9). Central adiposity is considered an even more accurate indicator of CVD risk in adults, and it was shown to predict coronary artery disease risk even among young people (10). Unfortunately, children with type 1 diabetes, who previously were not overweight, are no longer immune to obesity. Data from the SEARCH for Diabetes in Youth Study showed that 31.5% of non-Hispanic white (NHW), 46.6% of African Americans, and 45% of Hispanic youth with type 1 diabetes had a BMI ≥85th percentile (11). A sizeable proportion of such youth have also been shown to have elevated BP (12), dyslipidemia (13), and elevated albumin/creatinine ratio (ACR) (14). Moreover, a clustering of CV risk factors (i.e., at least two, in addition to hyperglycemia) were present in 14% of SEARCH youth with type 1 diabetes (12), clearly an excess compared with the general population.
There are currently no data examining the impact of CV risk factors and their clustering in youth with type 1 diabetes on subsequent CVD morbidity and mortality, though childhood and young adult MetS have been shown to predict arterial stiffness in adults (15,16). Thus, the aims of this report were: 1) to describe the progression of arterial stiffness, as measured by pulse wave velocity (PWV), over time, among youth with type 1 diabetes, and 2) to explore the association of CV risk factors and their clustering as MetS with PWV in this cohort.
RESEARCH DESIGN AND METHODS
Study design and participants
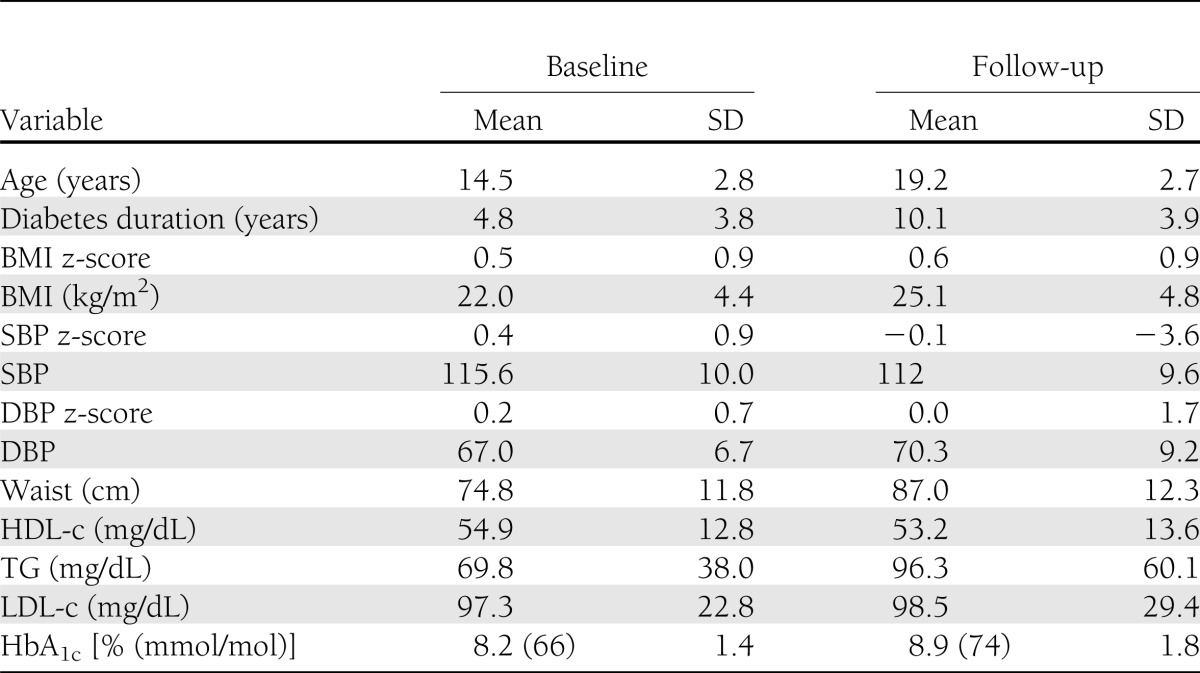
SEARCH CVD is an ancillary study to the SEARCH for Diabetes in Youth, conducted in Colorado and Ohio. SEARCH is a multicenter study that conducts population-based ascertainment of nongestational cases of physician-diagnosed diabetes in youth age <20 years at diagnosis (17). This report includes a cohort of 298 youth with physician-diagnosed type 1 diabetes from Colorado and Ohio who had data on PWV and CV risk factors measured ∼5 years apart, in 2004–2005 and 2009–2011, starting when youth were on average age 14.5 (SD 2.8) years and had a duration of diabetes of 4.8 (SD 3.8) years. Participants had data on demographic, anthropometric, and metabolic factors, including HbA1c. The study was reviewed and approved by the local institutional review boards that had jurisdiction over the local study population, and all participants provided signed informed consent or assent.
Anthropometric and metabolic measurements
SEARCH CVD participants had two outpatient research visits (baseline and follow-up), each following the same standard protocol. Visits were conducted after an 8-h overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit until after the blood draw was complete. Race/ethnicity was self-reported, and the participants were categorized into NHW and other racial/ethnic groups (including Hispanic, African American, and Asian/Pacific Islander racial/ethnic groups). Participants completed standardized questionnaires including medical history, medication inventory, daily insulin dose, family history of diabetes, and CVD. BMI was calculated as weight (kilograms) divided by height in meters squared, and age- and sex-specific BMI z-scores were derived (18). Waist circumference was measured to the nearest 0.1 cm with the National Health and Nutrition Examination Survey protocol (14). Resting systolic BP (SBP) and diastolic BP (DBP) were measured three times while the subjects were seated for at least 5 min, and the average of the three measurements was taken. Laboratory samples were obtained under conditions of metabolic stability, defined as no episode of diabetic ketoacidosis during the previous month. High-performance liquid chromatography (TOSOH Bioscience, Inc., San Francisco, CA) was used to measure HbA1c. Measurements of triglycerides (TGs), HDL cholesterol (HDL-c), and urinary creatinine were performed using Roche reagent on a Roche Modular P autoanalyzer (Roche Diagnostics, Indianapolis, IN). LDL-cholesterol (LDL-c) was calculated by the Friedewald equation for individuals with TG concentration <400 mg/dL and by the Beta Quantification procedure for those with TGs of ≥400 mg/dL. Urinary albumin was measured using Siemens reagent on a Siemens BNII nephelometer (Siemens Healthcare Diagnostics Inc., Newark, DE). Albumin and creatinine were measured in first morning urine samples and used to compute ACR (14). Threshold levels for CV risk factors were determined based on the Adult Treatment Panel III criteria (19) modified for children (20) and included SBP or DBP ≥95th percentile for age, sex, and height; waist circumference ≥90th percentile for age and sex; HDL-c ≤40 mg/dL; and TG ≥110 mg/dL. The MetS was considered to be present if at least two of the above occurred, since all youth had diabetes. Other CV risk factors were categorized as abnormal if LDL-c was ≥130 mg/dL (19), ACR was ≥30 µg/mg (21), and HbA1c was ≥7.5% (22).
Outcome measurement
PWV was measured in the carotid–femoral segment (trunk) with the SphygmoCor-Vx device (AtCor Medical, Itasca, IL) after 10 min of supine rest as previously described (23) and as recommended in the American Heart Association guidelines (24). Distance from the proximal (carotid) to the femoral artery recording site was measured to the nearest 0.1 cm twice and averaged. A tonometer was used to collect proximal and distal arterial waveforms gated by the R-wave on the simultaneously recorded electrocardiogram. The PWV was the difference in the carotid-to-femoral path length divided by the difference in R-wave-to-waveform foot times. Results were the average of three measures taken sequentially. Higher PWV (meters per second) indicated increased central stiffness. Repeated measures showed excellent reproducibility with coefficients of variability <7% (23). A Bland-Altman plot showing good reproducibility is included in Supplementary Fig. 1.
Statistical analyses
We used repeated-measures general linear models (SAS Proc Mixed; SAS Institute, Cary, NC) using all data from the initial and follow-up visits to explore CV risk factors associated with PWV and progression of PWV over time. These models adjust for the correlation between repeated observations in the same individual and have the advantage of handling a varying number of observations within individuals, thereby allowing for inclusion of the maximum number of data points (25). Further, this method allows for a random intercept and slope in disease duration to account for within-individual dependence. The outcome was log-transformed time-varying PWV. The base model explored whether time (duration of follow-up) was associated with PWV, adjusted for baseline age, sex, and race/ethnicity. This model provided us with a rate of progression in PWV over time. A second set of models explored whether baseline CV risk factors (MetS, large waist circumference, high BP, high TG levels, low HDL-c, high LDL-c, elevated ACR, and abnormal HbA1c) were associated with time-varying PWV, adjusted for age, sex, race/ethnicity, diabetes duration, and other significant baseline covariates as appropriate. A third set of models explored whether baseline CV risk factors were associated with the slope of change in PWV over time; statistically, this analysis explores significant interactions between determinants of interest (baseline CV factors) and time (duration of follow-up) on PWV, adjusted for covariates. Finally, the last set of models explored whether the change in CV risk factors, now treated as continuous time-varying variables, was associated with time-varying PWV, adjusted for covariates. Specifically, these models estimated the percent change in PWV per change in one interquartile range (IQR) of each CV risk factor. All statistical analyses were conducted using SAS software, version 9.3 (SAS Institute).
RESULTS
Characteristics of the SEARCH CVD longitudinal cohort are presented in Table 1. Youth were age 14.5 years (SD 2.8) and had an average disease duration of 4.8 (3.8) years at baseline, 46.3% were female, and 87.6% were of NHW race/ethnicity. At baseline, 10.0% had high BP, 10.9% had a large waist circumference, 11.6% had HDL-c ≤40 mg/dL, 10.9% had a TG level ≥110 mg/dL, and 7.0% had at least two of the above CV risk factors (MetS). In addition, 10.3% had LDL-c ≥130 mg/dL, 72.0% had an HbA1c ≥7.5% (58 mmol/mol), and 9.2% had ACR ≥30 μg/mL. Follow-up measures were obtained on average at age 19.2 years, when the average duration of diabetes was 10.1 (3.9) years.
Table 1.
Characteristics of 298 youth with type 1 diabetes participating in the longitudinal component of SEARCH CVD
Baseline CV risk factors and PWV over time
Over an average follow-up period of ∼5 years, there was a statistically significant increase of 0.7 m/s in PWV (from 5.2 to 5.9 m/s), representing an annual increase of 2.8% or 0.145 m/s. Table 2 shows the associations of various CV risk factors at baseline with PWV over time, adjusted for baseline age, sex, race/ethnicity, and duration of diabetes. Significant associations were seen for MetS (7.3% higher PVW; P = 0.0035), large waist (8.7% higher PWV; P < 0.0001), and high BP (7.1% higher PWV; P = 0.0003). Inclusion of baseline high BP in the model for large waist did not materially attenuate the association (right side, Table 2), nor did inclusion of large waist at baseline attenuate the association in the model for high BP. No significant associations were seen for high TG, low HDL-c, high LDL-c, elevated ACR, or suboptimal HbA1c at baseline.
Table 2.
Association of CV risk factors at baseline and PWV over time
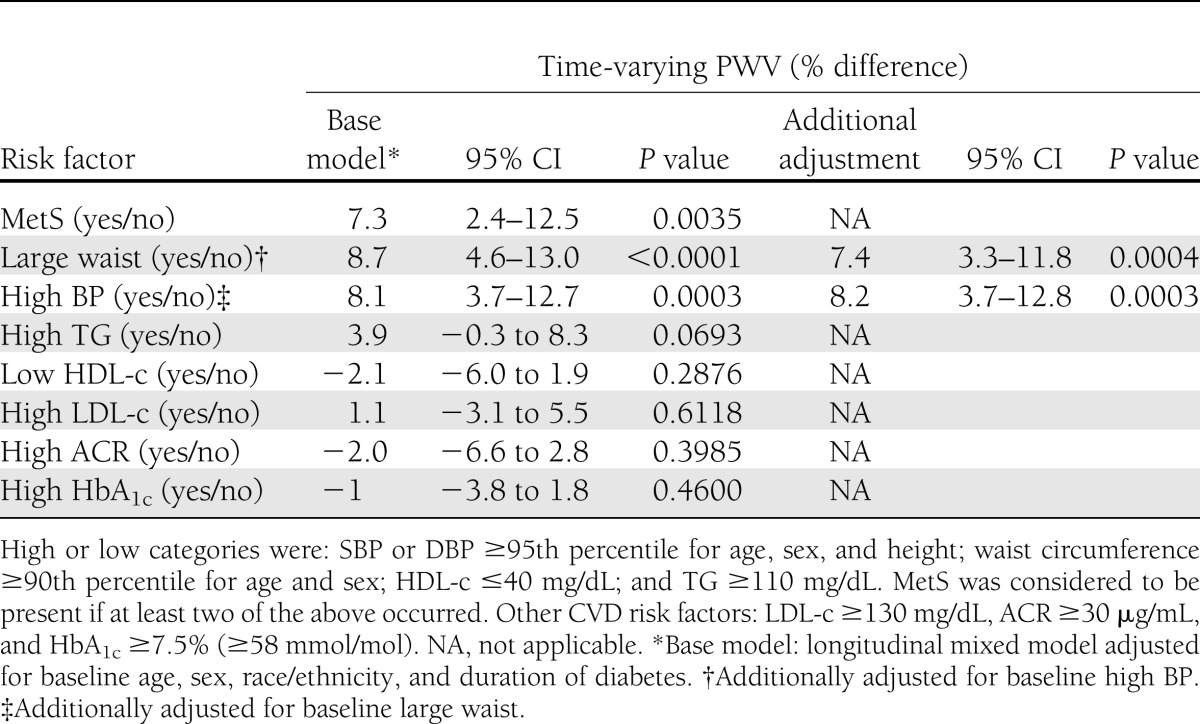
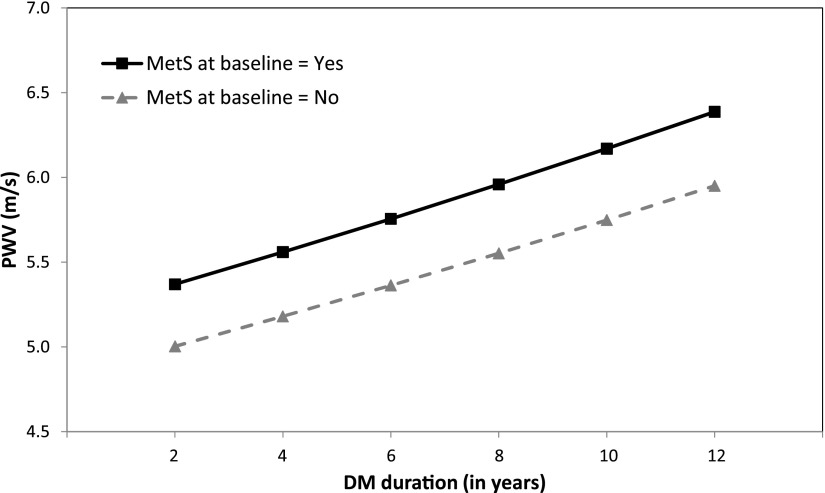
Figure 1 shows the model-based progression in PWV over time by presence of the MetS at baseline. The presence of MetS at baseline was associated with a 7.3% higher PWV over this time period (P = 0.0035); however, the presence of MetS at baseline did not influence the rate of progression in PWV (P = 0.855 for interaction between MetS and time on PWV). Similarly, large waist circumference (P < 0.0001) and high BP (P = 0.0003) at baseline were independently associated with higher PWV over time; however, they did not significantly influence PWV rate of change (interaction P = 0.391 for large waist; P = 0.662 for high BP).
Figure 1.
PWV over time by presence of MetS at baseline. Solid black line, MetS present; dashed gray line, MetS absent. P value for differences in intercept (level of PWV) = 0.0035; P value for difference in slopes = 0.85. DM, diabetes mellitus.
Changes in CV risk factors and PWV over time
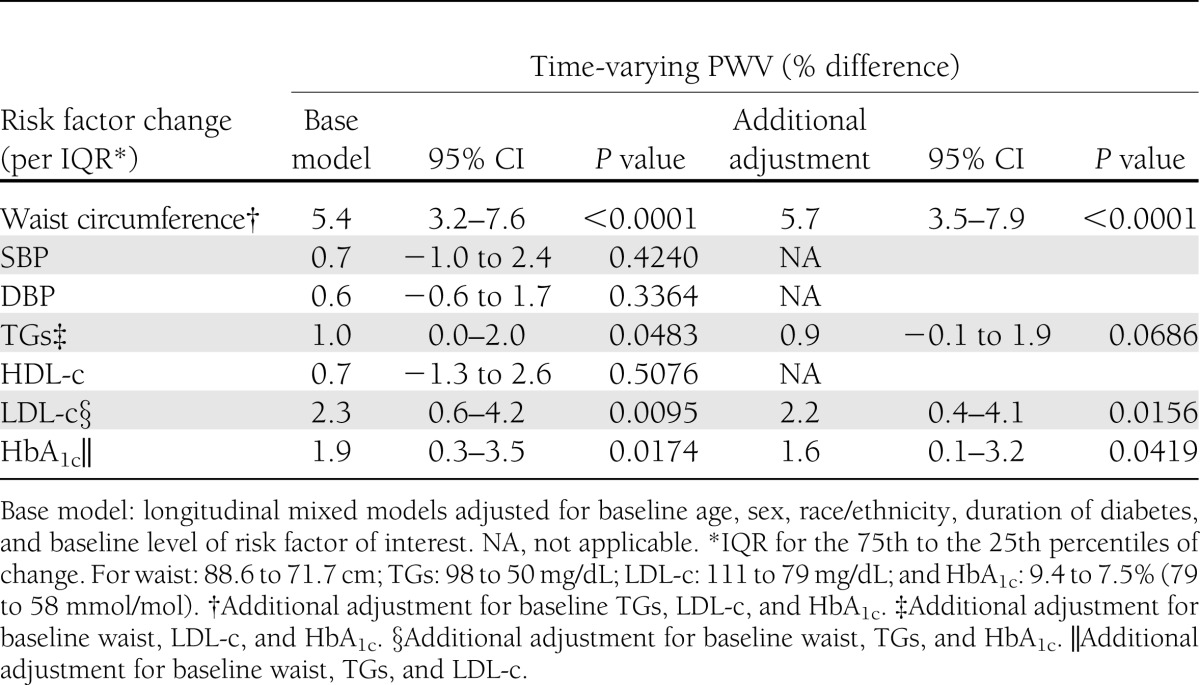
We next explored whether changes in CV risk factors (explored as continuous variables, with change standardized to the IQR of each variable) influenced PWV over time (Table 3). The models predicted that increases in waist circumference, TGs, LDL-c, and HbA1c levels were independently associated with significantly higher PWV over time, adjusted for age, sex, race/ethnicity, duration of diabetes, and baseline level of risk factor of interest. Specifically, for an increase of one IQR (from 25th to the 75th percentile) in the CV risk factor of interest over time, we found the following: 1) an increase in waist circumference of 17 cm resulted in a 5.4% higher PWV (P < 0.0001); 2) an increase in TG levels of 48 mg/dL resulted in a 1.0% higher PWV (P = 0.0483); 3) an increase in LDL-c of 32 mg/dL resulted in a 2.3% higher PWV (P = 0.0095); and 4) an increase in HbA1c of 2% resulted in a 1.9% higher PWV (P = 0.0174). When the model for waist circumference was further adjusted for baseline CV risk factors (Table 3, right side), no attenuation of the association was seen. Similarly, further adjustment of the LDL-c model and the HbA1c model for baseline CV risk factors made only slight changes in the estimates of the associations, which remained significant. Adjustment for baseline CV risk factors attenuated the TG association slightly, reducing the level of statistical significance to P = 0.0686, though with a very small decrease in the main effect size. Thus, it appears that changes in waist circumference, LDL-c, and HbA1c are independently associated with change in PWV over follow-up. No significant associations with PWV were seen for changes in SBP, DBP, or HDL-c over time. Of note, only four participants (1.3%) were on antihypertensive or dyslipidemia medications at baseline, and this increased to 27 participants (9.1%) at follow-up. However, medication use was not associated with PWV (P = 0.661), likely because of low usage in this population.
Table 3.
Changes in CVD risk factors and PWV over time
CONCLUSIONS
We report the first longitudinal study to evaluate risk factors for presence and progression of arterial stiffness over time in youth with type 1 diabetes. We estimated a significant increase of 0.145 m/s/year in PWV in youth with type 1 diabetes and short disease duration, over a period of only 5 years. Based on our data, if this rate of change is stable over time, the estimated average PWV by the time these youth enter their third decade of life will be 11.3 m/s, which was shown to be associated with a threefold increased hazard for major CV events (26). There are no similar studies in youth to compare these findings. In adults, the rate of change in PWV was 0.081 m/s/year in nondiabetic normotensive patients, although it was higher in hypertensive adults (0.147 m/s/year) (7). We also showed that the presence of central adiposity and elevated BP at baseline, as well as clustering of at least two CV risk factors, was associated with significantly worse PWV over time, although these baseline factors did not significantly influence the rate of change in PWV over this period of time. Changes in CV risk factors, specifically increases in central adiposity, LDL-c levels, and worsening glucose control, were independently associated with worse PWV over time.
There are a number of cross-sectional studies of arterial stiffness in youth and adults with type 1 diabetes (6), but few prospective studies, and only in adults. Prince et al. (27) examined risk markers of arterial stiffness among adults with youth-onset type 1 diabetes (aged in their mid-40s at follow-up). They found that prior presence of CV autonomic neuropathy, low HDL-c, higher HbA1c, and smoking history predicted worse measures of arterial stiffness 18 years later. Thus, arterial stiffness in adults with longstanding type 1 diabetes is predicted by some of the same variables we found, although not by central adiposity. Urbina et al. (28) have also reported higher arterial stiffness in youth aged 10–24 years with type 2 diabetes compared with lean or obese nondiabetic youth after adjusting for differences in CV risk factors cross-sectionally. With the exception of mean arterial BP, none of the other MetS variables were related to carotid–femoral PWV once obesity or diabetes was accounted for. Glycemia was not studied directly; however, those with type 2 diabetes had higher levels of fasting glucose and HbA1c than equally obese controls.
Three longitudinal studies have explored MetS and change in arterial stiffness in nondiabetic adults (8,29,30). Safar et al. (8) in 1,080 adults (average age of 51 years at baseline) found that the presence of MetS was associated with a worse annual rate of change in PWV over 6.5 years, such that persons with three or more CV risk factors (equivalent to MetS in individuals without diabetes) had an estimated rate of change of 0.148 m/s/year, similar to the rate we found. Interestingly, among those without MetS, slight decreases in PWV were seen. A higher rate of change with increasing numbers of MetS characteristics was noted (8), whereas we did not find a higher rate of change in youth with type 1 diabetes and the MetS compared with those without MetS. Of note, in this population, the baseline PWV levels of persons with the MetS were on average 12.2 m/s, compared with only 5.2 m/s in our study. Tomiyama et al. (29) studied 2,080 healthy Japanese male employees aged 42 years (range 29–76 years) with 3 years of follow-up. Among those with MetS at the baseline examination, ankle–brachial PWV was significantly higher and highest over time in the group with persistent MetS compared with those without MetS or to those with regression of MetS. These findings suggest that in larger populations of nondiabetic adults, MetS predicts a higher rate of change in PWV over time. Since our youth with type 1 diabetes and MetS had a higher baseline PWV than those without MetS (which continued to remain higher over the follow-up period), it seems likely that they must have had a higher rate of change than those without MetS at some point in time, perhaps prior to our baseline examination. Our inability to detect a difference in the rate of change in PWV in our youth with MetS (vs. those without MetS) may be due to several factors, including a combination of a relatively small sample size, short period of follow-up, and young age of the cohort (thus with lower baseline PWV levels).
In a study of healthy young adults aged 30 years at baseline with 2 years of follow-up, Wildman et al. (30) found an annual rate of change in PWV of 0.021 m/s/year, substantially lower than our finding. MetS was not directly studied, but weight change was highly associated with change in PWV, being ∼0.061 m/s/year higher for every 5 pounds of weight gain. Weight loss resulted in lower PWV over time as well. These results are consistent with our finding that a large waist circumference and increases in waist circumference were associated with higher PWV over time. A cross-sectional study of young adults aged 24–44 years in the Bogalusa Heart Study also found higher brachial to ankle PWV with increasing numbers of MetS components (16). The Cardiovascular Risk in Young Finns study explored childhood and young adult risk factors for arterial stiffness measured 27 years later in nondiabetic adults (15). Childhood SBP, glucose levels, adult SBP, insulin levels, and TG levels all independently predicted later arterial stiffness. There was a significant trend in both children and adults such that a higher number of risk factors (especially 3+) predicted higher arterial stiffness. Reduction in the number of risk factors over time and reduced obesity both predicted improved stiffness.
In addition to adults, studies in children and adolescents without diabetes have found associations between the MetS and components and arterial stiffness (31,32). Whincup et al. (31) studied 471 youth aged 13–15 years, with some longitudinal data from age 9 to 11 years in a subgroup. Using brachial distensibility as the measure of arterial stiffness, they found that BMI, insulin resistance by homeostasis model assessment, DBP, and LDL-c, as well as the number of MetS components, were associated with lower distensibility (increased stiffness) (31). In a study of 100 obese children aged 6–14 years, Iannuzzi et al. (32) found that MetS was strongly associated with carotid arterial stiffness compared with obese youth without MetS. Thus, among nondiabetic subjects across the entire age range, MetS and increasing numbers of MetS components have been shown to be associated with increased arterial stiffness (6). Our findings extend these observations to youth with type 1 diabetes at young ages and with short duration of diabetes.
Obesity, and especially central obesity, are major components of the MetS, and numerous studies have found associations between adiposity measures and arterial stiffness (6,33,34). We found that a larger baseline waist circumference and increases in waist circumference over time were associated with higher PWV levels in youth with type 1 diabetes, independent of other baseline CV risk factors. Since a much higher proportion of youth with type 1 diabetes are now overweight or obese (11), this additional risk factor for premature atherosclerosis must be addressed as part of any approach to CV risk reduction. We also found that increases in lipid levels, especially LDL-c, and worsening glycemic control were independently associated with increased arterial stiffness in youth with type 1 diabetes. While improved glycemic control is a central focus of diabetes care in youth, treatment of hypertension and dyslipidemia have received less attention, and few youth with type 1 diabetes received such medication.
Limitations and strengths
This study has some limitations. We only had two time points for assessment of arterial stiffness and risk factors, limiting our ability to determine the trajectory of changes and the full temporal sequence of events. For some participants, the second measurement was performed by a different technician; however, all technicians were trained centrally using the same standardized measurement protocol, and all PVW measures were conducted at the same locations in both centers. Only one HbA1c value was available at each point in time to assess glycemic control. However, we had a well-characterized group of contemporary youth with type 1 diabetes early in the course of their diabetes, allowing us to determine that arterial stiffness is elevated at a young age and stage of disease before other micro- and macrovascular complications have occurred. It is also the first prospective study of arterial stiffness and its’ determinants in such youth. Nevertheless, the combination of a relative small sample size, early in the evolution of the disease and followed over a relatively limited period of time may have reduced our ability to detect significant differences in the rate of progression of PWV according to baseline CV risk factors. Our further planned follow-up of this cohort will be able to address this aspect more conclusively.
In conclusion, we found that presence, clustering, and worsening of CV risk factors are associated with increased arterial stiffness over time in youth with type 1 diabetes. Whether improvement in CV risk factors early in life will slow the progression of arterial stiffness and reduce the burden of CV disease in this population requires further study.
Acknowledgments
The SEARCH CVD study was funded by National Institutes of Health grant R01-DK-078542 (to D.D.).
No potential conflicts of interest relevant to this article were reported.
D.D., R.P.W., E.M.U., and L.M.D. conducted the study and reviewed and edited the manuscript. J.W.T. and R.D. analyzed data. S.R.D. and R.F.H. contributed to the discussion and reviewed and edited the manuscript. S.M.M. conducted the laboratory analyses and reviewed and edited the manuscript. D.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented in abstract form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc13-0851/-/DC1.
References
- 1.Schram MT, Chaturvedi N, Fuller JH, Stehouwer CD, EURODIAB Prospective Complications Study Group Pulse pressure is associated with age and cardiovascular disease in type 1 diabetes: the Eurodiab Prospective Complications Study. J Hypertens 2003;21:2035–2044 [DOI] [PubMed] [Google Scholar]
- 2.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992-1999. Diabetologia 2006;49:660–666 [DOI] [PubMed] [Google Scholar]
- 3.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–2090 [DOI] [PubMed] [Google Scholar]
- 4.Gordin D, Wadén J, Forsblom C, et al. FinnDiane Study Group Pulse pressure predicts incident cardiovascular disease but not diabetic nephropathy in patients with type 1 diabetes (The FinnDiane Study). Diabetes Care 2011;34:886–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theilade S, Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P. Arterial stiffness and endothelial dysfunction independently and synergistically predict cardiovascular and renal outcome in patients with type 1 diabetes. Diabet Med 2012;29:990–994 [DOI] [PubMed] [Google Scholar]
- 6.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 2008;51:527–539 [DOI] [PubMed] [Google Scholar]
- 7.Benetos A, Adamopoulos C, Bureau JM, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002;105:1202–1207 [DOI] [PubMed] [Google Scholar]
- 8.Safar ME, Thomas F, Blacher J, et al. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol 2006;47:72–75 [DOI] [PubMed] [Google Scholar]
- 9.Bo S, Musso G, Gambino R, et al. Prognostic implications for insulin-sensitive and insulin-resistant normal-weight and obese individuals from a population-based cohort. Am J Clin Nutr 2012;96:962–969 [DOI] [PubMed] [Google Scholar]
- 10.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics 2001;108:712–718 [DOI] [PubMed] [Google Scholar]
- 11.Liu LL, Lawrence JM, Davis C, et al. SEARCH for Diabetes in Youth Study Group Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes 2010;11:4–11 [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care 2006;29:1891–1896 [DOI] [PubMed] [Google Scholar]
- 13.Kershnar AK, Daniels SR, Imperatore G, et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 2006;149:314–319 [DOI] [PubMed] [Google Scholar]
- 14.Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care 2007;30:2593–2598 [DOI] [PubMed] [Google Scholar]
- 15.Aatola H, Hutri-Kähönen N, Juonala M, et al. Lifetime risk factors and arterial pulse wave velocity in adulthood: the cardiovascular risk in young Finns study. Hypertension 2010;55:806–811 [DOI] [PubMed] [Google Scholar]
- 16.Li S, Chen W, Srinivasan SR, Berenson GS. Influence of metabolic syndrome on arterial stiffness and its age-related change in young adults: the Bogalusa Heart Study. Atherosclerosis 2005;180:349–354 [DOI] [PubMed] [Google Scholar]
- 17.SEARCH Study Group SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190 [PubMed] [Google Scholar]
- 19.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 20.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med 2003;157:821–827 [DOI] [PubMed] [Google Scholar]
- 21.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH. Diabetic nephropathy. Diabetes Care 2003;26(Suppl. 1):S94–S98 [DOI] [PubMed] [Google Scholar]
- 22.Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care 2005;28:186–212 [DOI] [PubMed] [Google Scholar]
- 23.Urbina EM, Wadwa RP, Davis C, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr 2010;156:731–737e1 [DOI] [PubMed] [Google Scholar]
- 24.Urbina EM, Williams RV, Alpert BS, et al. American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 2009;54:919–950 [DOI] [PubMed] [Google Scholar]
- 25.Brown H, Prescott R. Applied Mixed Models in Medicine. West Sussex, England, John Wiley & Sons, 2006 [Google Scholar]
- 26.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 2010;33:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens 2010;28:1692–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomiyama H, Hirayama Y, Hashimoto H, et al. The effects of changes in the metabolic syndrome detection status on arterial stiffening: a prospective study. Hypertens Res 2006;29:673–678 [DOI] [PubMed] [Google Scholar]
- 30.Wildman RP, Farhat GN, Patel AS, et al. Weight change is associated with change in arterial stiffness among healthy young adults. Hypertension 2005;45:187–192 [DOI] [PubMed] [Google Scholar]
- 31.Whincup PH, Gilg JA, Donald AE, et al. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation 2005;112:1789–1797 [DOI] [PubMed] [Google Scholar]
- 32.Iannuzzi A, Licenziati MR, Acampora C, et al. Carotid artery stiffness in obese children with the metabolic syndrome. Am J Cardiol 2006;97:528–531 [DOI] [PubMed] [Google Scholar]
- 33.Sakuragi S, Abhayaratna K, Gravenmaker KJ, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension 2009;53:611–616 [DOI] [PubMed] [Google Scholar]
- 34.Sutton-Tyrrell K, Newman A, Simonsick EM, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension 2001;38:429–433 [DOI] [PubMed] [Google Scholar]